 Found 76 hits with Last Name = 'holloway' and Initial = 'ac'
Found 76 hits with Last Name = 'holloway' and Initial = 'ac' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM50509915

(CHEMBL4441579)Show InChI InChI=1S/C17H13Cl2N3/c18-16-5-1-13(2-6-16)11-15(12-22-10-9-20-21-22)14-3-7-17(19)8-4-14/h1-11H,12H2/b15-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50023460
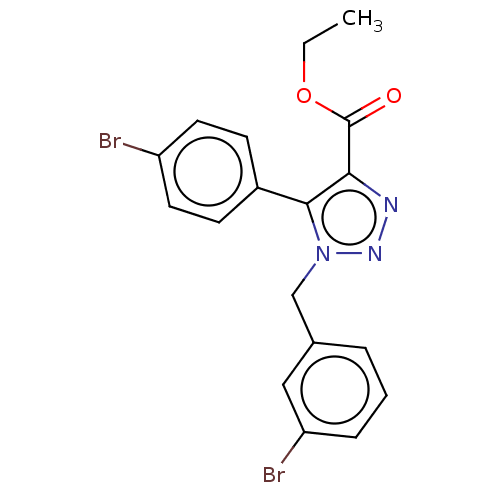
(CHEMBL3326517)Show SMILES CCOC(=O)c1nnn(Cc2cccc(Br)c2)c1-c1ccc(Br)cc1 Show InChI InChI=1S/C18H15Br2N3O2/c1-2-25-18(24)16-17(13-6-8-14(19)9-7-13)23(22-21-16)11-12-4-3-5-15(20)10-12/h3-10H,2,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human CYP19A1 assessed as inhibition of DBF conversion to fluorescein by-product by fluorometry |
Bioorg Med Chem Lett 24: 4586-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.083
BindingDB Entry DOI: 10.7270/Q27W6DRV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442766
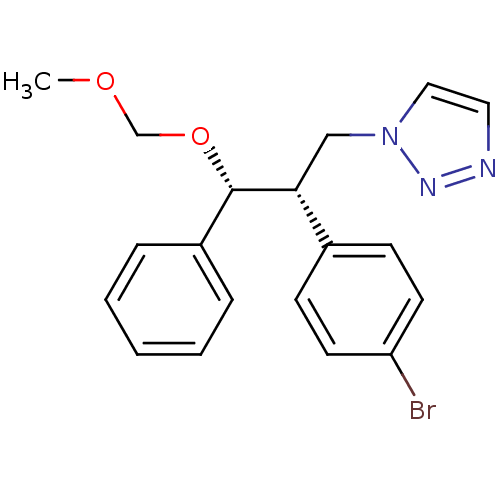
(CHEMBL2443358)Show SMILES COCO[C@H]([C@H](Cn1ccnn1)c1ccc(Br)cc1)c1ccccc1 |r| Show InChI InChI=1S/C19H20BrN3O2/c1-24-14-25-19(16-5-3-2-4-6-16)18(13-23-12-11-21-22-23)15-7-9-17(20)10-8-15/h2-12,18-19H,13-14H2,1H3/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442776
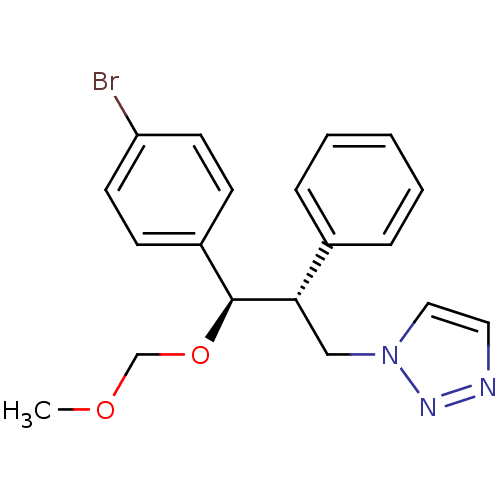
(CHEMBL2443348)Show SMILES COCO[C@H]([C@@H](Cn1ccnn1)c1ccccc1)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C19H20BrN3O2/c1-24-14-25-19(16-7-9-17(20)10-8-16)18(13-23-12-11-21-22-23)15-5-3-2-4-6-15/h2-12,18-19H,13-14H2,1H3/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50023460

