 Found 163 hits with Last Name = 'hong' and Initial = 'xj'
Found 163 hits with Last Name = 'hong' and Initial = 'xj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301603
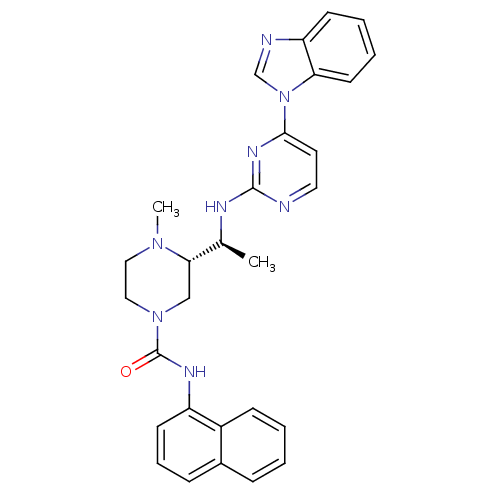
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)[C@@H]1CN(CCN1C)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C29H30N8O/c1-20(32-28-30-15-14-27(34-28)37-19-31-24-11-5-6-13-25(24)37)26-18-36(17-16-35(26)2)29(38)33-23-12-7-9-21-8-3-4-10-22(21)23/h3-15,19-20,26H,16-18H2,1-2H3,(H,33,38)(H,30,32,34)/t20-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301619
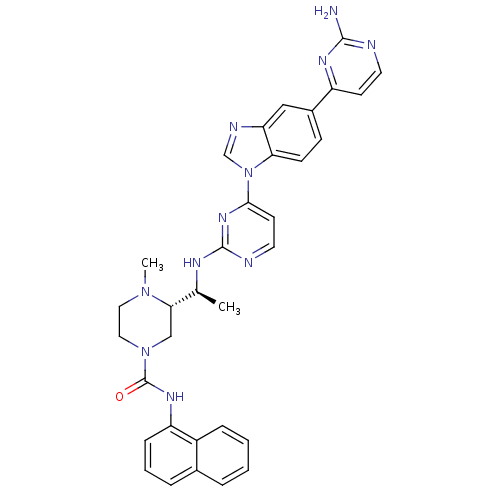
((S)-3-((S)-1-(4-(5-(2-aminopyrimidin-4-yl)-1H-benz...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2cc(ccc12)-c1ccnc(N)n1)[C@@H]1CN(CCN1C)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C33H33N11O/c1-21(29-19-43(17-16-42(29)2)33(45)40-26-9-5-7-22-6-3-4-8-24(22)26)38-32-36-15-13-30(41-32)44-20-37-27-18-23(10-11-28(27)44)25-12-14-35-31(34)39-25/h3-15,18,20-21,29H,16-17,19H2,1-2H3,(H,40,45)(H2,34,35,39)(H,36,38,41)/t21-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301604
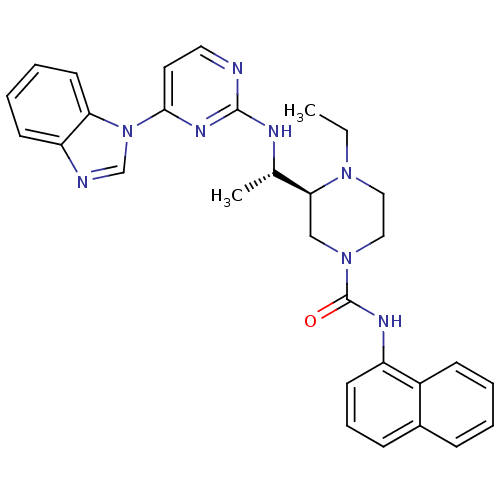
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES CCN1CCN(C[C@H]1[C@H](C)Nc1nccc(n1)-n1cnc2ccccc12)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C30H32N8O/c1-3-36-17-18-37(30(39)34-24-13-8-10-22-9-4-5-11-23(22)24)19-27(36)21(2)33-29-31-16-15-28(35-29)38-20-32-25-12-6-7-14-26(25)38/h4-16,20-21,27H,3,17-19H2,1-2H3,(H,34,39)(H,31,33,35)/t21-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301624
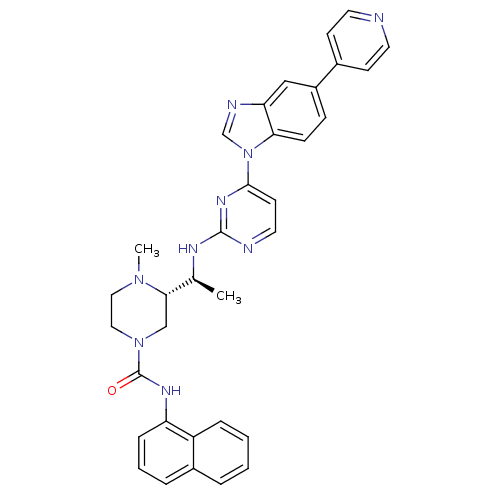
((S)-4-methyl-N(S)-4-methyl-N-(naphthalen-1-yl)-3-(...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2cc(ccc12)-c1ccncc1)[C@@H]1CN(CCN1C)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C34H33N9O/c1-23(31-21-42(19-18-41(31)2)34(44)39-28-9-5-7-25-6-3-4-8-27(25)28)38-33-36-17-14-32(40-33)43-22-37-29-20-26(10-11-30(29)43)24-12-15-35-16-13-24/h3-17,20,22-23,31H,18-19,21H2,1-2H3,(H,39,44)(H,36,38,40)/t23-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301605
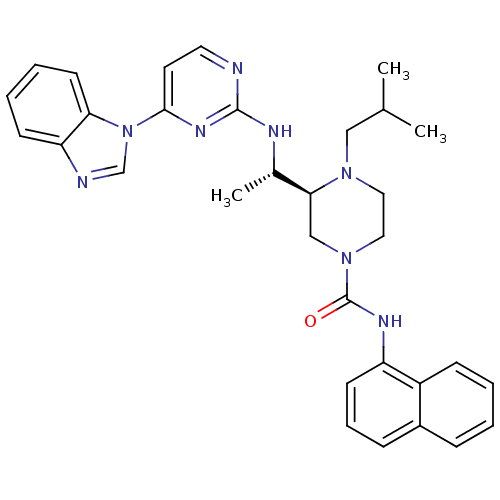
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES CC(C)CN1CCN(C[C@H]1[C@H](C)Nc1nccc(n1)-n1cnc2ccccc12)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C32H36N8O/c1-22(2)19-38-17-18-39(32(41)36-26-13-8-10-24-9-4-5-11-25(24)26)20-29(38)23(3)35-31-33-16-15-30(37-31)40-21-34-27-12-6-7-14-28(27)40/h4-16,21-23,29H,17-20H2,1-3H3,(H,36,41)(H,33,35,37)/t23-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301607
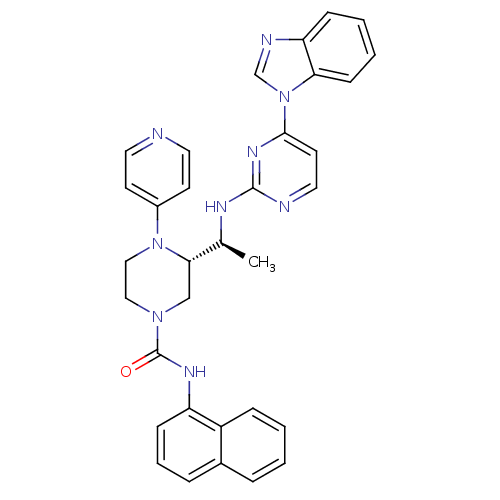
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)[C@@H]1CN(CCN1c1ccncc1)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C33H31N9O/c1-23(37-32-35-18-15-31(39-32)42-22-36-28-10-4-5-12-29(28)42)30-21-40(19-20-41(30)25-13-16-34-17-14-25)33(43)38-27-11-6-8-24-7-2-3-9-26(24)27/h2-18,22-23,30H,19-21H2,1H3,(H,38,43)(H,35,37,39)/t23-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301594
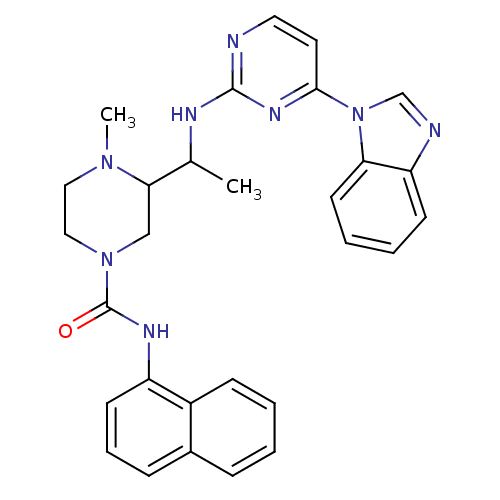
(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CN(CCN1C)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C29H30N8O/c1-20(32-28-30-15-14-27(34-28)37-19-31-24-11-5-6-13-25(24)37)26-18-36(17-16-35(26)2)29(38)33-23-12-7-9-21-8-3-4-10-22(21)23/h3-15,19-20,26H,16-18H2,1-2H3,(H,33,38)(H,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301594

