

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50303032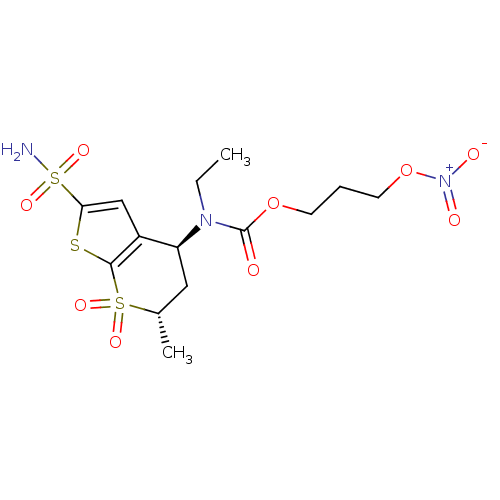 (CHEMBL584939 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50303030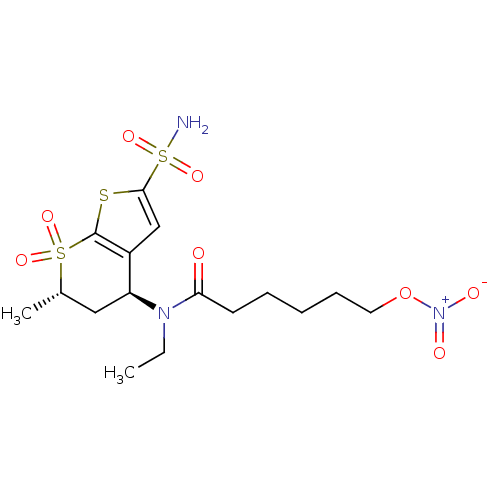 (6-Nitrooxy-hexanoic acid ethyl-((4S,6S)-6-methyl-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50303031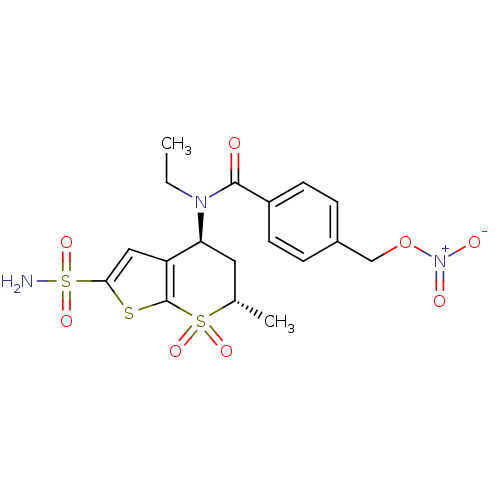 (CHEMBL570894 | N-Ethyl-N-((4S,6S)-6-methyl-7,7-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50303034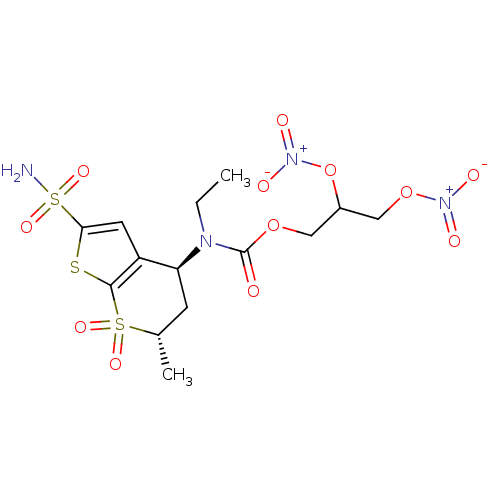 (CHEMBL571337 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50303031 (CHEMBL570894 | N-Ethyl-N-((4S,6S)-6-methyl-7,7-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50303033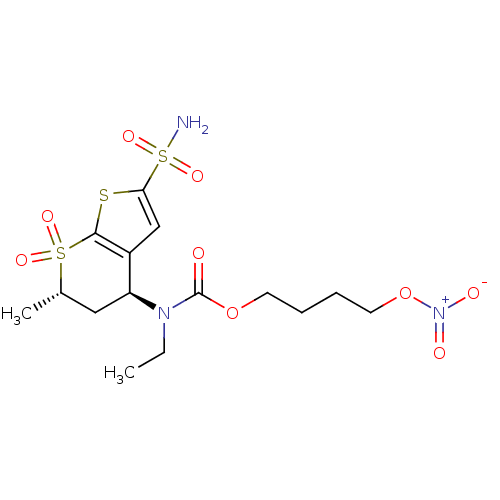 (CHEMBL585756 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50303032 (CHEMBL584939 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50303033 (CHEMBL585756 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50303032 (CHEMBL584939 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50303031 (CHEMBL570894 | N-Ethyl-N-((4S,6S)-6-methyl-7,7-dio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50303033 (CHEMBL585756 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 