(CHEMBL3326517)Show SMILES CCOC(=O)c1nnn(Cc2cccc(Br)c2)c1-c1ccc(Br)cc1 Show InChI InChI=1S/C18H15Br2N3O2/c1-2-25-18(24)16-17(13-6-8-14(19)9-7-13)23(22-21-16)11-12-4-3-5-15(20)10-12/h3-10H,2,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human CYP19A1 assessed as inhibition of DBF conversion to fluorescein by-product by fluorometry |
Bioorg Med Chem Lett 24: 4586-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.083
BindingDB Entry DOI: 10.7270/Q27W6DRV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50509909
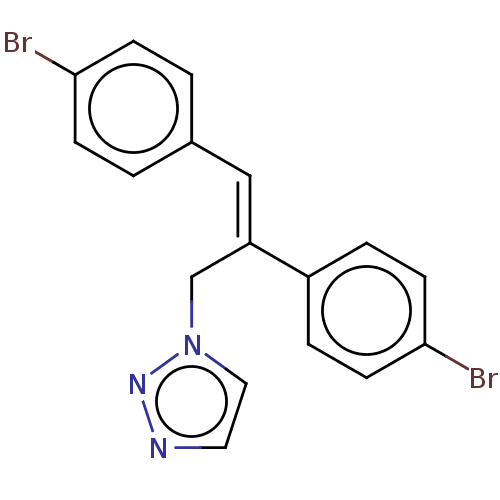
(CHEMBL4450923)Show InChI InChI=1S/C17H13Br2N3/c18-16-5-1-13(2-6-16)11-15(12-22-10-9-20-21-22)14-3-7-17(19)8-4-14/h1-11H,12H2/b15-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50314498
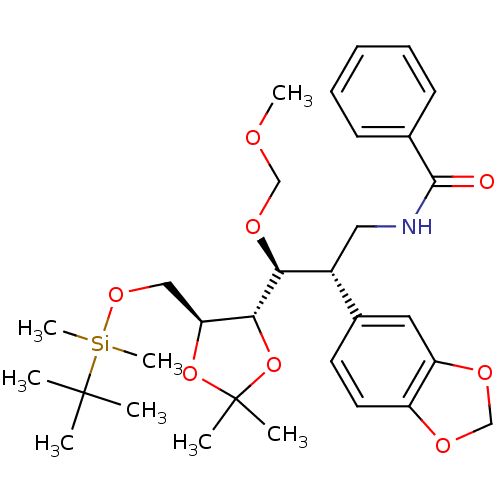
(CHEMBL1089299 | N-((2S,3S)-2-(benzo[d][1,3]dioxol-...)Show SMILES COCO[C@@H]([C@H](CNC(=O)c1ccccc1)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1CO[Si](C)(C)C(C)(C)C |r| Show InChI InChI=1S/C31H45NO8Si/c1-30(2,3)41(7,8)38-18-26-28(40-31(4,5)39-26)27(37-19-34-6)23(17-32-29(33)21-12-10-9-11-13-21)22-14-15-24-25(16-22)36-20-35-24/h9-16,23,26-28H,17-20H2,1-8H3,(H,32,33)/t23-,26+,27+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-hydroxyquinoline to 7-benzyloxyquinoline measured every minute for 15 mins ... |
Bioorg Med Chem Lett 20: 2335-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.157
BindingDB Entry DOI: 10.7270/Q21V5F4M |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50023465
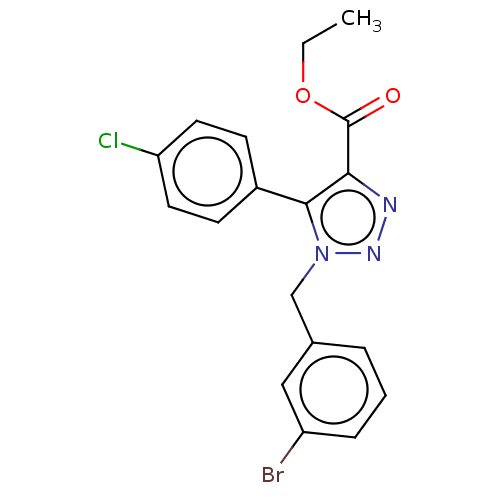
(CHEMBL3326520)Show SMILES CCOC(=O)c1nnn(Cc2cccc(Br)c2)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C18H15BrClN3O2/c1-2-25-18(24)16-17(13-6-8-15(20)9-7-13)23(22-21-16)11-12-4-3-5-14(19)10-12/h3-10H,2,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 assessed as inhibition of DBF conversion to fluorescein by-product by fluorometry |
Bioorg Med Chem Lett 24: 4586-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.083
BindingDB Entry DOI: 10.7270/Q27W6DRV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50300539
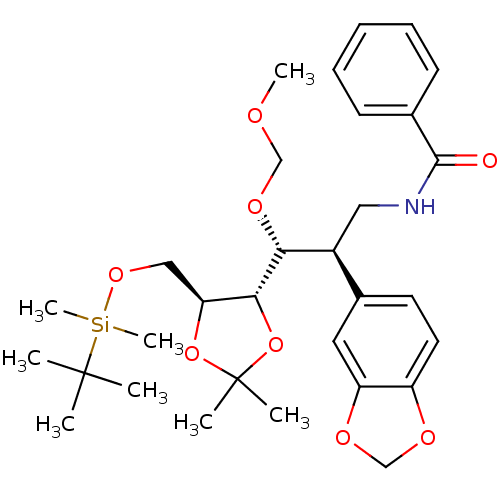
(CHEMBL573430 | N-((2R,3R)-2-(benzo[d][1,3]dioxol-5...)Show SMILES COCO[C@H]([C@@H](CNC(=O)c1ccccc1)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1CO[Si](C)(C)C(C)(C)C |r| Show InChI InChI=1S/C31H45NO8Si/c1-30(2,3)41(7,8)38-18-26-28(40-31(4,5)39-26)27(37-19-34-6)23(17-32-29(33)21-12-10-9-11-13-21)22-14-15-24-25(16-22)36-20-35-24/h9-16,23,26-28H,17-20H2,1-8H3,(H,32,33)/t23-,26-,27+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline to 7-hydroxyquinoline measured every 15 mins by fluoresc... |
Bioorg Med Chem Lett 19: 5607-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.032
BindingDB Entry DOI: 10.7270/Q2CF9Q4D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50023465