(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CN(CCN1C)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C29H30N8O/c1-20(32-28-30-15-14-27(34-28)37-19-31-24-11-5-6-13-25(24)37)26-18-36(17-16-35(26)2)29(38)33-23-12-7-9-21-8-3-4-10-22(21)23/h3-15,19-20,26H,16-18H2,1-2H3,(H,33,38)(H,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301588
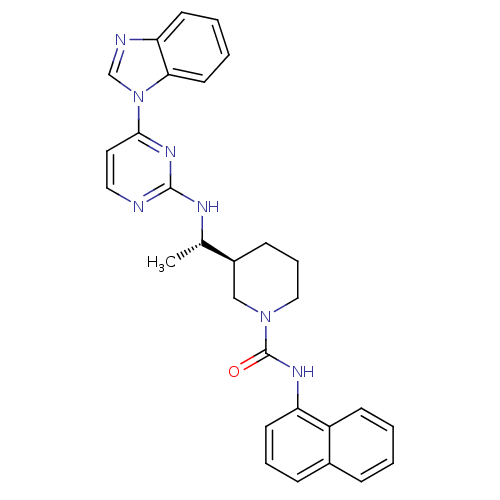
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)[C@H]1CCCN(C1)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C29H29N7O/c1-20(32-28-30-16-15-27(34-28)36-19-31-25-12-4-5-14-26(25)36)22-10-7-17-35(18-22)29(37)33-24-13-6-9-21-8-2-3-11-23(21)24/h2-6,8-9,11-16,19-20,22H,7,10,17-18H2,1H3,(H,33,37)(H,30,32,34)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301618
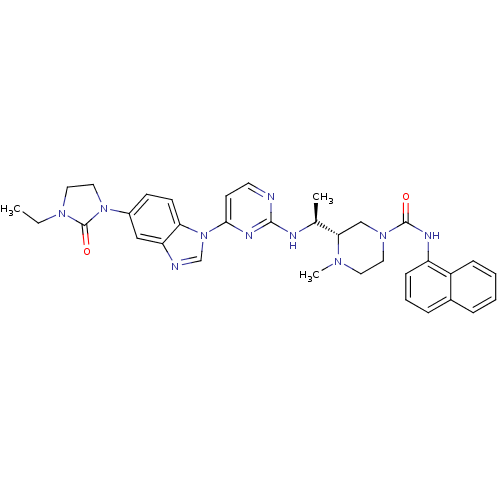
((S)-3-((S)-1-(4-(5-(3-ethyl-2-oxoimidazolidin-1-yl...)Show SMILES CCN1CCN(C1=O)c1ccc2n(cnc2c1)-c1ccnc(N[C@@H](C)[C@@H]2CN(CCN2C)C(=O)Nc2cccc3ccccc23)n1 |r| Show InChI InChI=1S/C34H38N10O2/c1-4-41-18-19-43(34(41)46)25-12-13-29-28(20-25)36-22-44(29)31-14-15-35-32(39-31)37-23(2)30-21-42(17-16-40(30)3)33(45)38-27-11-7-9-24-8-5-6-10-26(24)27/h5-15,20,22-23,30H,4,16-19,21H2,1-3H3,(H,38,45)(H,35,37,39)/t23-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301608
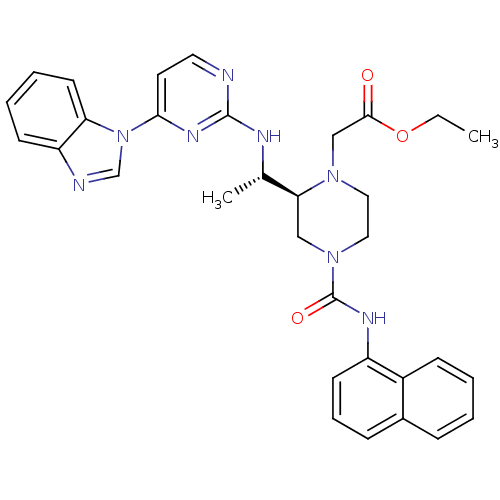
(CHEMBL566507 | ethyl 2-((S)-2-((S)-1-(4-(1H-benzo[...)Show SMILES CCOC(=O)CN1CCN(C[C@H]1[C@H](C)Nc1nccc(n1)-n1cnc2ccccc12)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C32H34N8O3/c1-3-43-30(41)20-38-17-18-39(32(42)36-25-13-8-10-23-9-4-5-11-24(23)25)19-28(38)22(2)35-31-33-16-15-29(37-31)40-21-34-26-12-6-7-14-27(26)40/h4-16,21-22,28H,3,17-20H2,1-2H3,(H,36,42)(H,33,35,37)/t22-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301587
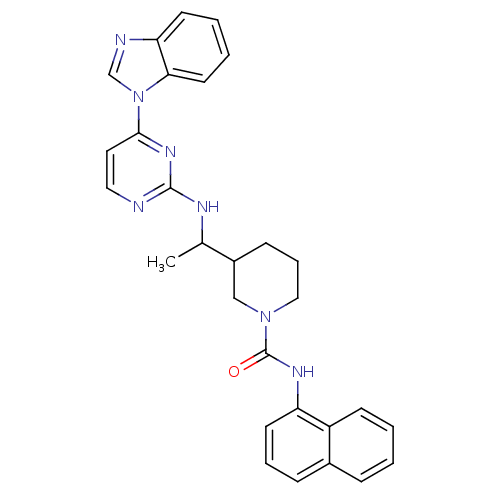
(CHEMBL567885 | rac 3-(1-(4-(1H-benzo[d]imidazol-1-...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CCCN(C1)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C29H29N7O/c1-20(32-28-30-16-15-27(34-28)36-19-31-25-12-4-5-14-26(25)36)22-10-7-17-35(18-22)29(37)33-24-13-6-9-21-8-2-3-11-23(21)24/h2-6,8-9,11-16,19-20,22H,7,10,17-18H2,1H3,(H,33,37)(H,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301621

