

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235302 (CHEMBL4099771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235302 (CHEMBL4099771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235301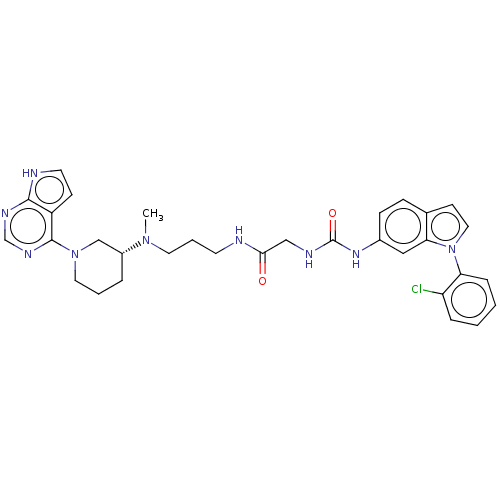 (CHEMBL4081752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50400274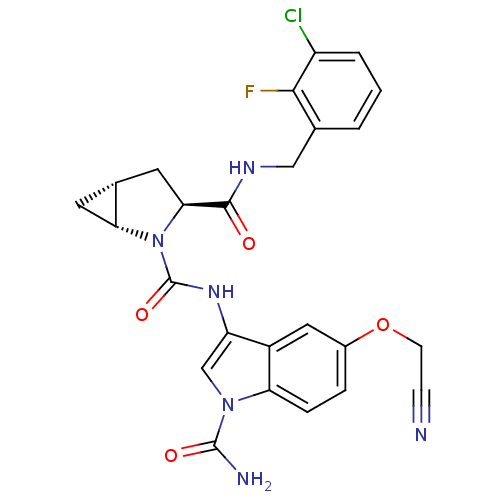 (CHEMBL2180765 | US9085555, 373) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154078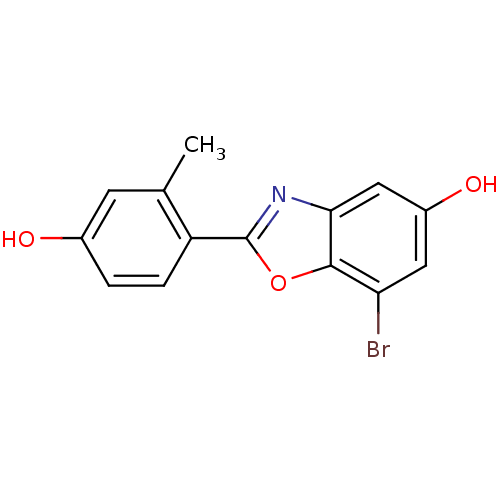 (7-Bromo-2-(4-hydroxy-2-methyl-phenyl)-benzooxazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of estrogen receptor beta | J Med Chem 51: 2481-91 (2008) Article DOI: 10.1021/jm701314u BindingDB Entry DOI: 10.7270/Q2GX4CF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154137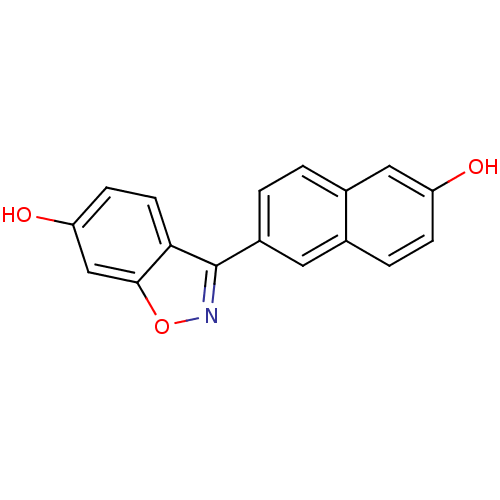 (3-(6-HYDROXY-NAPHTHALEN-2-YL)-BENZO[D]ISOOXAZOL-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of estrogen receptor beta | J Med Chem 51: 2481-91 (2008) Article DOI: 10.1021/jm701314u BindingDB Entry DOI: 10.7270/Q2GX4CF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50400273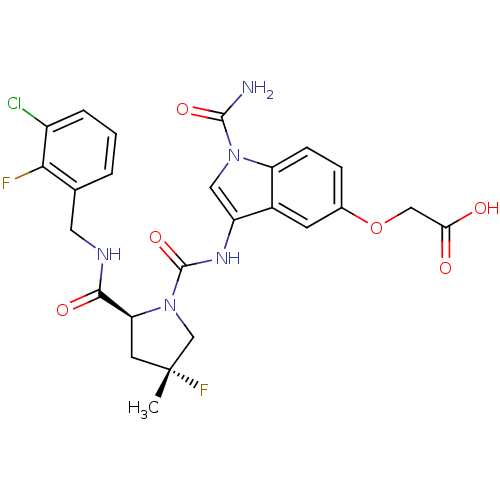 (CHEMBL2180764 | US9085555, 375) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170890 (US9085555, 318) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235302 (CHEMBL4099771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L in human HeLa cells assessed as reduction in H3K79me2 level after 72 hrs by ELISA | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50400275 (CHEMBL2180766 | US9085555, 127) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170864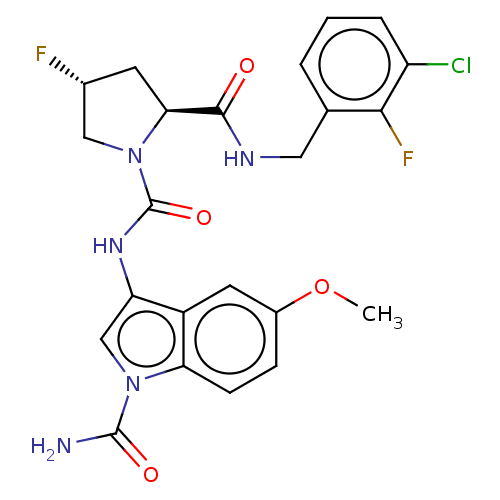 (US9085555, 292) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171305 (US9085555, 735) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171262 (US9085555, 692) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM254483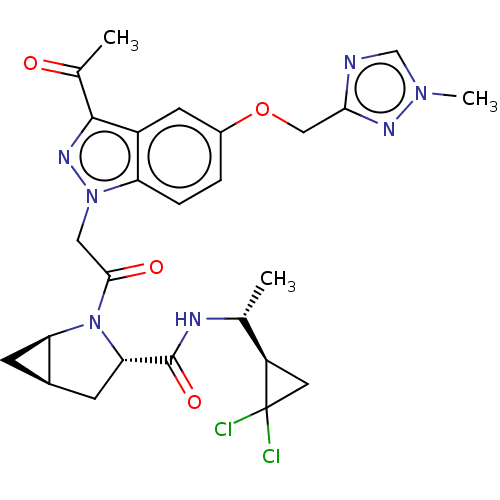 (US9468661, 61) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Method 1: Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compoun... | US Patent US9468661 (2016) BindingDB Entry DOI: 10.7270/Q23N22B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171311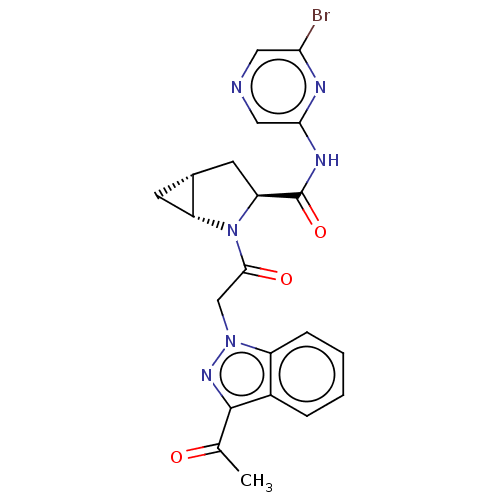 (US9085555, 741 | US9085555, 757) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM254498 (US9468661, 109 | US9468661, 76) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Method 1: Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compoun... | US Patent US9468661 (2016) BindingDB Entry DOI: 10.7270/Q23N22B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170943 (US9085555, 371) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171267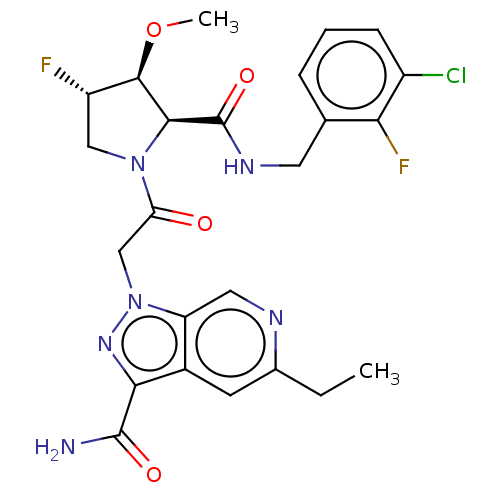 (US9085555, 697) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170945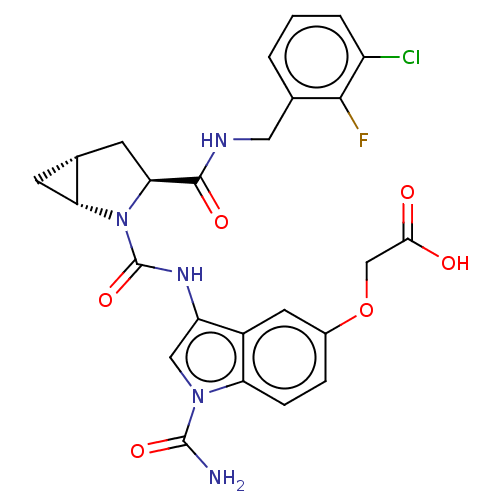 (US9085555, 374) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM254486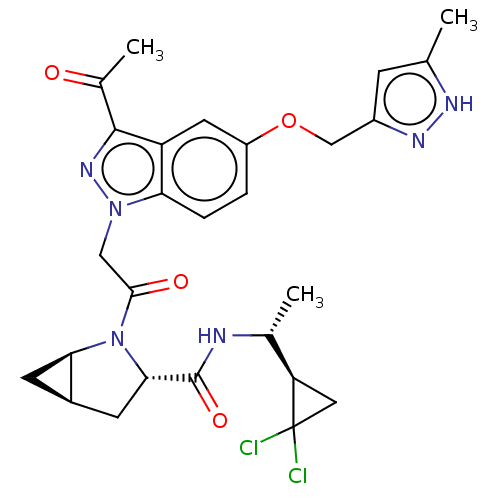 (US9468661, 64) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Method 1: Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compoun... | US Patent US9468661 (2016) BindingDB Entry DOI: 10.7270/Q23N22B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170738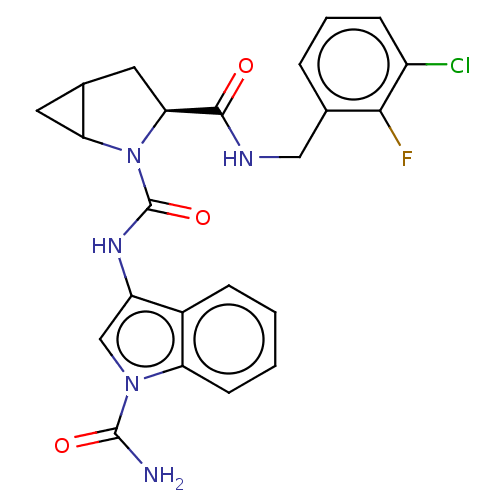 (US9085555, 166) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170872 (US9085555, 300) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170953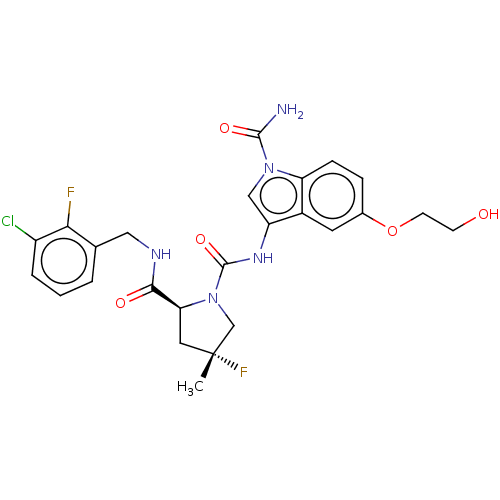 (US9085555, 383) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170701 (US9085555, 129) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170878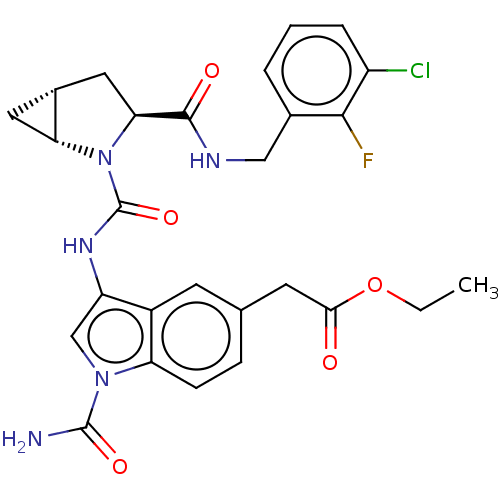 (US9085555, 306) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM254475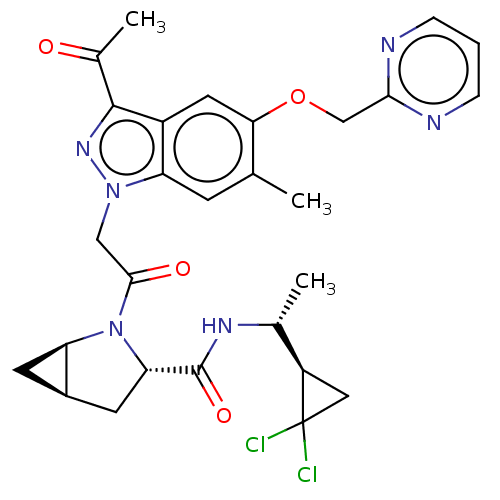 (US9468661, 53) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Method 1: Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compoun... | US Patent US9468661 (2016) BindingDB Entry DOI: 10.7270/Q23N22B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM254471 (US9468661, 49) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Method 1: Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compoun... | US Patent US9468661 (2016) BindingDB Entry DOI: 10.7270/Q23N22B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235300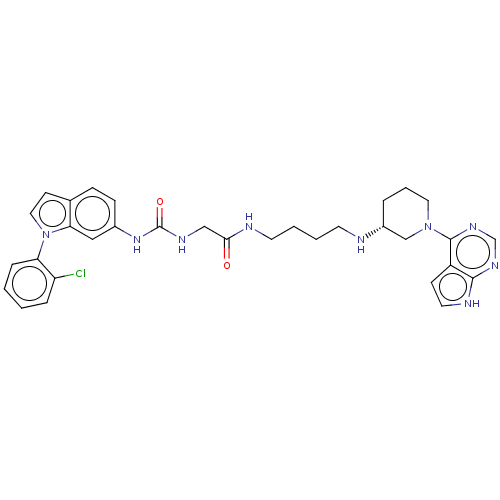 (CHEMBL4087730) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235299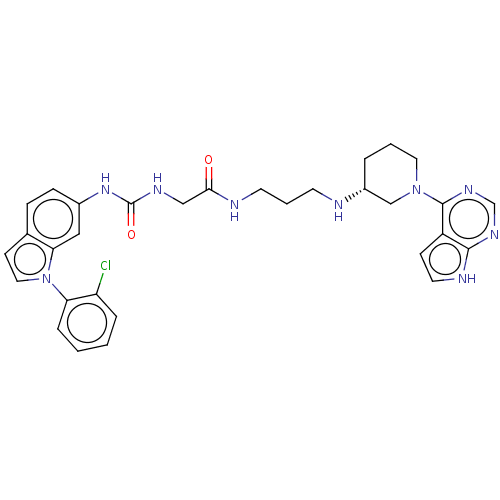 (CHEMBL4066397) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235300 (CHEMBL4087730) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235299 (CHEMBL4066397) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171334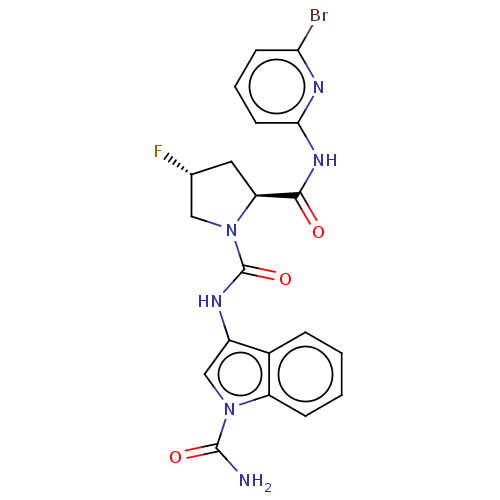 (US9085555, 764) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171319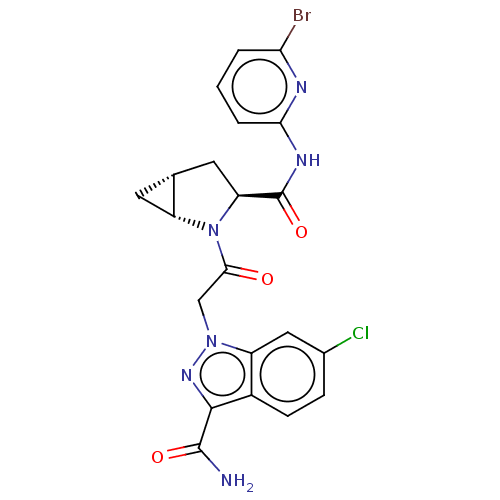 (US9085555, 749) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171318 (US9085555, 748) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM254488 (US9468661, 66) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Method 1: Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compoun... | US Patent US9468661 (2016) BindingDB Entry DOI: 10.7270/Q23N22B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171314 (US9085555, 744) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171304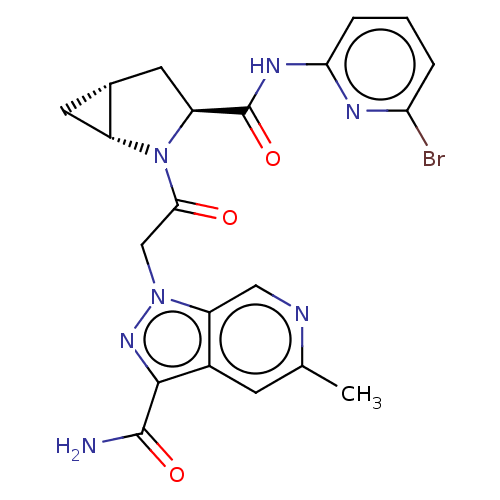 (US9085555, 734) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171315 (US9085555, 745) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154062 (2-(5-HYDROXY-NAPHTHALEN-1-YL)-1,3-BENZOOXAZOL-6-OL...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of estrogen receptor beta | J Med Chem 51: 2481-91 (2008) Article DOI: 10.1021/jm701314u BindingDB Entry DOI: 