(CHEMBL3326520)Show SMILES CCOC(=O)c1nnn(Cc2cccc(Br)c2)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C18H15BrClN3O2/c1-2-25-18(24)16-17(13-6-8-15(20)9-7-13)23(22-21-16)11-12-4-3-5-14(19)10-12/h3-10H,2,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 assessed as inhibition of DBF conversion to fluorescein by-product by fluorometry |
Bioorg Med Chem Lett 24: 4586-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.083
BindingDB Entry DOI: 10.7270/Q27W6DRV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50509907
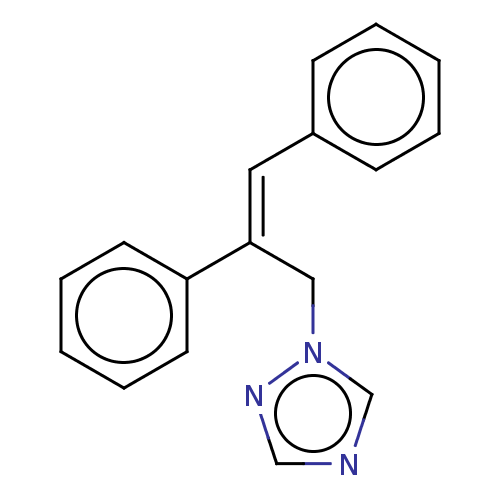
(CHEMBL4545917)Show InChI InChI=1S/C17H15N3/c1-3-7-15(8-4-1)11-17(12-20-14-18-13-19-20)16-9-5-2-6-10-16/h1-11,13-14H,12H2/b17-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50360383
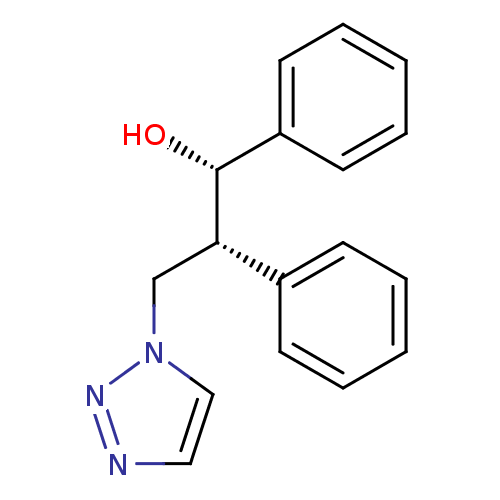
(CHEMBL1933700)Show SMILES O[C@@H]([C@@H](Cn1ccnn1)c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C17H17N3O/c21-17(15-9-5-2-6-10-15)16(13-20-12-11-18-19-20)14-7-3-1-4-8-14/h1-12,16-17,21H,13H2/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... |
Bioorg Med Chem Lett 22: 718-22 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.039
BindingDB Entry DOI: 10.7270/Q2PV6KS2 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50509911
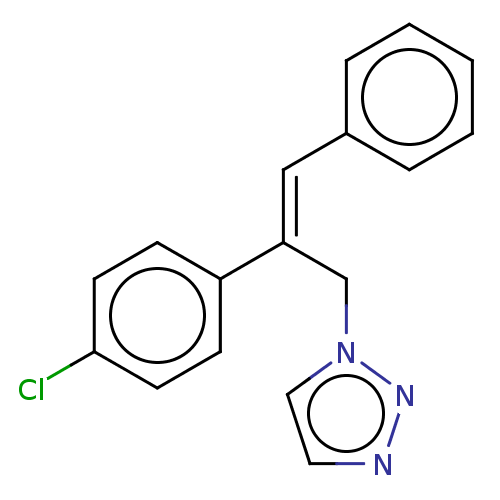
(CHEMBL4561656)Show InChI InChI=1S/C17H14ClN3/c18-17-8-6-15(7-9-17)16(13-21-11-10-19-20-21)12-14-4-2-1-3-5-14/h1-12H,13H2/b16-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50360381
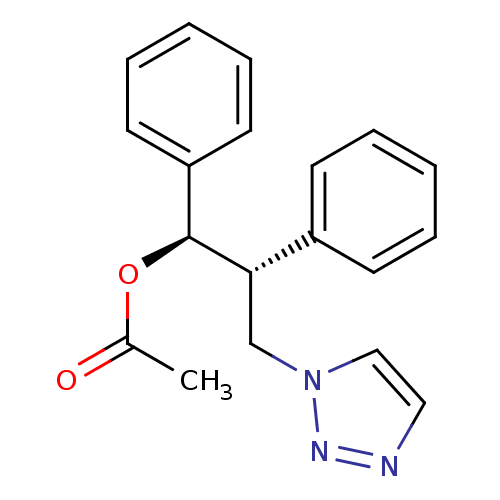
(CHEMBL1933694)Show SMILES CC(=O)O[C@H]([C@@H](Cn1ccnn1)c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C19H19N3O2/c1-15(23)24-19(17-10-6-3-7-11-17)18(14-22-13-12-20-21-22)16-8-4-2-5-9-16/h2-13,18-19H,14H2,1H3/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... |
Bioorg Med Chem Lett 22: 718-22 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.039
BindingDB Entry DOI: 10.7270/Q2PV6KS2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50314501
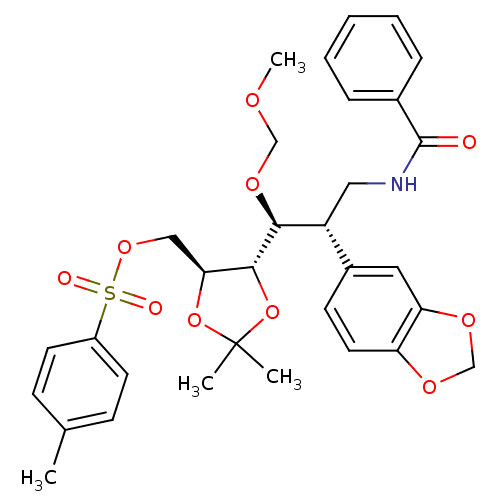
(((4S,5S)-5-((1S,2S)-3-benzamido-2-(benzo[d][1,3]di...)Show SMILES COCO[C@@H]([C@H](CNC(=O)c1ccccc1)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1COS(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C32H37NO10S/c1-21-10-13-24(14-11-21)44(35,36)41-18-28-30(43-32(2,3)42-28)29(40-19-37-4)25(17-33-31(34)22-8-6-5-7-9-22)23-12-15-26-27(16-23)39-20-38-26/h5-16,25,28-30H,17-20H2,1-4H3,(H,33,34)/t25-,28+,29+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-hydroxyquinoline to 7-benzyloxyquinoline measured every minute for 15 mins ... |
Bioorg Med Chem Lett 20: 2335-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.157
BindingDB Entry DOI: 10.7270/Q21V5F4M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50300543
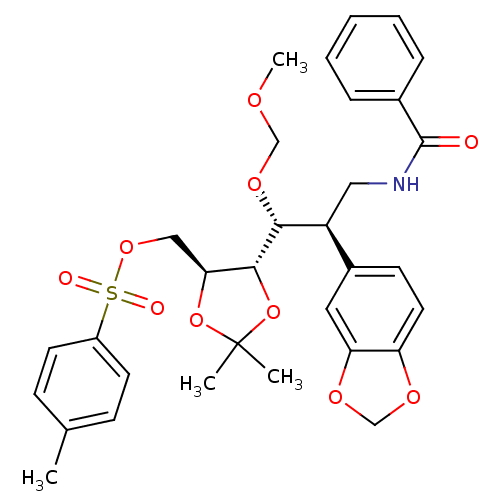
(((4S,5S)-5-((1R,2R)-3-benzamido-2-(benzo[d][1,3]di...)Show SMILES COCO[C@H]([C@@H](CNC(=O)c1ccccc1)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1COS(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C32H37NO10S/c1-21-10-13-24(14-11-21)44(35,36)41-18-28-30(43-32(2,3)42-28)29(40-19-37-4)25(17-33-31(34)22-8-6-5-7-9-22)23-12-15-26-27(16-23)39-20-38-26/h5-16,25,28-30H,17-20H2,1-4H3,(H,33,34)/t25-,28-,29+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline to 7-hydroxyquinoline measured every 15 mins by fluoresc... |
Bioorg Med Chem Lett 19: 5607-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.032
BindingDB Entry DOI: 10.7270/Q2CF9Q4D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442767
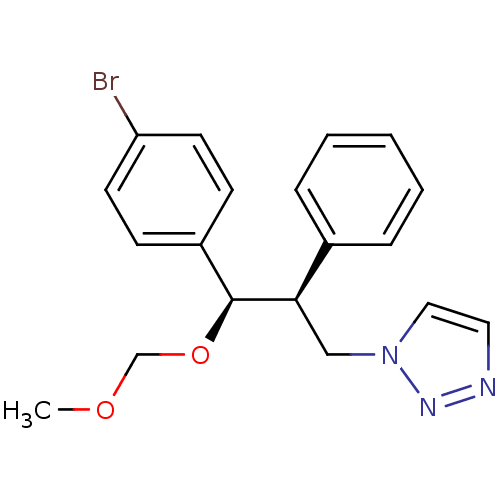
(CHEMBL2443357)Show SMILES COCO[C@H]([C@H](Cn1ccnn1)c1ccccc1)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C19H20BrN3O2/c1-24-14-25-19(16-7-9-17(20)10-8-16)18(13-23-12-11-21-22-23)15-5-3-2-4-6-15/h2-12,18-19H,13-14H2,1H3/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50509910