((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)[C@]1(C)CN(CCN1C)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C30H32N8O/c1-21(33-28-31-16-15-27(35-28)38-20-32-25-12-6-7-14-26(25)38)30(2)19-37(18-17-36(30)3)29(39)34-24-13-8-10-22-9-4-5-11-23(22)24/h4-16,20-21H,17-19H2,1-3H3,(H,34,39)(H,31,33,35)/t21-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301617
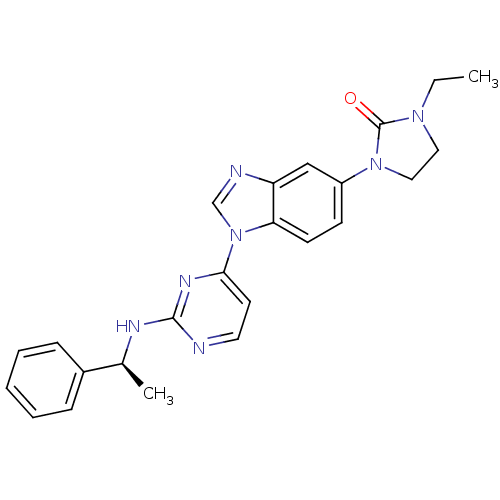
((S)-1-ethyl-3-(1-(2-(1-phenylethylamino)pyrimidin-...)Show SMILES CCN1CCN(C1=O)c1ccc2n(cnc2c1)-c1ccnc(N[C@@H](C)c2ccccc2)n1 |r| Show InChI InChI=1S/C24H25N7O/c1-3-29-13-14-30(24(29)32)19-9-10-21-20(15-19)26-16-31(21)22-11-12-25-23(28-22)27-17(2)18-7-5-4-6-8-18/h4-12,15-17H,3,13-14H2,1-2H3,(H,25,27,28)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301616
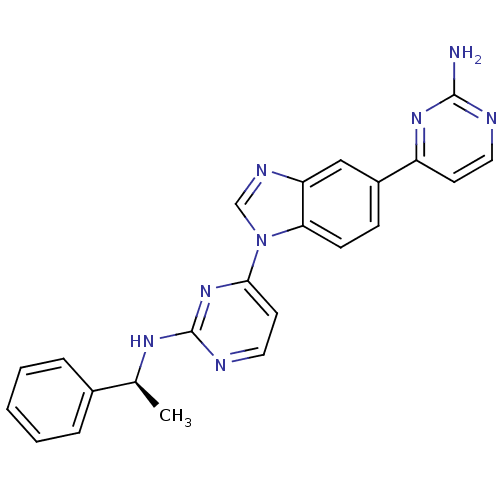
((S)-4-(5-(2-aminopyrimidin-4-yl)-1H-benzo[d]imidaz...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2cc(ccc12)-c1ccnc(N)n1)c1ccccc1 |r| Show InChI InChI=1S/C23H20N8/c1-15(16-5-3-2-4-6-16)28-23-26-12-10-21(30-23)31-14-27-19-13-17(7-8-20(19)31)18-9-11-25-22(24)29-18/h2-15H,1H3,(H2,24,25,29)(H,26,28,30)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301606
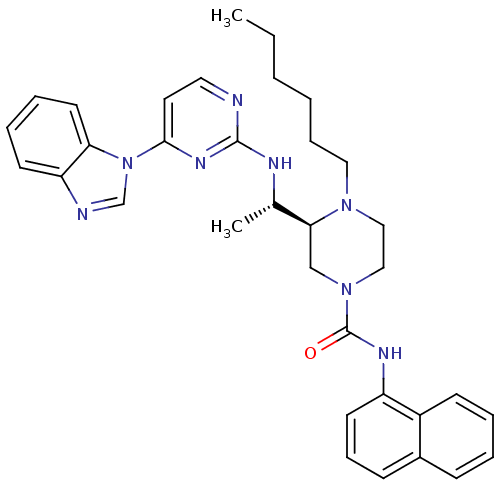
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES CCCCCCN1CCN(C[C@H]1[C@H](C)Nc1nccc(n1)-n1cnc2ccccc12)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C34H40N8O/c1-3-4-5-10-20-40-21-22-41(34(43)38-28-16-11-13-26-12-6-7-14-27(26)28)23-31(40)25(2)37-33-35-19-18-32(39-33)42-24-36-29-15-8-9-17-30(29)42/h6-9,11-19,24-25,31H,3-5,10,20-23H2,1-2H3,(H,38,43)(H,35,37,39)/t25-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301595
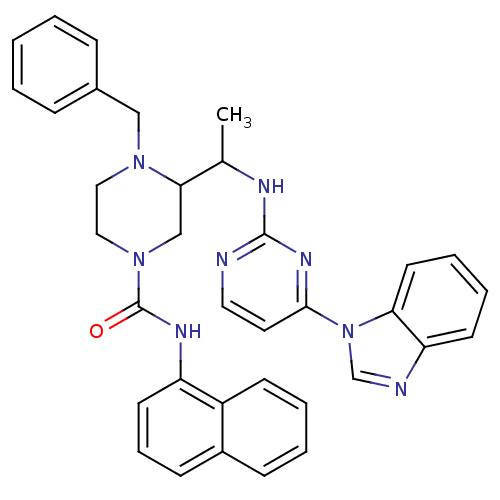
(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CN(CCN1Cc1ccccc1)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C35H34N8O/c1-25(38-34-36-19-18-33(40-34)43-24-37-30-15-7-8-17-31(30)43)32-23-42(21-20-41(32)22-26-10-3-2-4-11-26)35(44)39-29-16-9-13-27-12-5-6-14-28(27)29/h2-19,24-25,32H,20-23H2,1H3,(H,39,44)(H,36,38,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301623
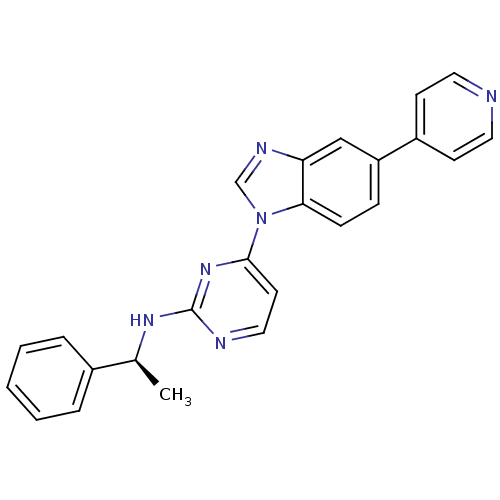
((S)-N-(1-phenylethyl)-4-(5-(pyridin-4-yl)-1H-benzo...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2cc(ccc12)-c1ccncc1)c1ccccc1 |r| Show InChI InChI=1S/C24H20N6/c1-17(18-5-3-2-4-6-18)28-24-26-14-11-23(29-24)30-16-27-21-15-20(7-8-22(21)30)19-9-12-25-13-10-19/h2-17H,1H3,(H,26,28,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125504
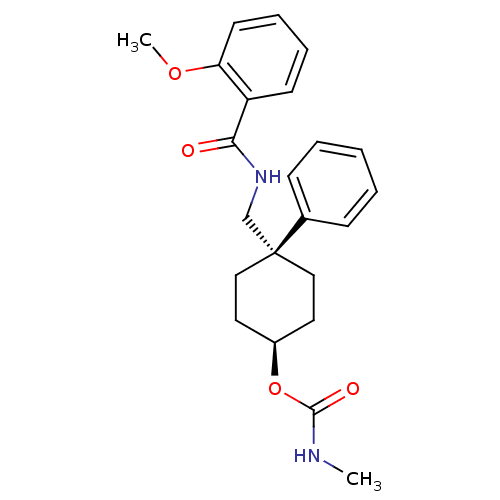
(CHEMBL16806 | Methyl-carbamic acid 4-[(2-methoxy-b...)Show SMILES CNC(=O)O[C@H]1CC[C@](CNC(=O)c2ccccc2OC)(CC1)c1ccccc1 |wU:8.8,wD:5.4,(-6.12,-5.94,;-4.77,-5.17,;-3.45,-5.96,;-3.47,-7.5,;-2.11,-5.2,;-2.1,-3.66,;-.77,-2.89,;-.77,-1.35,;-2.1,-.58,;-.77,.19,;.57,-.58,;1.89,.19,;1.89,1.72,;3.24,-.58,;3.24,-2.13,;4.55,-2.89,;5.9,-2.13,;5.9,-.58,;4.55,.19,;4.55,1.72,;3.24,2.49,;-3.44,-1.35,;-3.44,-2.89,;-3.44,.19,;-3.44,1.72,;-4.76,2.49,;-6.1,1.72,;-6.1,.19,;-4.77,-.58,)| Show InChI InChI=1S/C23H28N2O4/c1-24-22(27)29-18-12-14-23(15-13-18,17-8-4-3-5-9-17)16-25-21(26)19-10-6-7-11-20(19)28-2/h3-11,18H,12-16H2,1-2H3,(H,24,27)(H,25,26)/t18-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DiTc binding to Kv1.3 channel in human brain membranes |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125521
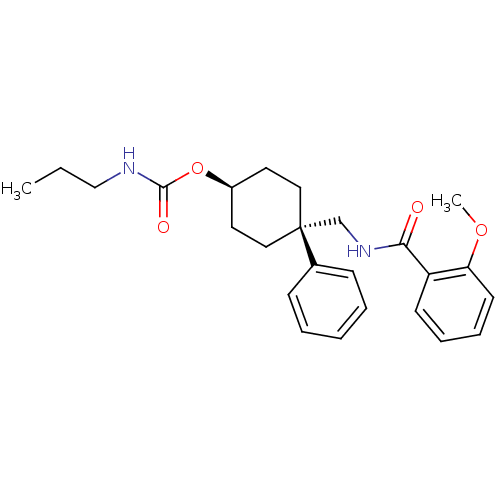
(CHEMBL16804 | Propyl-carbamic acid 4-[(2-methoxy-b...)Show SMILES CCCNC(=O)O[C@H]1CC[C@](CNC(=O)c2ccccc2OC)(CC1)c1ccccc1 |wU:10.10,wD:7.6,(-9.05,-5.79,;-9.05,-7.33,;-7.74,-8.13,;-6.39,-7.36,;-5.06,-8.15,;-5.08,-9.69,;-3.7,-7.4,;-3.7,-5.86,;-2.37,-5.09,;-2.37,-3.53,;-3.7,-2.76,;-2.37,-1.99,;-1.02,-2.76,;.31,-1.99,;.31,-.45,;1.64,-2.76,;1.64,-4.32,;2.97,-5.09,;4.32,-4.32,;4.32,-2.76,;2.97,-1.99,;2.97,-.45,;1.64,.32,;-5.03,-3.53,;-5.03,-5.09,;-5.03,-1.99,;-6.39,-2.76,;-7.72,-1.99,;-7.72,-.45,;-6.36,.32,;-5.03,-.45,)| Show InChI InChI=1S/C25H32N2O4/c1-3-17-26-24(29)31-20-13-15-25(16-14-20,19-9-5-4-6-10-19)18-27-23(28)21-11-7-8-12-22(21)30-2/h4-12,20H,3,13-18H2,1-2H3,(H,26,29)(H,27,28)/t20-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DiTc binding to Kv1.3 channel in human brain membranes |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301593