10.7270/Q2GX4CF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170866 (US9085555, 294) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171311 (US9085555, 741 | US9085555, 757) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171313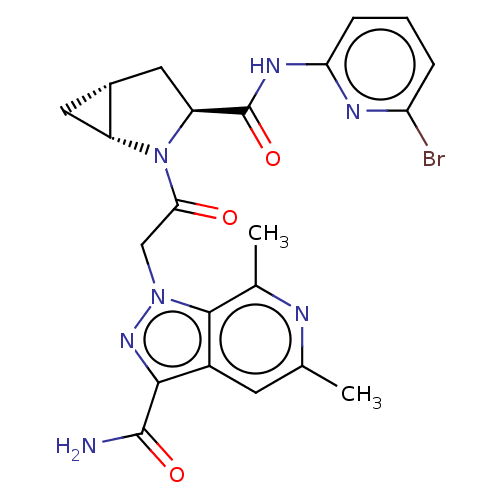 (US9085555, 743) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170934 (US9085555, 362) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171208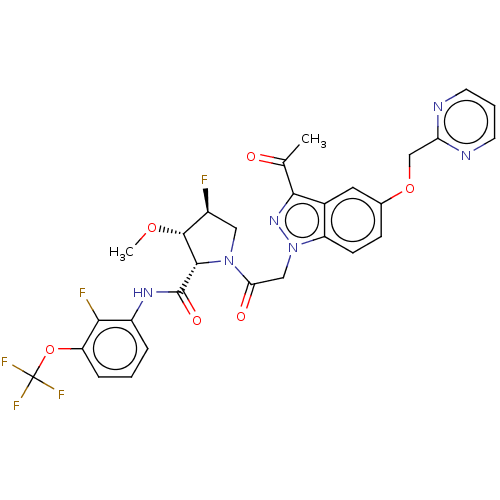 (US9085555, 638) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170949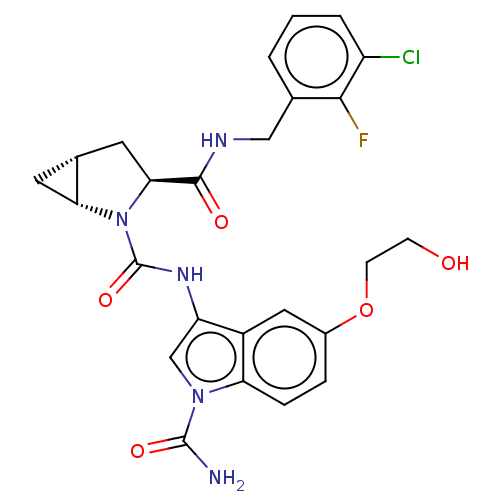 (US9085555, 379) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM254528 (US9468661, 106) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description Method 1: Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compoun... | US Patent US9468661 (2016) BindingDB Entry DOI: 10.7270/Q23N22B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170734 (US9085555, 162) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171255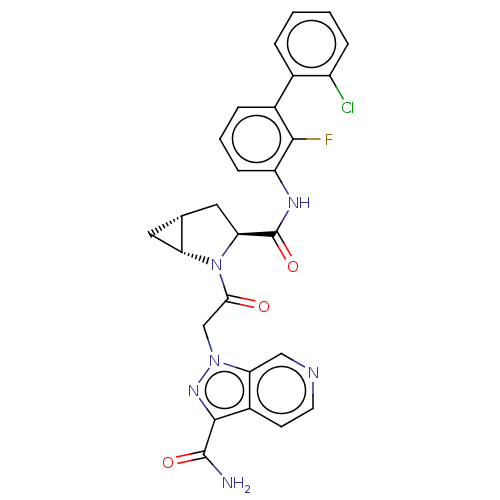 (US9085555, 685) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1366 total ) | Next | Last >> |