(CHEMBL4468933)Show InChI InChI=1S/C17H14BrN3/c18-17-8-6-15(7-9-17)16(13-21-11-10-19-20-21)12-14-4-2-1-3-5-14/h1-12H,13H2/b16-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50509914
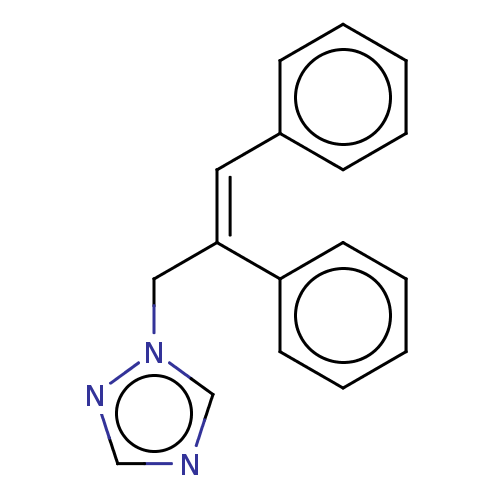
(CHEMBL4526477)Show InChI InChI=1S/C17H15N3/c1-3-7-15(8-4-1)11-17(12-20-14-18-13-19-20)16-9-5-2-6-10-16/h1-11,13-14H,12H2/b17-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50360382

(CHEMBL1933699)Show SMILES COCO[C@@H]([C@@H](Cn1ccnn1)c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C19H21N3O2/c1-23-15-24-19(17-10-6-3-7-11-17)18(14-22-13-12-20-21-22)16-8-4-2-5-9-16/h2-13,18-19H,14-15H2,1H3/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... |
Bioorg Med Chem Lett 22: 718-22 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.039
BindingDB Entry DOI: 10.7270/Q2PV6KS2 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442777
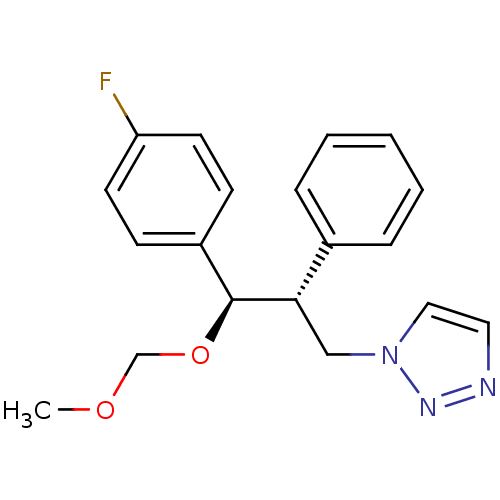
(CHEMBL2443365)Show SMILES COCO[C@H]([C@@H](Cn1ccnn1)c1ccccc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H20FN3O2/c1-24-14-25-19(16-7-9-17(20)10-8-16)18(13-23-12-11-21-22-23)15-5-3-2-4-6-15/h2-12,18-19H,13-14H2,1H3/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50509916
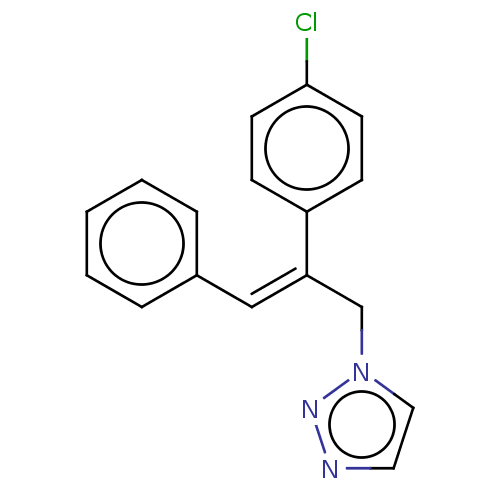
(CHEMBL4468397)Show InChI InChI=1S/C17H14ClN3/c18-17-8-6-15(7-9-17)16(13-21-11-10-19-20-21)12-14-4-2-1-3-5-14/h1-12H,13H2/b16-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442770