((3-((4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylami...)Show SMILES CN1CCN(CC1CNc1nccc(n1)-n1cnc2ccccc12)C(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C28H27N7O/c1-33-14-15-34(27(36)22-11-10-20-6-2-3-7-21(20)16-22)18-23(33)17-30-28-29-13-12-26(32-28)35-19-31-24-8-4-5-9-25(24)35/h2-13,16,19,23H,14-15,17-18H2,1H3,(H,29,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301583

(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CCCN(C1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C25H27N7O/c1-18(19-8-7-15-31(16-19)25(33)29-20-9-3-2-4-10-20)28-24-26-14-13-23(30-24)32-17-27-21-11-5-6-12-22(21)32/h2-6,9-14,17-19H,7-8,15-16H2,1H3,(H,29,33)(H,26,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301596
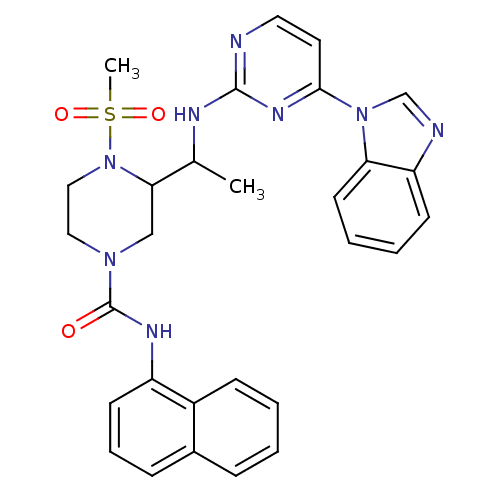
(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CN(CCN1S(C)(=O)=O)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C29H30N8O3S/c1-20(32-28-30-15-14-27(34-28)36-19-31-24-11-5-6-13-25(24)36)26-18-35(16-17-37(26)41(2,39)40)29(38)33-23-12-7-9-21-8-3-4-10-22(21)23/h3-15,19-20,26H,16-18H2,1-2H3,(H,33,38)(H,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125498
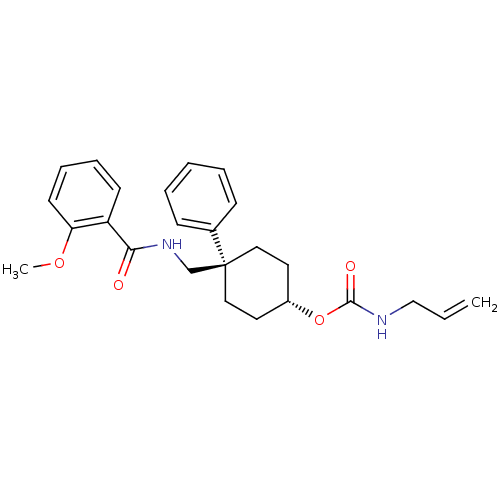
(Allyl-carbamic acid 4-[(2-methoxy-benzoylamino)-me...)Show SMILES COc1ccccc1C(=O)NC[C@]1(CC[C@@H](CC1)OC(=O)NCC=C)c1ccccc1 |wU:12.12,wD:15.19,(3.96,3.63,;5.29,2.86,;5.29,1.32,;6.63,.55,;6.63,-1.01,;5.29,-1.78,;3.96,-1.01,;3.96,.55,;2.62,1.32,;2.62,2.86,;1.29,.55,;-.05,1.32,;-1.38,.55,;-.05,-.22,;-.05,-1.78,;-1.38,-2.55,;-2.7,-1.78,;-2.7,-.22,;-1.39,-4.09,;-2.73,-4.83,;-2.75,-6.37,;-4.06,-4.04,;-5.41,-4.81,;-6.73,-4.02,;-8.08,-4.79,;-2.72,1.32,;-4.06,.55,;-5.39,1.32,;-5.39,2.86,;-4.05,3.63,;-2.72,2.86,)| Show InChI InChI=1S/C25H30N2O4/c1-3-17-26-24(29)31-20-13-15-25(16-14-20,19-9-5-4-6-10-19)18-27-23(28)21-11-7-8-12-22(21)30-2/h3-12,20H,1,13-18H2,2H3,(H,26,29)(H,27,28)/t20-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.3 ion channel. Measured in the Rb_Kv assay: [86Rb+] efflux from CHO cells stably transfected with Kv1.3 channel. |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301615
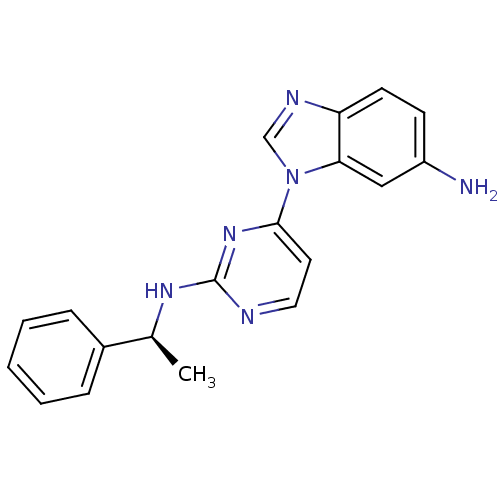
((S)-1-(2-(1-phenylethylamino)pyrimidin-4-yl)-1H-be...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccc(N)cc12)c1ccccc1 |r| Show InChI InChI=1S/C19H18N6/c1-13(14-5-3-2-4-6-14)23-19-21-10-9-18(24-19)25-12-22-16-8-7-15(20)11-17(16)25/h2-13H,20H2,1H3,(H,21,23,24)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301599