705 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50303034 (CHEMBL571337 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50303030 (6-Nitrooxy-hexanoic acid ethyl-((4S,6S)-6-methyl-7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50303034 (CHEMBL571337 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50303030 (6-Nitrooxy-hexanoic acid ethyl-((4S,6S)-6-methyl-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay | Bioorg Med Chem Lett 19: 6565-70 (2009) Article DOI: 10.1016/j.bmcl.2009.10.036 BindingDB Entry DOI: 10.7270/Q2X92C80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294427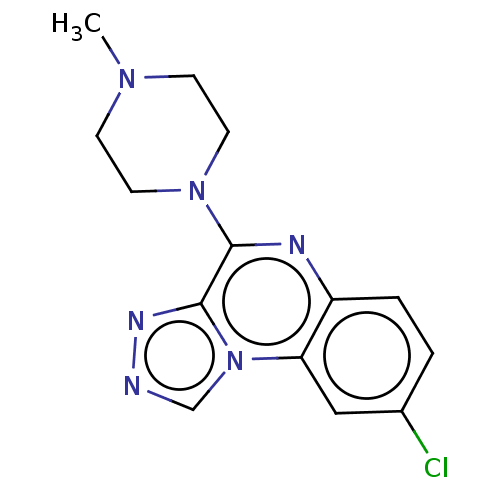 (US9586959, Compound disclosed in WO 2010030785, Ex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294407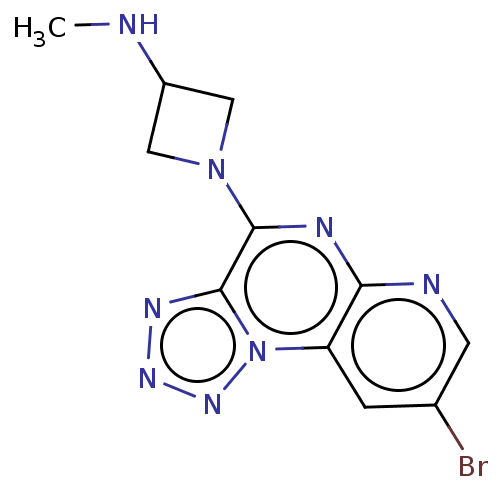 (US9586959, Compound 69 | US9586959, Compound 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294407 (US9586959, Compound 69 | US9586959, Compound 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at recombinant human histamine H4 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine induced st... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294418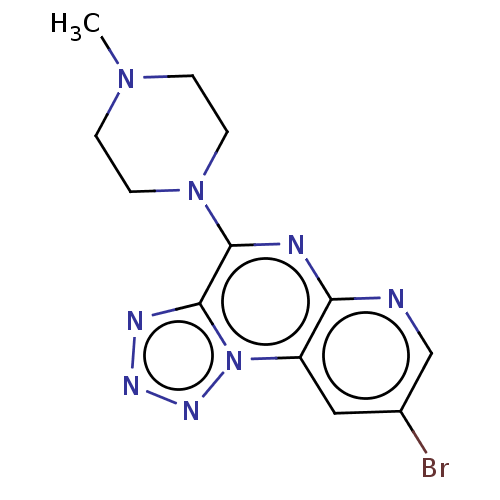 (US9586959, Compound 96) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294386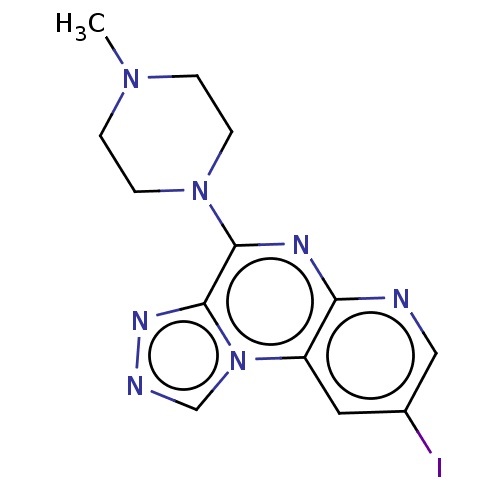 (US9586959, Compound 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294377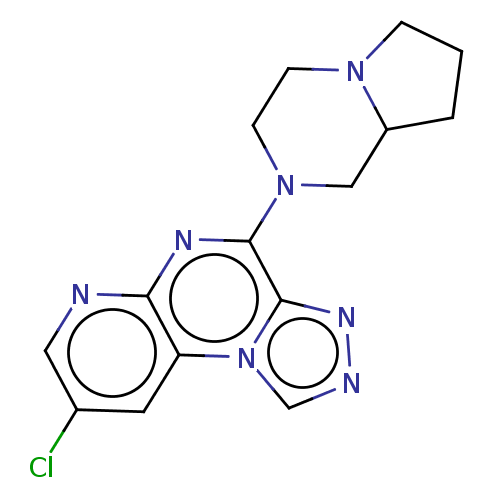 (US9586959, Compound 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294415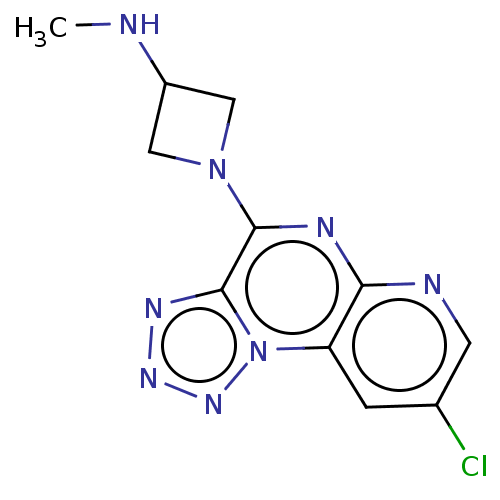 (US9586959, Compound 93) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM294361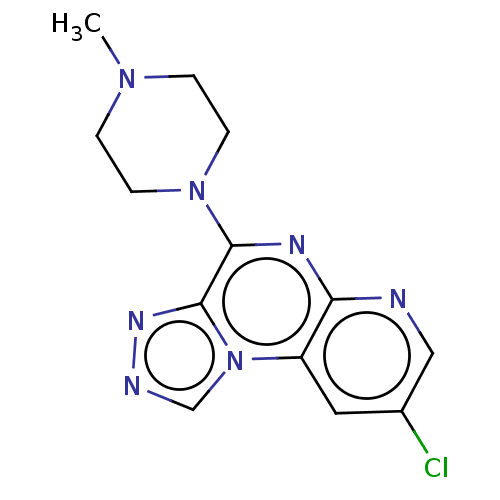 (US9586959, Compound 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]BRL 43694 from human recombinant 5-HT3 receptor after 120 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294416 (US9586959, Compound 94) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294368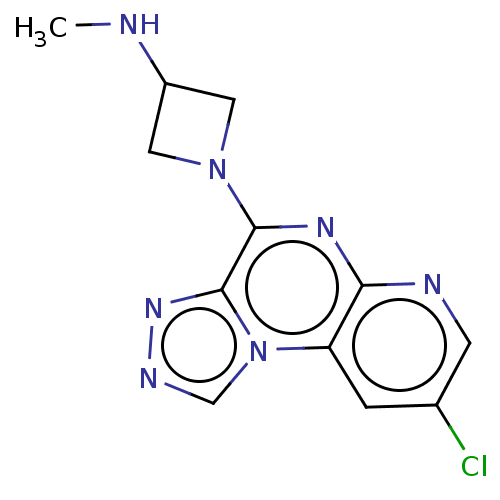 (US9586959, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294364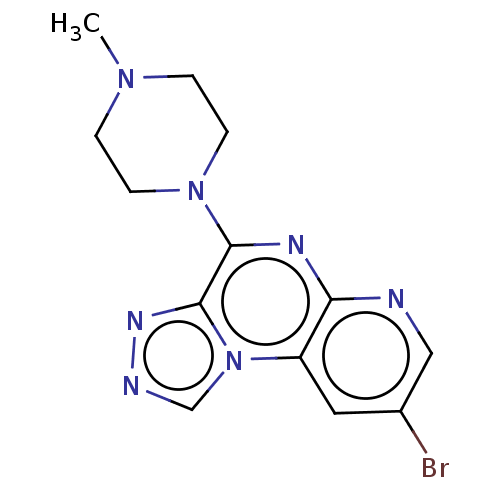 (US9586959, Compound 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294361 (US9586959, Compound 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294362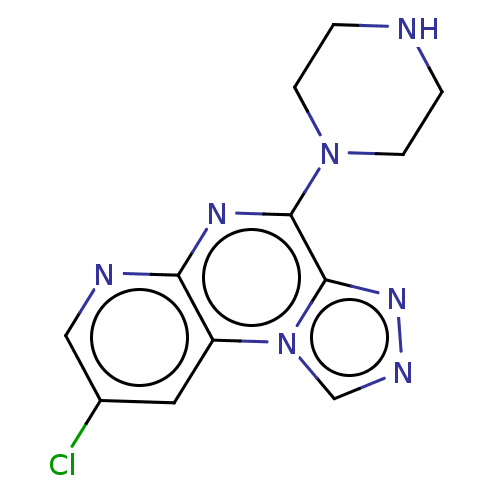 (US9586959, Compound 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50563967 (CHEMBL4798729) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294380 (US9586959, Compound 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294367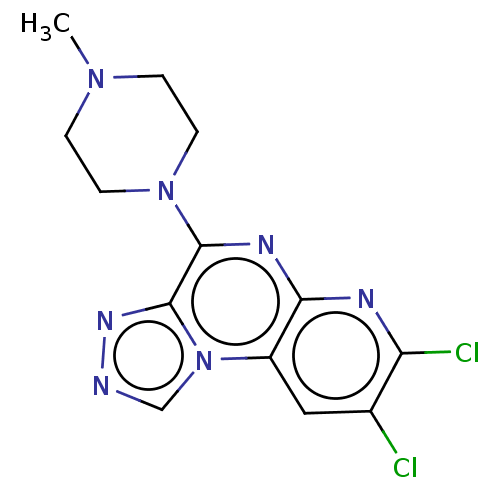 (US9586959, Compound 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50563970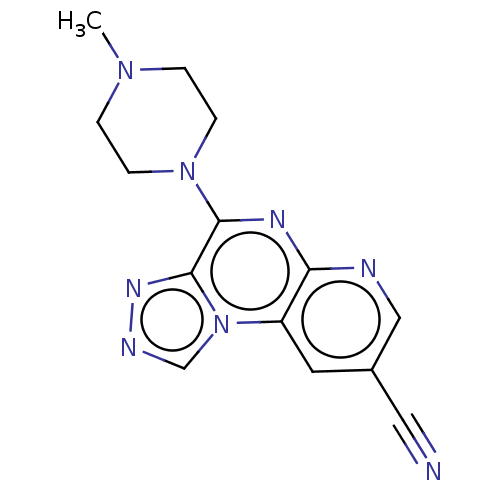 (CHEMBL4793425) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294398 (US9586959, Compound 64) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50563971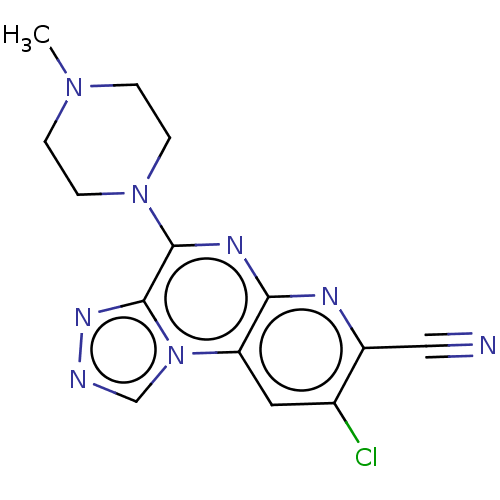 (CHEMBL4784683) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294390 (US9586959, Compound 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Mus musculus (mouse)) | BDBM294407 (US9586959, Compound 69 | US9586959, Compound 95) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant mouse histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294401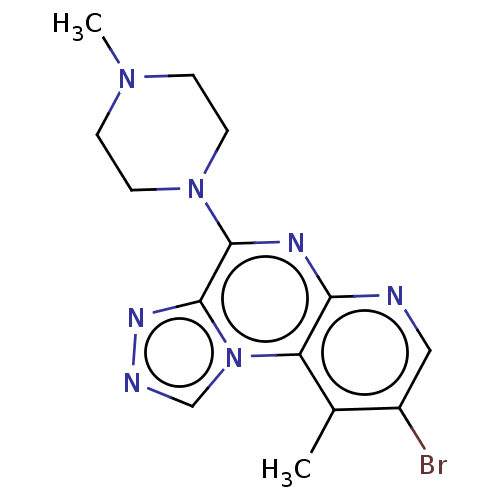 (US9586959, Compound 66) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294370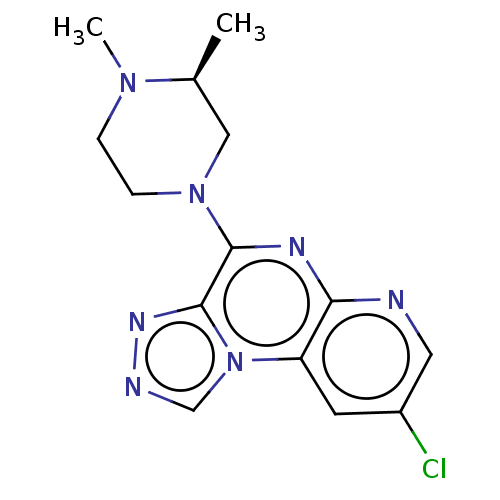 (US9586959, Compound 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294411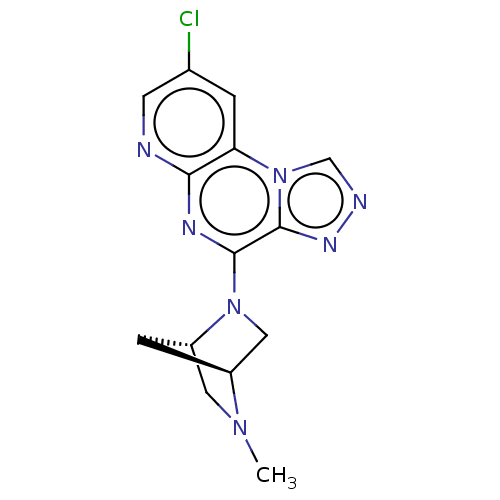 (US9586959, Compound 78) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294410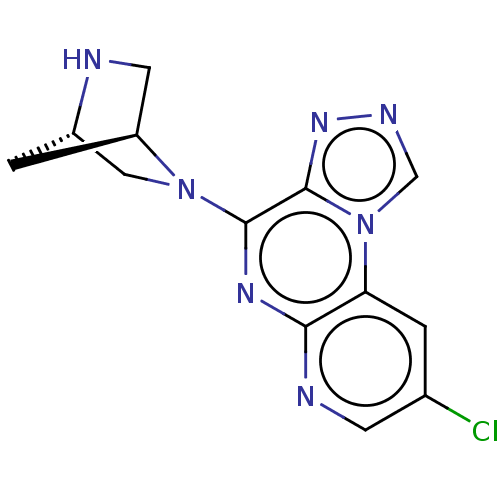 (US9586959, Compound 77) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294360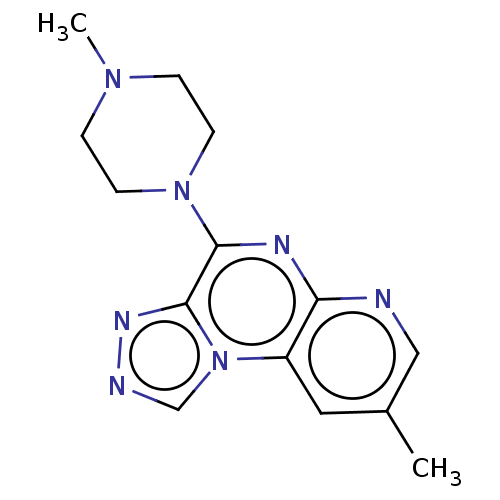 (US9586959, Compound 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294372 (US9586959, Compound 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50563968 (CHEMBL4776004) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily B member 1 (Homo sapiens (Human)) | BDBM50088573 (CHEMBL3577208) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of Kv2.1 channel in human INS1 cells assessed as inhibition of outward K+ current by electrophysiology/patch clamp technique | J Nat Prod 78: 363-7 (2015) Article DOI: 10.1021/np5007586 BindingDB Entry DOI: 10.7270/Q20P11R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Mus musculus (mouse)) | BDBM294407 (US9586959, Compound 69 | US9586959, Compound 95) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at recombinant mouse histamine H4 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine induced st... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294369 (US9586959, Compound 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 138 total ) | Next | Last >> |