(CHEMBL2443354)Show SMILES CC(=O)O[C@H]([C@@H](Cn1ccnn1)c1ccccc1)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C19H18BrN3O2/c1-14(24)25-19(16-7-9-17(20)10-8-16)18(13-23-12-11-21-22-23)15-5-3-2-4-6-15/h2-12,18-19H,13H2,1H3/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50509918

(CHEMBL4453517)Show InChI InChI=1S/C17H13Cl2N3/c18-16-5-1-13(2-6-16)11-15(12-22-10-9-20-21-22)14-3-7-17(19)8-4-14/h1-11H,12H2/b15-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50360380
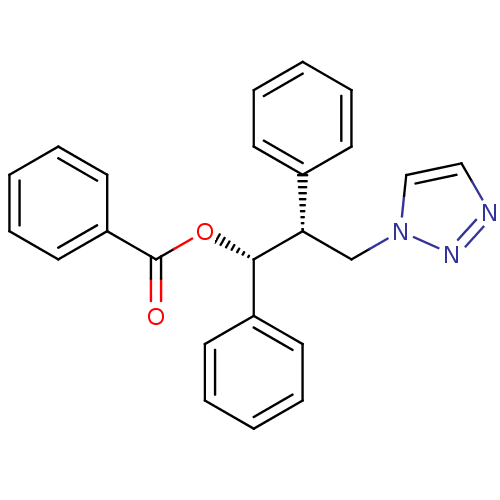
(CHEMBL1933693)Show SMILES O=C(O[C@H]([C@@H](Cn1ccnn1)c1ccccc1)c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C24H21N3O2/c28-24(21-14-8-3-9-15-21)29-23(20-12-6-2-7-13-20)22(18-27-17-16-25-26-27)19-10-4-1-5-11-19/h1-17,22-23H,18H2/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... |
Bioorg Med Chem Lett 22: 718-22 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.039
BindingDB Entry DOI: 10.7270/Q2PV6KS2 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50509913

(CHEMBL4437348)Show InChI InChI=1S/C17H15N3/c1-3-7-15(8-4-1)13-17(14-20-12-11-18-19-20)16-9-5-2-6-10-16/h1-13H,14H2/b17-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442771
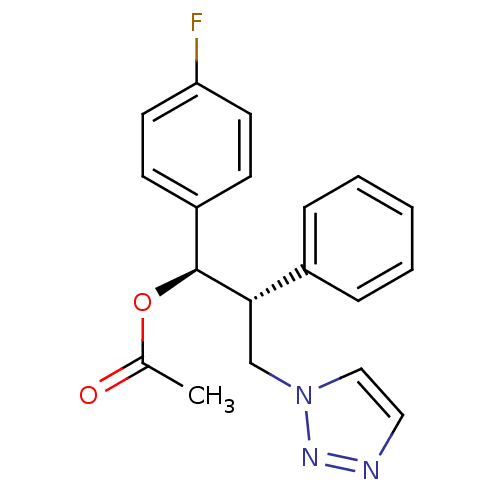
(CHEMBL2443353)Show SMILES CC(=O)O[C@H]([C@@H](Cn1ccnn1)c1ccccc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H18FN3O2/c1-14(24)25-19(16-7-9-17(20)10-8-16)18(13-23-12-11-21-22-23)15-5-3-2-4-6-15/h2-12,18-19H,13H2,1H3/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442775

(CHEMBL2443349)Show SMILES COCO[C@H]([C@@H](Cn1ccnn1)c1ccc(Br)cc1)c1ccccc1 |r| Show InChI InChI=1S/C19H20BrN3O2/c1-24-14-25-19(16-5-3-2-4-6-16)18(13-23-12-11-21-22-23)15-7-9-17(20)10-8-15/h2-12,18-19H,13-14H2,1H3/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50509912
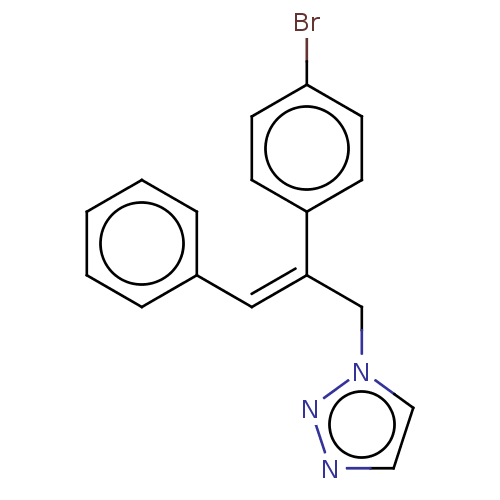
(CHEMBL4562457)Show InChI InChI=1S/C17H14BrN3/c18-17-8-6-15(7-9-17)16(13-21-11-10-19-20-21)12-14-4-2-1-3-5-14/h1-12H,13H2/b16-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442764

(CHEMBL2443360)Show SMILES O[C@H]([C@H](Cn1ccnn1)c1ccccc1)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C17H16BrN3O/c18-15-8-6-14(7-9-15)17(22)16(12-21-11-10-19-20-21)13-4-2-1-3-5-13/h1-11,16-17,22H,12H2/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50023463
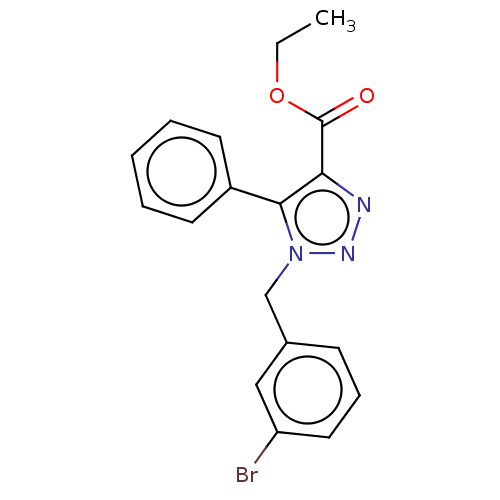
(CHEMBL3326522)Show InChI InChI=1S/C18H16BrN3O2/c1-2-24-18(23)16-17(14-8-4-3-5-9-14)22(21-20-16)12-13-7-6-10-15(19)11-13/h3-11H,2,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 assessed as inhibition of DBF conversion to fluorescein by-product by fluorometry |
Bioorg Med Chem Lett 24: 4586-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.083
BindingDB Entry DOI: 10.7270/Q27W6DRV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50023463