(4-(1H-benzo[d]imidazol-1-yl)-N-(1-(1-methyl-4-(met...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CN(CCN1C)S(C)(=O)=O Show InChI InChI=1S/C19H25N7O2S/c1-14(17-12-25(29(3,27)28)11-10-24(17)2)22-19-20-9-8-18(23-19)26-13-21-15-6-4-5-7-16(15)26/h4-9,13-14,17H,10-12H2,1-3H3,(H,20,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 22.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301597
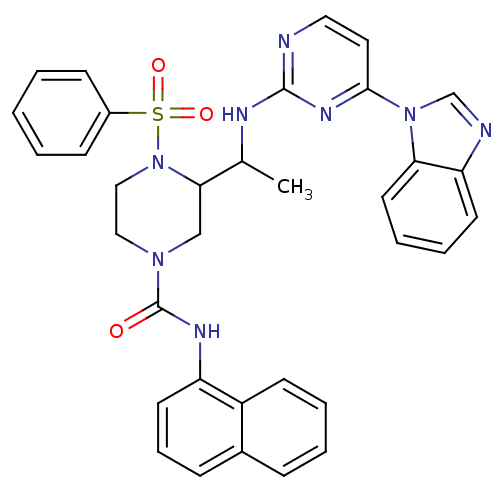
(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CN(CCN1S(=O)(=O)c1ccccc1)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C34H32N8O3S/c1-24(37-33-35-19-18-32(39-33)41-23-36-29-15-7-8-17-30(29)41)31-22-40(20-21-42(31)46(44,45)26-12-3-2-4-13-26)34(43)38-28-16-9-11-25-10-5-6-14-27(25)28/h2-19,23-24,31H,20-22H2,1H3,(H,38,43)(H,35,37,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125501
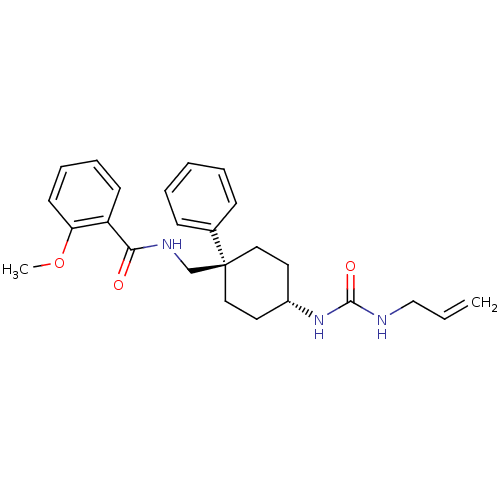
(CHEMBL16255 | N-[4-(3-Allyl-ureido)-1-phenyl-cyclo...)Show SMILES COc1ccccc1C(=O)NC[C@]1(CC[C@@H](CC1)NC(=O)NCC=C)c1ccccc1 |wU:12.12,wD:15.19,(2.5,-.08,;3.83,-.85,;3.83,-2.39,;5.18,-3.16,;5.18,-4.71,;3.83,-5.48,;2.5,-4.71,;2.5,-3.16,;1.15,-2.39,;1.15,-.85,;-.18,-3.16,;-1.52,-2.39,;-2.85,-3.16,;-1.52,-3.94,;-1.52,-5.48,;-2.85,-6.25,;-4.19,-5.48,;-4.19,-3.94,;-2.85,-7.79,;-1.52,-8.57,;-.19,-7.79,;-1.52,-10.11,;-.19,-10.88,;1.15,-10.11,;2.48,-10.88,;-3.97,-2.07,;-5.42,-2.49,;-6.52,-1.39,;-6.13,.09,;-4.68,.51,;-3.58,-.59,)| Show InChI InChI=1S/C25H31N3O3/c1-3-17-26-24(30)28-20-13-15-25(16-14-20,19-9-5-4-6-10-19)18-27-23(29)21-11-7-8-12-22(21)31-2/h3-12,20H,1,13-18H2,2H3,(H,27,29)(H2,26,28,30)/t20-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DiTc binding to Kv1.3 channel in human brain membranes |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301586
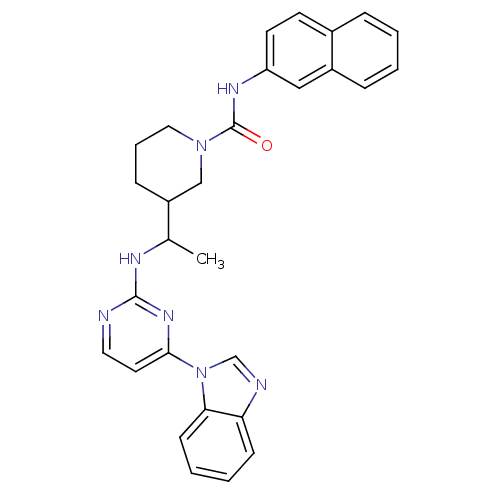
(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CCCN(C1)C(=O)Nc1ccc2ccccc2c1 Show InChI InChI=1S/C29H29N7O/c1-20(32-28-30-15-14-27(34-28)36-19-31-25-10-4-5-11-26(25)36)23-9-6-16-35(18-23)29(37)33-24-13-12-21-7-2-3-8-22(21)17-24/h2-5,7-8,10-15,17,19-20,23H,6,9,16,18H2,1H3,(H,33,37)(H,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125509

(Allyl-carbamic acid 4-[(5-fluoro-2-methoxy-benzoyl...)Show SMILES COc1ccc(F)cc1C(=O)NC[C@]1(CC[C@@H](CC1)OC(=O)NCC=C)c1ccccc1 |wU:13.13,wD:16.20,(2.06,.16,;3.38,-.59,;3.38,-2.14,;4.71,-2.91,;4.71,-4.45,;3.38,-5.22,;3.36,-6.76,;2.05,-4.45,;2.05,-2.91,;.71,-2.14,;.71,-.59,;-.62,-2.91,;-1.95,-2.14,;-3.28,-2.91,;-1.95,-3.68,;-1.95,-5.22,;-3.28,-5.99,;-4.61,-5.22,;-4.61,-3.68,;-3.28,-7.53,;-1.96,-8.3,;-.63,-7.53,;-1.96,-9.83,;-3.28,-10.6,;-4.62,-9.83,;-5.95,-10.6,;-4.39,-1.82,;-5.84,-2.22,;-6.93,-1.15,;-6.53,.32,;-5.09,.74,;-4,-.33,)| Show InChI InChI=1S/C25H29FN2O4/c1-3-15-27-24(30)32-20-11-13-25(14-12-20,18-7-5-4-6-8-18)17-28-23(29)21-16-19(26)9-10-22(21)31-2/h3-10,16,20H,1,11-15,17H2,2H3,(H,27,30)(H,28,29)/t20-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.3 ion channel. Measured in the Rb_Kv assay: [86Rb+] efflux from CHO cells stably transfected with Kv1.3 channel. |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301585
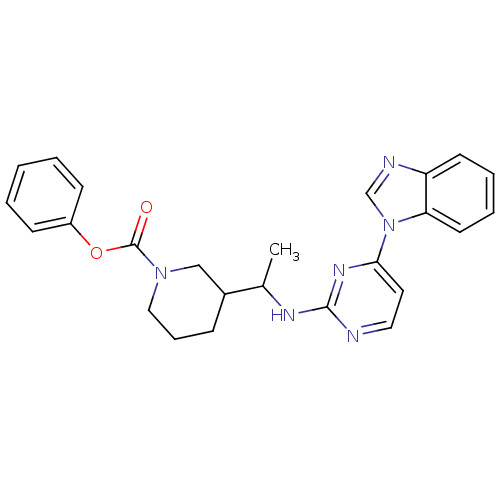
(CHEMBL567880 | phenyl 3-(1-(4-(1H-benzo[d]imidazol...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CCCN(C1)C(=O)Oc1ccccc1 Show InChI InChI=1S/C25H26N6O2/c1-18(19-8-7-15-30(16-19)25(32)33-20-9-3-2-4-10-20)28-24-26-14-13-23(29-24)31-17-27-21-11-5-6-12-22(21)31/h2-6,9-14,17-19H,7-8,15-16H2,1H3,(H,26,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301575
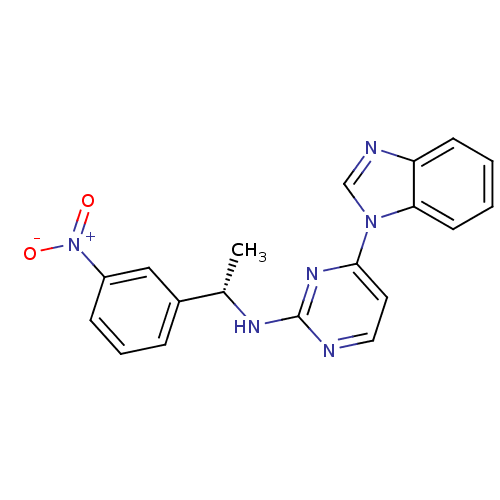
((S)-4-(1H-benzo[d]imidazol-1-yl)-N-(1-(3-nitrophen...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)c1cccc(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C19H16N6O2/c1-13(14-5-4-6-15(11-14)25(26)27)22-19-20-10-9-18(23-19)24-12-21-16-7-2-3-8-17(16)24/h2-13H,1H3,(H,20,22,23)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125521

(CHEMBL16804 | Propyl-carbamic acid 4-[(2-methoxy-b...)Show SMILES CCCNC(=O)O[C@H]1CC[C@](CNC(=O)c2ccccc2OC)(CC1)c1ccccc1 |wU:10.10,wD:7.6,(-9.05,-5.79,;-9.05,-7.33,;-7.74,-8.13,;-6.39,-7.36,;-5.06,-8.15,;-5.08,-9.69,;-3.7,-7.4,;-3.7,-5.86,;-2.37,-5.09,;-2.37,-3.53,;-3.7,-2.76,;-2.37,-1.99,;-1.02,-2.76,;.31,-1.99,;.31,-.45,;1.64,-2.76,;1.64,-4.32,;2.97,-5.09,;4.32,-4.32,;4.32,-2.76,;2.97,-1.99,;2.97,-.45,;1.64,.32,;-5.03,-3.53,;-5.03,-5.09,;-5.03,-1.99,;-6.39,-2.76,;-7.72,-1.99,;-7.72,-.45,;-6.36,.32,;-5.03,-.45,)| Show InChI InChI=1S/C25H32N2O4/c1-3-17-26-24(29)31-20-13-15-25(16-14-20,19-9-5-4-6-10-19)18-27-23(28)21-11-7-8-12-22(21)30-2/h4-12,20H,3,13-18H2,1-2H3,(H,26,29)(H,27,28)/t20-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [86Rb+] efflux from CHO cells stably transfected with Kv1.3 channel |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125504

(CHEMBL16806 | Methyl-carbamic acid 4-[(2-methoxy-b...)Show SMILES CNC(=O)O[C@H]1CC[C@](CNC(=O)c2ccccc2OC)(CC1)c1ccccc1 |wU:8.8,wD:5.4,(-6.12,-5.94,;-4.77,-5.17,;-3.45,-5.96,;-3.47,-7.5,;-2.11,-5.2,;-2.1,-3.66,;-.77,-2.89,;-.77,-1.35,;-2.1,-.58,;-.77,.19,;.57,-.58,;1.89,.19,;1.89,1.72,;3.24,-.58,;3.24,-2.13,;4.55,-2.89,;5.9,-2.13,;5.9,-.58,;4.55,.19,;4.55,1.72,;3.24,2.49,;-3.44,-1.35,;-3.44,-2.89,;-3.44,.19,;-3.44,1.72,;-4.76,2.49,;-6.1,1.72,;-6.1,.19,;-4.77,-.58,)| Show InChI InChI=1S/C23H28N2O4/c1-24-22(27)29-18-12-14-23(15-13-18,17-8-4-3-5-9-17)16-25-21(26)19-10-6-7-11-20(19)28-2/h3-11,18H,12-16H2,1-2H3,(H,24,27)(H,25,26)/t18-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [86Rb+] efflux from CHO cells stably transfected with Kv1.3 channel |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301612

((S)-4-(6-methyl-1H-benzo[d]imidazol-1-yl)-N-(1-phe...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccc(C)cc12)c1ccccc1 |r| Show InChI InChI=1S/C20H19N5/c1-14-8-9-17-18(12-14)25(13-22-17)19-10-11-21-20(24-19)23-15(2)16-6-4-3-5-7-16/h3-13,15H,1-2H3,(H,21,23,24)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125498

(Allyl-carbamic acid 4-[(2-methoxy-benzoylamino)-me...)Show SMILES COc1ccccc1C(=O)NC[C@]1(CC[C@@H](CC1)OC(=O)NCC=C)c1ccccc1 |wU:12.12,wD:15.19,(3.96,3.63,;5.29,2.86,;5.29,1.32,;6.63,.55,;6.63,-1.01,;5.29,-1.78,;3.96,-1.01,;3.96,.55,;2.62,1.32,;2.62,2.86,;1.29,.55,;-.05,1.32,;-1.38,.55,;-.05,-.22,;-.05,-1.78,;-1.38,-2.55,;-2.7,-1.78,;-2.7,-.22,;-1.39,-4.09,;-2.73,-4.83,;-2.75,-6.37,;-4.06,-4.04,;-5.41,-4.81,;-6.73,-4.02,;-8.08,-4.79,;-2.72,1.32,;-4.06,.55,;-5.39,1.32,;-5.39,2.86,;-4.05,3.63,;-2.72,2.86,)| Show InChI InChI=1S/C25H30N2O4/c1-3-17-26-24(29)31-20-13-15-25(16-14-20,19-9-5-4-6-10-19)18-27-23(28)21-11-7-8-12-22(21)30-2/h3-12,20H,1,13-18H2,2H3,(H,26,29)(H,27,28)/t20-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [86Rb+] efflux from CHO cells stably transfected with Kv1.3 channel |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125513
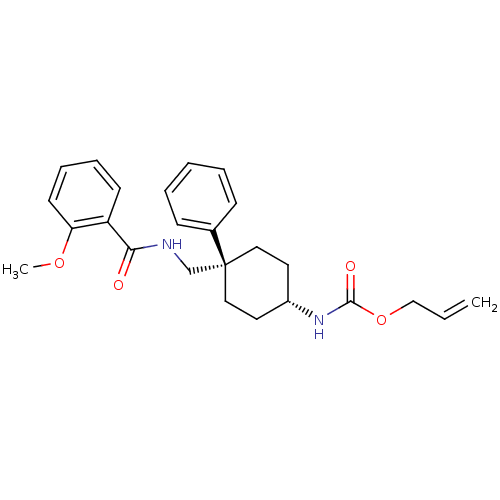
(CHEMBL277598 | {4-[(2-Methoxy-benzoylamino)-methyl...)Show SMILES COc1ccccc1C(=O)NC[C@@]1(CC[C@@H](CC1)NC(=O)OCC=C)c1ccccc1 |wU:12.12,15.19,(12.7,1.28,;14.04,.51,;14.04,-1.04,;15.37,-1.81,;15.37,-3.35,;14.04,-4.12,;12.7,-3.35,;12.7,-1.81,;11.35,-1.04,;11.35,.53,;10.02,-1.81,;8.68,-1.04,;7.35,-1.81,;6.02,-2.58,;6.02,-4.12,;7.35,-4.89,;8.68,-4.12,;8.68,-2.58,;7.35,-6.44,;8.68,-7.21,;10.02,-6.44,;8.68,-8.75,;10.02,-9.52,;10.02,-11.07,;11.35,-11.84,;6.23,-.71,;6.62,.76,;5.53,1.86,;4.07,1.44,;3.68,-.03,;4.77,-1.13,)| Show InChI InChI=1S/C25H30N2O4/c1-3-17-31-24(29)27-20-13-15-25(16-14-20,19-9-5-4-6-10-19)18-26-23(28)21-11-7-8-12-22(21)30-2/h3-12,20H,1,13-18H2,2H3,(H,26,28)(H,27,29)/t20-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of T-cell proliferation was determined by a human T-cell assay |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125512
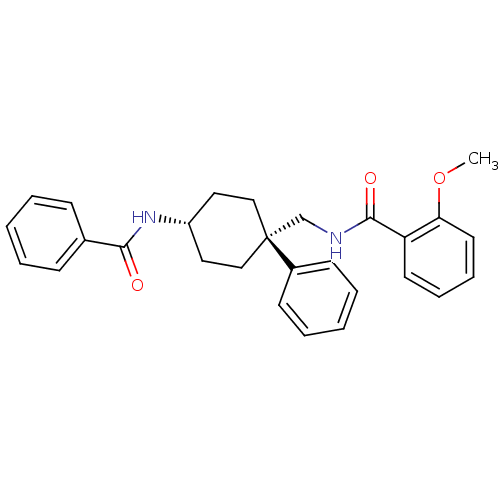
(CHEMBL16468 | N-(4-Benzoylamino-1-phenyl-cyclohexy...)Show SMILES COc1ccccc1C(=O)NC[C@@]1(CC[C@@H](CC1)NC(=O)c1ccccc1)c1ccccc1 |wU:12.12,15.19,(.54,3.3,;1.87,2.53,;1.87,.98,;3.2,.21,;3.2,-1.33,;1.87,-2.1,;.54,-1.33,;.54,.21,;-.82,.98,;-.82,2.55,;-2.15,.21,;-3.48,.98,;-4.82,.21,;-6.15,-.56,;-6.15,-2.1,;-4.82,-2.88,;-3.48,-2.1,;-3.48,-.56,;-4.82,-4.42,;-3.48,-5.19,;-2.15,-4.42,;-3.48,-6.73,;-4.84,-7.49,;-4.84,-9.05,;-3.48,-9.82,;-2.15,-9.05,;-2.15,-7.49,;-5.94,1.31,;-5.54,2.78,;-6.64,3.9,;-8.11,3.46,;-8.49,1.99,;-7.4,.89,)| Show InChI InChI=1S/C28H30N2O3/c1-33-25-15-9-8-14-24(25)27(32)29-20-28(22-12-6-3-7-13-22)18-16-23(17-19-28)30-26(31)21-10-4-2-5-11-21/h2-15,23H,16-20H2,1H3,(H,29,32)(H,30,31)/t23-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.3 ion channel. Measured in the Rb_Kv assay: [86Rb+] efflux from CHO cells stably transfected with Kv1.3 channel. |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125531

(CHEMBL16848 | Isopropyl-carbamic acid 4-[(2-methox...)Show SMILES COc1ccccc1C(=O)NC[C@]1(CC[C@@H](CC1)OC(=O)NC(C)C)c1ccccc1 |wU:12.12,wD:15.19,(1.14,2.86,;2.47,2.09,;2.47,.55,;3.81,-.22,;3.81,-1.77,;2.47,-2.54,;1.14,-1.77,;1.14,-.22,;-.2,.55,;-.2,2.09,;-1.53,-.22,;-2.87,.55,;-4.2,-.22,;-2.87,-.99,;-2.87,-2.54,;-4.2,-3.31,;-5.53,-2.54,;-5.53,-.99,;-4.21,-4.85,;-5.55,-5.6,;-5.57,-7.14,;-6.88,-4.81,;-8.23,-5.58,;-9.55,-4.79,;-8.25,-7.12,;-5.54,.55,;-6.88,-.22,;-8.22,.55,;-8.22,2.09,;-6.87,2.86,;-5.54,2.09,)| Show InChI InChI=1S/C25H32N2O4/c1-18(2)27-24(29)31-20-13-15-25(16-14-20,19-9-5-4-6-10-19)17-26-23(28)21-11-7-8-12-22(21)30-3/h4-12,18,20H,13-17H2,1-3H3,(H,26,28)(H,27,29)/t20-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.3 ion channel. Measured in the Rb_Kv assay: [86Rb+] efflux from CHO cells stably transfected with Kv1.3 channel. |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301598