(CHEMBL3326522)Show InChI InChI=1S/C18H16BrN3O2/c1-2-24-18(23)16-17(14-8-4-3-5-9-14)22(21-20-16)12-13-7-6-10-15(19)11-13/h3-11H,2,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 assessed as inhibition of DBF conversion to fluorescein by-product by fluorometry |
Bioorg Med Chem Lett 24: 4586-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.083
BindingDB Entry DOI: 10.7270/Q27W6DRV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442768
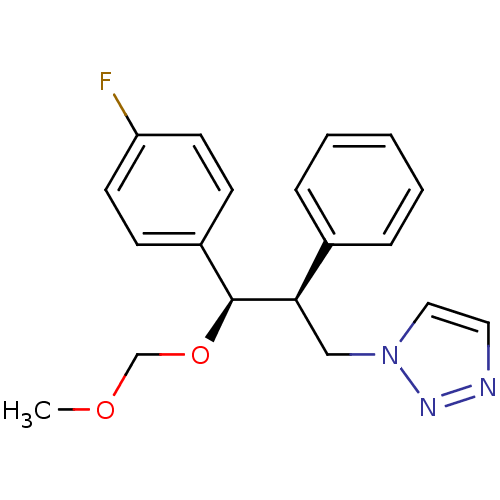
(CHEMBL2443356)Show SMILES COCO[C@H]([C@H](Cn1ccnn1)c1ccccc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H20FN3O2/c1-24-14-25-19(16-7-9-17(20)10-8-16)18(13-23-12-11-21-22-23)15-5-3-2-4-6-15/h2-12,18-19H,13-14H2,1H3/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50300538

(CHEMBL574914 | N-((2R,3R)-2-(benzo[d][1,3]dioxol-5...)Show SMILES CC(C)(C)[Si](C)(C)OC[C@@H]1OC(C)(C)O[C@H]1[C@H](O)[C@@H](CNC(=O)c1ccccc1)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C29H41NO7Si/c1-28(2,3)38(6,7)35-17-24-26(37-29(4,5)36-24)25(31)21(16-30-27(32)19-11-9-8-10-12-19)20-13-14-22-23(15-20)34-18-33-22/h8-15,21,24-26,31H,16-18H2,1-7H3,(H,30,32)/t21-,24-,25+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline to 7-hydroxyquinoline measured every 15 mins by fluoresc... |
Bioorg Med Chem Lett 19: 5607-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.032
BindingDB Entry DOI: 10.7270/Q2CF9Q4D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442773
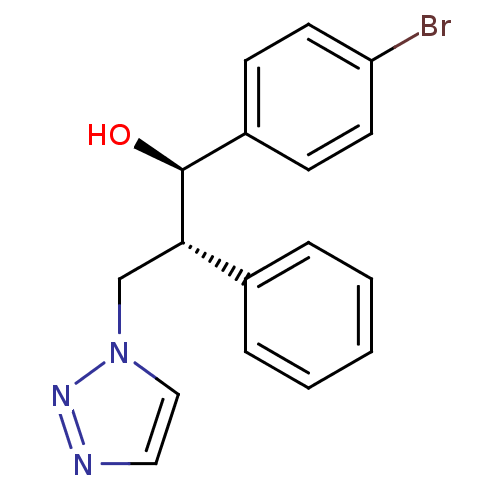
(CHEMBL2443351)Show SMILES O[C@H]([C@@H](Cn1ccnn1)c1ccccc1)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C17H16BrN3O/c18-15-8-6-14(7-9-15)17(22)16(12-21-11-10-19-20-21)13-4-2-1-3-5-13/h1-11,16-17,22H,12H2/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50509917
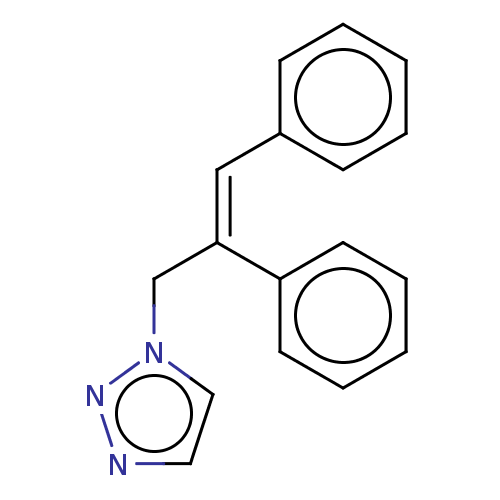
(CHEMBL4454530)Show InChI InChI=1S/C17H15N3/c1-3-7-15(8-4-1)13-17(14-20-12-11-18-19-20)16-9-5-2-6-10-16/h1-13H,14H2/b17-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442765
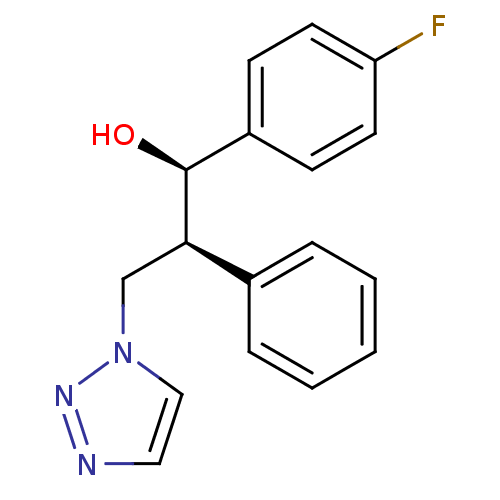
(CHEMBL2443359)Show SMILES O[C@H]([C@H](Cn1ccnn1)c1ccccc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C17H16FN3O/c18-15-8-6-14(7-9-15)17(22)16(12-21-11-10-19-20-21)13-4-2-1-3-5-13/h1-11,16-17,22H,12H2/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50293601
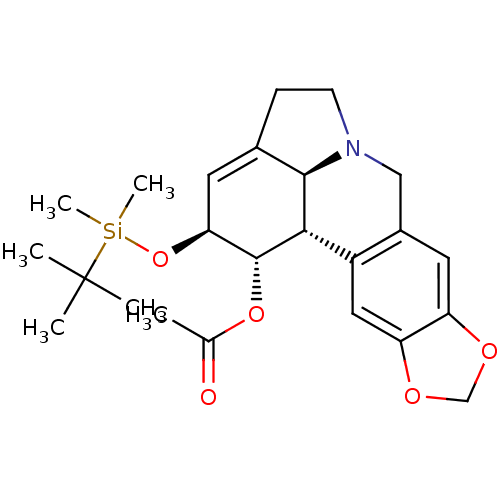
((1S,2S,3a1S,12bS)-2-(tert-butyldimethylsilyloxy)-2...)Show SMILES CC(=O)O[C@@H]1[C@@H](O[Si](C)(C)C(C)(C)C)C=C2CCN3Cc4cc5OCOc5cc4[C@H]1[C@@H]23 |r,t:14| Show InChI InChI=1S/C24H33NO5Si/c1-14(26)29-23-20(30-31(5,6)24(2,3)4)9-15-7-8-25-12-16-10-18-19(28-13-27-18)11-17(16)21(23)22(15)25/h9-11,20-23H,7-8,12-13H2,1-6H3/t20-,21-,22+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 3233-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.086
BindingDB Entry DOI: 10.7270/Q2X92BBG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442769
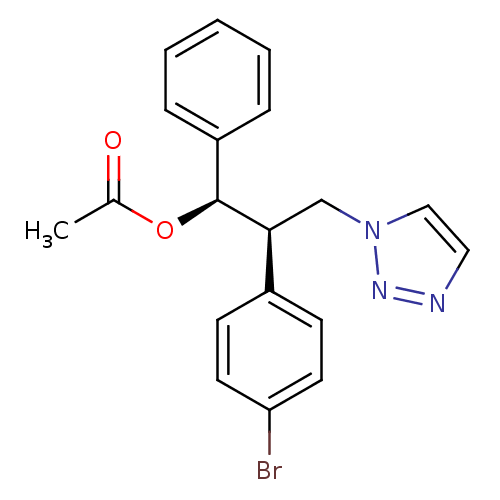
(CHEMBL2443355)Show SMILES CC(=O)O[C@H]([C@@H](Cn1ccnn1)c1ccc(Br)cc1)c1ccccc1 |r| Show InChI InChI=1S/C19H18BrN3O2/c1-14(24)25-19(16-5-3-2-4-6-16)18(13-23-12-11-21-22-23)15-7-9-17(20)10-8-15/h2-12,18-19H,13H2,1H3/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 237 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50314499