(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CN(CCN1C)C(=O)Nc1ccc2ccccc2c1 Show InChI InChI=1S/C29H30N8O/c1-20(32-28-30-14-13-27(34-28)37-19-31-24-9-5-6-10-25(24)37)26-18-36(16-15-35(26)2)29(38)33-23-12-11-21-7-3-4-8-22(21)17-23/h3-14,17,19-20,26H,15-16,18H2,1-2H3,(H,33,38)(H,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125516

(CHEMBL279606 | Propyl-carbamic acid 4-[(2-methoxy-...)Show SMILES CCCNC(=O)O[C@H]1CC[C@@](CNC(=O)c2ccccc2OC)(CC1)c1ccccc1 |wU:10.10,7.6,(-3.03,-11.46,;-1.71,-10.69,;-1.72,-9.15,;-3.06,-8.38,;-3.06,-6.83,;-1.72,-6.06,;-4.39,-6.06,;-4.39,-4.51,;-5.72,-3.74,;-5.72,-2.18,;-4.39,-1.41,;-3.06,-.64,;-1.71,-1.41,;-.37,-.64,;-.37,.9,;.97,-1.41,;.97,-2.97,;2.3,-3.74,;3.65,-2.97,;3.65,-1.41,;2.3,-.64,;2.3,.9,;.97,1.68,;-3.06,-2.18,;-3.06,-3.74,;-5.49,-.33,;-6.96,-.73,;-8.04,.35,;-7.66,1.82,;-6.21,2.24,;-5.11,1.16,)| Show InChI InChI=1S/C25H32N2O4/c1-3-17-26-24(29)31-20-13-15-25(16-14-20,19-9-5-4-6-10-19)18-27-23(28)21-11-7-8-12-22(21)30-2/h4-12,20H,3,13-18H2,1-2H3,(H,26,29)(H,27,28)/t20-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of T-cell proliferation was determined by a human T-cell assay |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125526

(CHEMBL16683 | N-[4-(3-Allyl-ureido)-1-phenyl-cyclo...)Show SMILES COc1ccccc1C(=O)NC[C@@]1(CC[C@@H](CC1)NC(=O)NCC=C)c1ccccc1 |wU:12.12,15.19,(11.14,1.75,;12.47,.97,;12.47,-.57,;13.8,-1.34,;13.8,-2.88,;12.47,-3.66,;11.14,-2.88,;11.14,-1.34,;9.78,-.57,;9.78,1,;8.45,-1.34,;7.12,-.57,;5.78,-1.34,;4.45,-2.11,;4.45,-3.66,;5.78,-4.43,;7.12,-3.66,;7.12,-2.11,;5.78,-5.97,;7.12,-6.74,;8.45,-5.97,;7.12,-8.29,;8.45,-9.06,;8.45,-10.6,;9.78,-11.37,;4.66,-.24,;5.06,1.23,;3.96,2.33,;2.51,1.91,;2.11,.44,;3.21,-.66,)| Show InChI InChI=1S/C25H31N3O3/c1-3-17-26-24(30)28-20-13-15-25(16-14-20,19-9-5-4-6-10-19)18-27-23(29)21-11-7-8-12-22(21)31-2/h3-12,20H,1,13-18H2,2H3,(H,27,29)(H2,26,28,30)/t20-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DiTc binding to Kv1.3 channel in human brain membranes |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301614
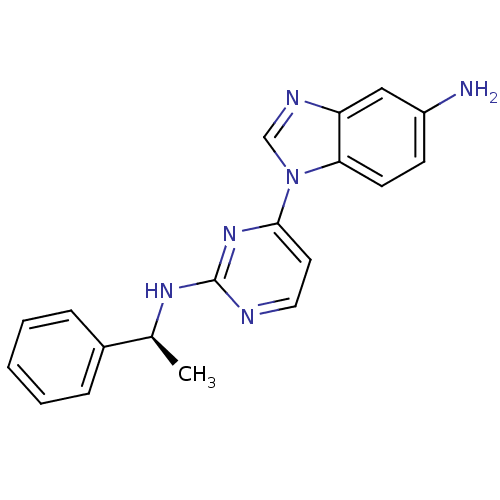
((S)-1-(2-(1-phenylethylamino)pyrimidin-4-yl)-1H-be...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2cc(N)ccc12)c1ccccc1 |r| Show InChI InChI=1S/C19H18N6/c1-13(14-5-3-2-4-6-14)23-19-21-10-9-18(24-19)25-12-22-16-11-15(20)7-8-17(16)25/h2-13H,20H2,1H3,(H,21,23,24)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125502

((2-Methyl-allyl)-carbamic acid 4-[(2-methoxy-benzo...)Show SMILES COc1ccccc1C(=O)NC[C@]1(CC[C@@H](CC1)OC(=O)NCC(C)=C)c1ccccc1 |wU:12.12,wD:15.19,(4.67,2.7,;6,1.93,;6,.39,;7.35,-.38,;7.35,-1.94,;6,-2.71,;4.67,-1.94,;4.67,-.38,;3.34,.39,;3.34,1.93,;2.01,-.38,;.66,.39,;-.67,-.38,;.66,-1.15,;.66,-2.71,;-.67,-3.48,;-2,-2.71,;-2,-1.15,;-.68,-5.02,;-2.02,-5.77,;-2.05,-7.31,;-3.35,-4.98,;-4.71,-5.75,;-6.02,-4.95,;-6.01,-3.41,;-7.37,-5.72,;-2.01,.39,;-2.01,1.93,;-3.34,2.7,;-4.68,1.93,;-4.68,.39,;-3.35,-.38,)| Show InChI InChI=1S/C26H32N2O4/c1-19(2)17-27-25(30)32-21-13-15-26(16-14-21,20-9-5-4-6-10-20)18-28-24(29)22-11-7-8-12-23(22)31-3/h4-12,21H,1,13-18H2,2-3H3,(H,27,30)(H,28,29)/t21-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of T-cell proliferation was determined by a human T-cell assay |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125524
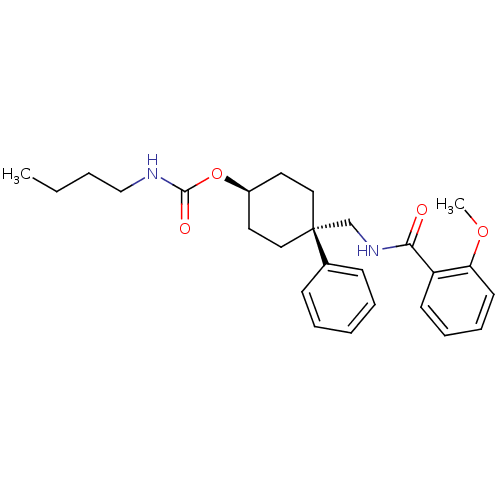
(Butyl-carbamic acid 4-[(2-methoxy-benzoylamino)-me...)Show SMILES CCCCNC(=O)O[C@H]1CC[C@](CNC(=O)c2ccccc2OC)(CC1)c1ccccc1 |wU:11.11,wD:8.7,(-.65,-13.17,;-.65,-11.61,;-1.99,-10.83,;-1.99,-9.29,;-3.34,-8.52,;-3.34,-6.98,;-1.99,-6.2,;-4.65,-6.2,;-4.65,-4.66,;-3.34,-3.89,;-3.34,-2.32,;-4.65,-1.55,;-3.34,-.78,;-1.98,-1.55,;-.65,-.78,;-.65,.76,;.7,-1.55,;.7,-3.12,;2.03,-3.89,;3.37,-3.12,;3.37,-1.55,;2.03,-.78,;2.03,.76,;.7,1.54,;-5.99,-2.32,;-5.99,-3.89,;-5.77,-.48,;-5.38,1.02,;-6.48,2.1,;-7.93,1.68,;-8.32,.2,;-7.22,-.87,)| Show InChI InChI=1S/C26H34N2O4/c1-3-4-18-27-25(30)32-21-14-16-26(17-15-21,20-10-6-5-7-11-20)19-28-24(29)22-12-8-9-13-23(22)31-2/h5-13,21H,3-4,14-19H2,1-2H3,(H,27,30)(H,28,29)/t21-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of T-cell proliferation was determined by a human T-cell assay |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125513

(CHEMBL277598 | {4-[(2-Methoxy-benzoylamino)-methyl...)Show SMILES COc1ccccc1C(=O)NC[C@@]1(CC[C@@H](CC1)NC(=O)OCC=C)c1ccccc1 |wU:12.12,15.19,(12.7,1.28,;14.04,.51,;14.04,-1.04,;15.37,-1.81,;15.37,-3.35,;14.04,-4.12,;12.7,-3.35,;12.7,-1.81,;11.35,-1.04,;11.35,.53,;10.02,-1.81,;8.68,-1.04,;7.35,-1.81,;6.02,-2.58,;6.02,-4.12,;7.35,-4.89,;8.68,-4.12,;8.68,-2.58,;7.35,-6.44,;8.68,-7.21,;10.02,-6.44,;8.68,-8.75,;10.02,-9.52,;10.02,-11.07,;11.35,-11.84,;6.23,-.71,;6.62,.76,;5.53,1.86,;4.07,1.44,;3.68,-.03,;4.77,-1.13,)| Show InChI InChI=1S/C25H30N2O4/c1-3-17-31-24(29)27-20-13-15-25(16-14-20,19-9-5-4-6-10-19)18-26-23(28)21-11-7-8-12-22(21)30-2/h3-12,20H,1,13-18H2,2H3,(H,26,28)(H,27,29)/t20-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-(D-Pro10)-Dynorphin A binding to human kappa opioid receptor from membranes of HEK293 cells |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125510
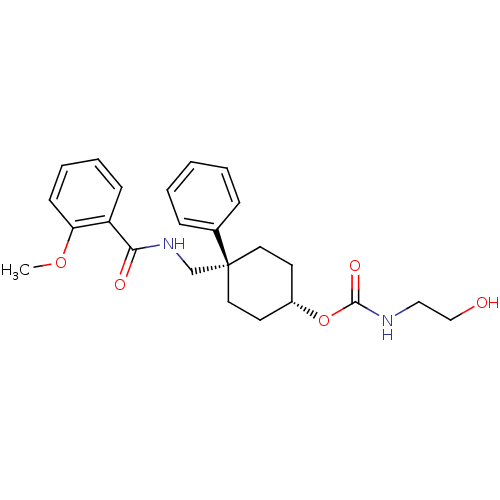
((2-Hydroxy-ethyl)-carbamic acid 4-[(2-methoxy-benz...)Show SMILES COc1ccccc1C(=O)NC[C@@]1(CC[C@@H](CC1)OC(=O)NCCO)c1ccccc1 |wU:12.12,15.19,(-1.58,6.53,;-.25,5.76,;-.25,4.24,;1.1,3.45,;1.1,1.91,;-.25,1.14,;-1.58,1.91,;-1.58,3.45,;-2.92,4.24,;-2.92,5.76,;-4.25,3.45,;-5.59,4.24,;-6.92,3.45,;-8.25,2.68,;-8.25,1.14,;-6.92,.37,;-5.59,1.14,;-5.59,2.68,;-6.92,-1.17,;-5.59,-1.94,;-4.26,-1.17,;-5.59,-3.5,;-4.26,-4.27,;-4.25,-5.81,;-2.91,-6.56,;-8.03,4.54,;-9.49,4.13,;-10.57,5.22,;-10.19,6.69,;-8.74,7.11,;-7.65,6.04,)| Show InChI InChI=1S/C24H30N2O5/c1-30-21-10-6-5-9-20(21)22(28)26-17-24(18-7-3-2-4-8-18)13-11-19(12-14-24)31-23(29)25-15-16-27/h2-10,19,27H,11-17H2,1H3,(H,25,29)(H,26,28)/t19-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.3 ion channel. Measured in the Rb_Kv assay: [86Rb+] efflux from CHO cells stably transfected with Kv1.3 channel. |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125522
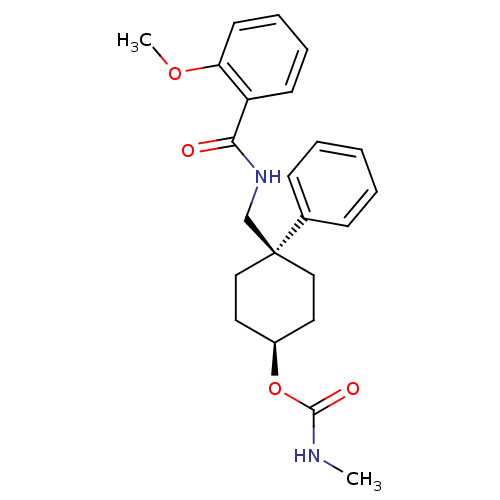
(CHEMBL16945 | Methyl-carbamic acid 4-[(2-methoxy-b...)Show SMILES CNC(=O)O[C@H]1CC[C@@](CNC(=O)c2ccccc2OC)(CC1)c1ccccc1 |wU:8.8,5.4,(-1.82,-8.49,;-3.15,-7.72,;-3.15,-6.16,;-1.82,-5.39,;-4.48,-5.39,;-4.48,-3.84,;-5.81,-3.07,;-5.81,-1.52,;-4.48,-.75,;-3.15,.02,;-1.8,-.75,;-.47,.02,;-.47,1.57,;.87,-.75,;.87,-2.3,;2.21,-3.07,;3.55,-2.3,;3.55,-.75,;2.21,.02,;2.21,1.57,;.87,2.34,;-3.15,-1.52,;-3.15,-3.07,;-5.59,.34,;-5.21,1.82,;-6.31,2.91,;-7.75,2.49,;-8.14,1.02,;-7.05,-.05,)| Show InChI InChI=1S/C23H28N2O4/c1-24-22(27)29-18-12-14-23(15-13-18,17-8-4-3-5-9-17)16-25-21(26)19-10-6-7-11-20(19)28-2/h3-11,18H,12-16H2,1-2H3,(H,24,27)(H,25,26)/t18-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of T-cell proliferation was determined by a human T-cell assay |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125507

(CHEMBL277064 | N-[4-((E)-But-2-enoylamino)-1-pheny...)Show SMILES COc1ccccc1C(=O)NC[C@@]1(CC[C@@H](CC1)NC(=O)\C=C\C)c1ccccc1 |wU:12.12,15.19,(2.11,1.93,;3.44,1.16,;3.44,-.38,;4.78,-1.15,;4.78,-2.7,;3.44,-3.47,;2.11,-2.7,;2.11,-1.15,;.76,-.38,;.76,1.18,;-.58,-1.15,;-1.91,-.38,;-3.24,-1.15,;-4.58,-1.93,;-4.58,-3.47,;-3.24,-4.24,;-1.91,-3.47,;-1.91,-1.93,;-3.24,-5.78,;-1.91,-6.55,;-.58,-5.78,;-1.91,-8.1,;-.58,-8.87,;-.58,-10.41,;-4.36,-.05,;-3.97,1.43,;-5.07,2.52,;-6.52,2.1,;-6.91,.62,;-5.81,-.47,)| Show InChI InChI=1S/C25H30N2O3/c1-3-9-23(28)27-20-14-16-25(17-15-20,19-10-5-4-6-11-19)18-26-24(29)21-12-7-8-13-22(21)30-2/h3-13,20H,14-18H2,1-2H3,(H,26,29)(H,27,28)/b9-3+/t20-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of T-cell proliferation was determined by a human T-cell assay |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50125516

(CHEMBL279606 | Propyl-carbamic acid 4-[(2-methoxy-...)Show SMILES CCCNC(=O)O[C@H]1CC[C@@](CNC(=O)c2ccccc2OC)(CC1)c1ccccc1 |wU:10.10,7.6,(-3.03,-11.46,;-1.71,-10.69,;-1.72,-9.15,;-3.06,-8.38,;-3.06,-6.83,;-1.72,-6.06,;-4.39,-6.06,;-4.39,-4.51,;-5.72,-3.74,;-5.72,-2.18,;-4.39,-1.41,;-3.06,-.64,;-1.71,-1.41,;-.37,-.64,;-.37,.9,;.97,-1.41,;.97,-2.97,;2.3,-3.74,;3.65,-2.97,;3.65,-1.41,;2.3,-.64,;2.3,.9,;.97,1.68,;-3.06,-2.18,;-3.06,-3.74,;-5.49,-.33,;-6.96,-.73,;-8.04,.35,;-7.66,1.82,;-6.21,2.24,;-5.11,1.16,)| Show InChI InChI=1S/C25H32N2O4/c1-3-17-26-24(29)31-20-13-15-25(16-14-20,19-9-5-4-6-10-19)18-27-23(28)21-11-7-8-12-22(21)30-2/h4-12,20H,3,13-18H2,1-2H3,(H,26,29)(H,27,28)/t20-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [86Rb+] efflux from CHO cells stably transfected with Kv1.3 channel |
Bioorg Med Chem Lett 13: 1161-4 (2003)
BindingDB Entry DOI: 10.7270/Q2W09596 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data