(CHEMBL1089300 | tert-butyl(2S,3S)-2-(benzo[d][1,3]...)Show SMILES COCO[C@@H]([C@H](CNC(=O)OC(C)(C)C)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1CO[Si](C)(C)C(C)(C)C |r| Show InChI InChI=1S/C29H49NO9Si/c1-27(2,3)39-26(31)30-15-20(19-12-13-21-22(14-19)34-18-33-21)24(35-17-32-9)25-23(37-29(7,8)38-25)16-36-40(10,11)28(4,5)6/h12-14,20,23-25H,15-18H2,1-11H3,(H,30,31)/t20-,23+,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-hydroxyquinoline to 7-benzyloxyquinoline measured every minute for 15 mins ... |
Bioorg Med Chem Lett 20: 2335-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.157
BindingDB Entry DOI: 10.7270/Q21V5F4M |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50360384
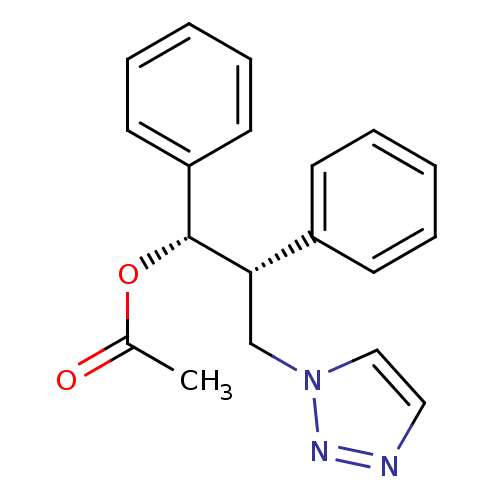
(CHEMBL1933701)Show SMILES CC(=O)O[C@@H]([C@@H](Cn1ccnn1)c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C19H19N3O2/c1-15(23)24-19(17-10-6-3-7-11-17)18(14-22-13-12-20-21-22)16-8-4-2-5-9-16/h2-13,18-19H,14H2,1H3/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... |
Bioorg Med Chem Lett 22: 718-22 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.039
BindingDB Entry DOI: 10.7270/Q2PV6KS2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50293600
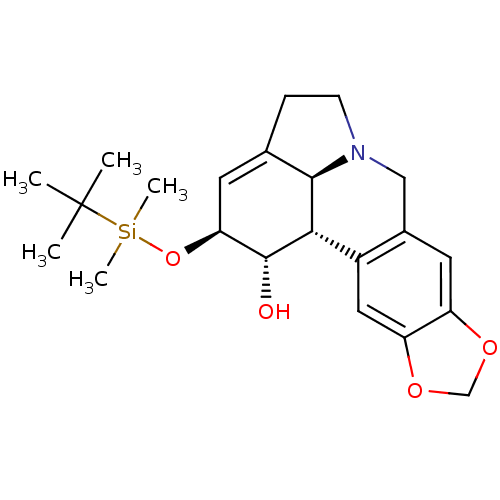
((1S,2S,3a1S,12bS)-2-(tert-butyldimethylsilyloxy)-2...)Show SMILES CC(C)(C)[Si](C)(C)O[C@H]1C=C2CCN3Cc4cc5OCOc5cc4[C@@H]([C@@H]23)[C@@H]1O |r,t:9| Show InChI InChI=1S/C22H31NO4Si/c1-22(2,3)28(4,5)27-18-8-13-6-7-23-11-14-9-16-17(26-12-25-16)10-15(14)19(20(13)23)21(18)24/h8-10,18-21,24H,6-7,11-12H2,1-5H3/t18-,19-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 3233-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.086
BindingDB Entry DOI: 10.7270/Q2X92BBG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50509908
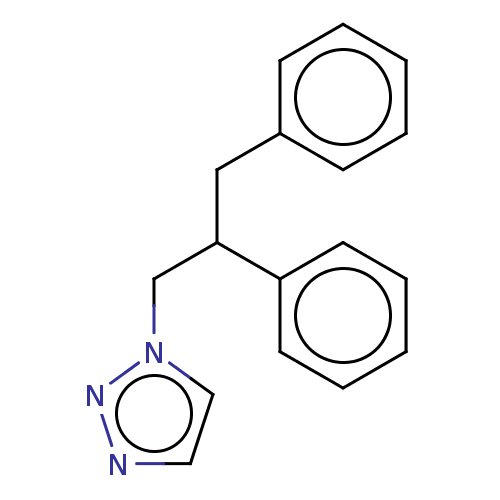
(CHEMBL4582591)Show InChI InChI=1S/C17H17N3/c1-3-7-15(8-4-1)13-17(14-20-12-11-18-19-20)16-9-5-2-6-10-16/h1-12,17H,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 29: 1395-1398 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.033
BindingDB Entry DOI: 10.7270/Q2F1932J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50300541

(CHEMBL583953 | tert-butyl(2R,3R)-2-(benzo[d][1,3]d...)Show SMILES COCO[C@H]([C@@H](CNC(=O)OC(C)(C)C)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1CO[Si](C)(C)C(C)(C)C |r| Show InChI InChI=1S/C29H49NO9Si/c1-27(2,3)39-26(31)30-15-20(19-12-13-21-22(14-19)34-18-33-21)24(35-17-32-9)25-23(37-29(7,8)38-25)16-36-40(10,11)28(4,5)6/h12-14,20,23-25H,15-18H2,1-11H3,(H,30,31)/t20-,23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline to 7-hydroxyquinoline measured every 15 mins by fluoresc... |
Bioorg Med Chem Lett 19: 5607-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.032
BindingDB Entry DOI: 10.7270/Q2CF9Q4D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442774
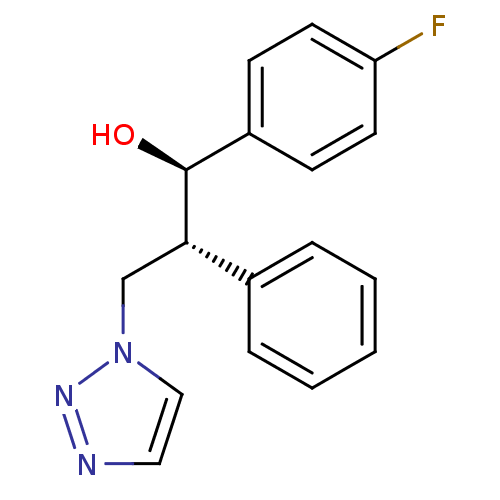
(CHEMBL2443350)Show SMILES O[C@H]([C@@H](Cn1ccnn1)c1ccccc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C17H16FN3O/c18-15-8-6-14(7-9-15)17(22)16(12-21-11-10-19-20-21)13-4-2-1-3-5-13/h1-11,16-17,22H,12H2/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Aromatase
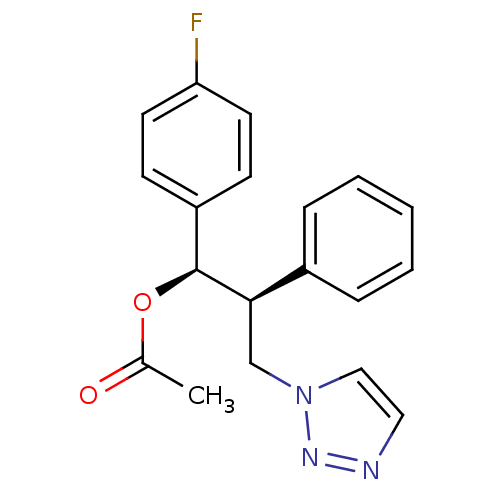
(Homo sapiens (Human)) | BDBM50442762
(CHEMBL2443362)Show SMILES CC(=O)O[C@H]([C@H](Cn1ccnn1)c1ccccc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H18FN3O2/c1-14(24)25-19(16-7-9-17(20)10-8-16)18(13-23-12-11-21-22-23)15-5-3-2-4-6-15/h2-12,18-19H,13H2,1H3/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assay |
Bioorg Med Chem Lett 23: 6060-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.030
BindingDB Entry DOI: 10.7270/Q2QJ7JQB |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50023464

(CHEMBL3326521)Show InChI InChI=1S/C18H16ClN3O2/c1-2-24-18(23)16-17(14-8-10-15(19)11-9-14)22(21-20-16)12-13-6-4-3-5-7-13/h3-11H,2,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 assessed as inhibition of DBF conversion to fluorescein by-product by fluorometry |
Bioorg Med Chem Lett 24: 4586-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.083
BindingDB Entry DOI: 10.7270/Q27W6DRV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50023464

(CHEMBL3326521)Show InChI InChI=1S/C18H16ClN3O2/c1-2-24-18(23)16-17(14-8-10-15(19)11-9-14)22(21-20-16)12-13-6-4-3-5-7-13/h3-11H,2,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP19A1 assessed as inhibition of DBF conversion to fluorescein by-product by fluorometry |
Bioorg Med Chem Lett 24: 4586-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.083
BindingDB Entry DOI: 10.7270/Q27W6DRV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... |
Bioorg Med Chem Lett 22: 718-22 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.039
BindingDB Entry DOI: 10.7270/Q2PV6KS2 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... |
Bioorg Med Chem Lett 22: 718-22 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.039
BindingDB Entry DOI: 10.7270/Q2PV6KS2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data