

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27188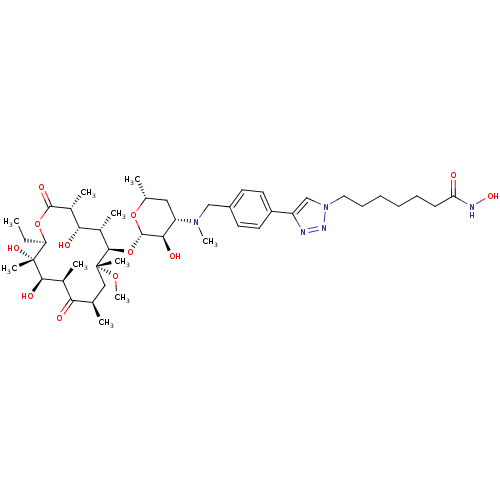 (triazole-linked clarithromycin-based compound, 24d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27187 (triazole-linked clarithromycin-based compound, 24c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27180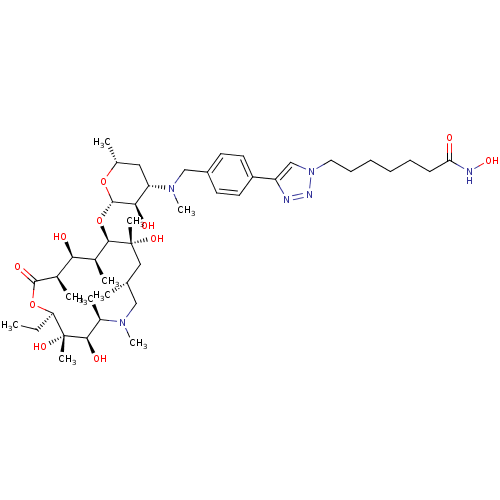 (7-{4-[4-({[(2S,3R,4S,6R)-2-{[(2R,3S,4R,5R,8R,10R,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10.6 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27179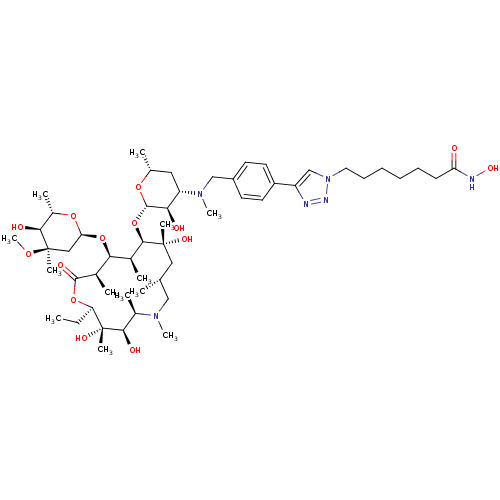 (triazole-linked azithromycin-based compound, 16c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13.9 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50491819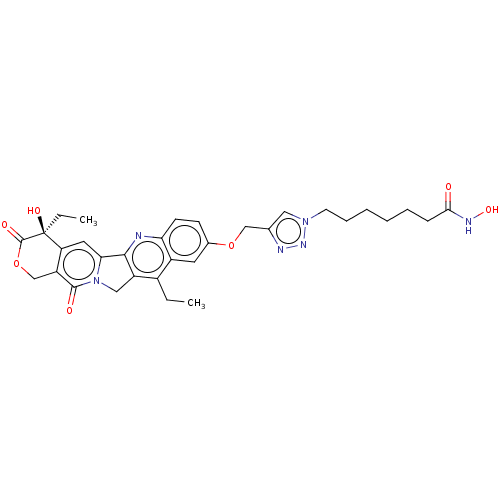 (CHEMBL2386908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27185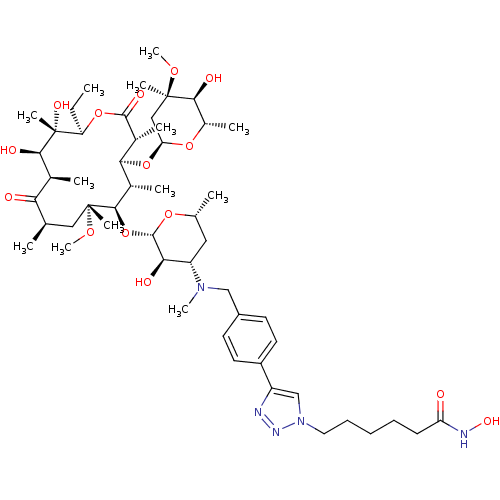 (triazole-linked clarithromycin-based compound, 24a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50491817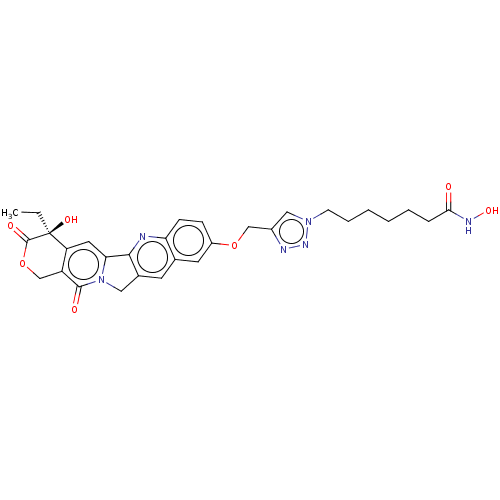 (CHEMBL2386912) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50491820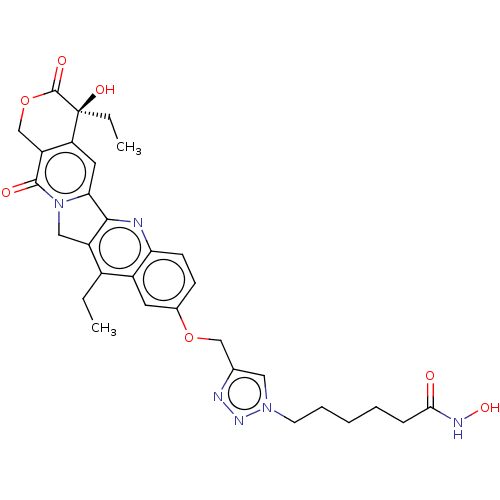 (CHEMBL2386907) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27186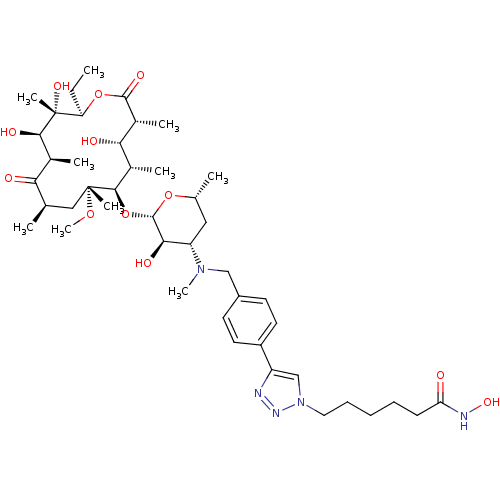 (triazole-linked clarithromycin-based compound, 24b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 44.3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50491819 (CHEMBL2386908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27189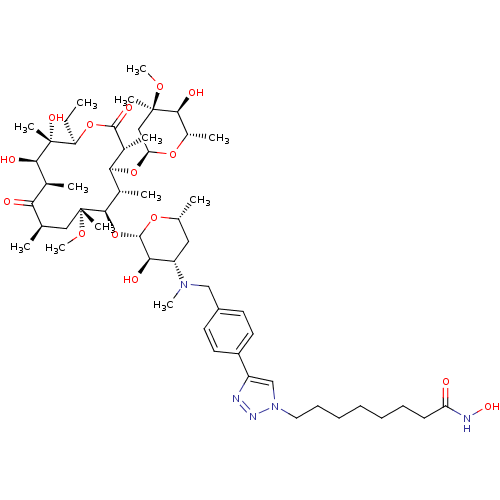 (triazole-linked clarithromycin-based compound, 24e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 55.6 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50491817 (CHEMBL2386912) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27181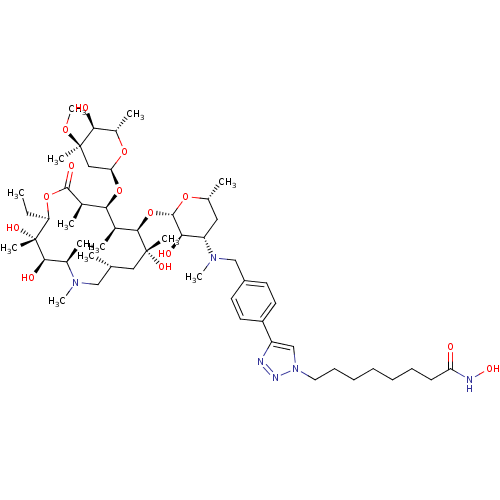 (triazole-linked azithromycin-based compound, 16e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 58.9 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50491819 (CHEMBL2386908) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27182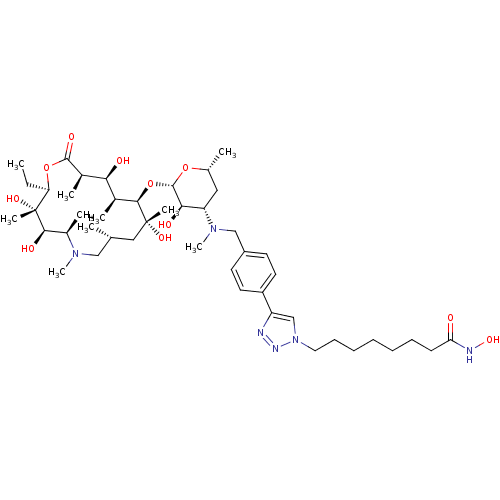 (8-{4-[4-({[(2S,3R,4S,6R)-2-{[(2R,3S,4R,5R,8R,10R,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72.4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50491822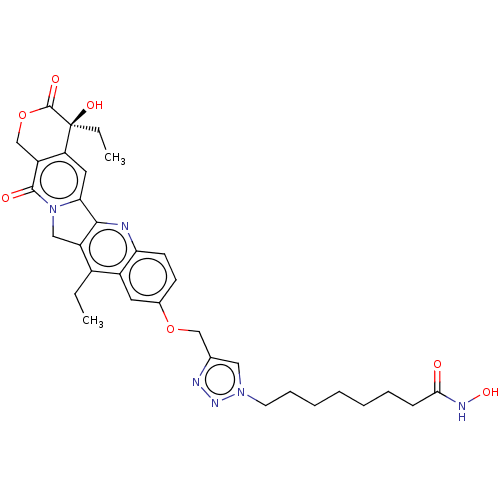 (CHEMBL2386909) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50491817 (CHEMBL2386912) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50491818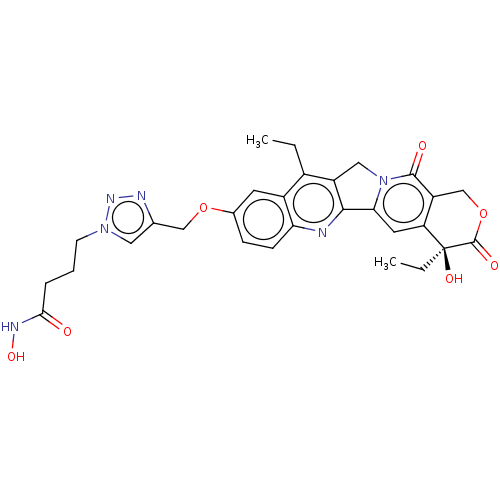 (CHEMBL2386905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27178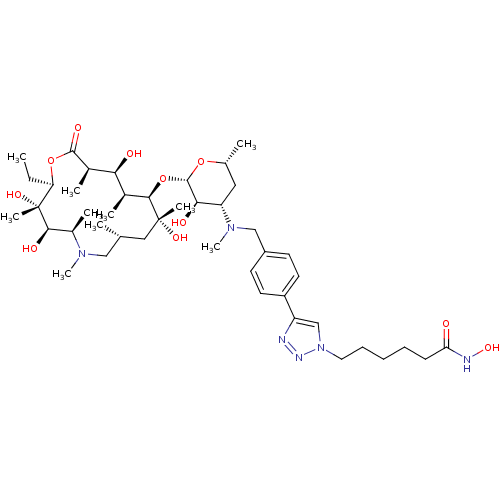 (6-{4-[4-({[(2S,3R,4S,6R)-2-{[(2R,3S,4R,5R,8R,10R,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 88.8 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27177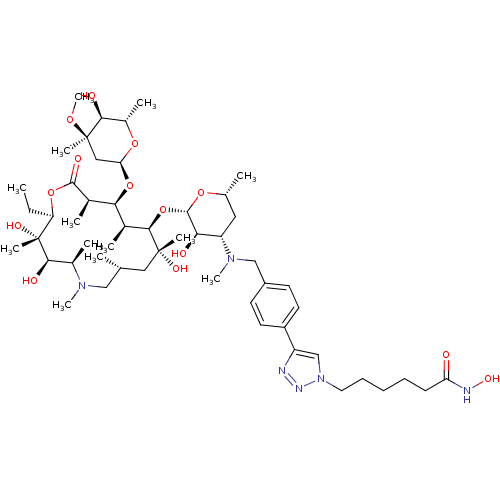 (triazole-linked azithromycin-based compound, 16a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91.6 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27175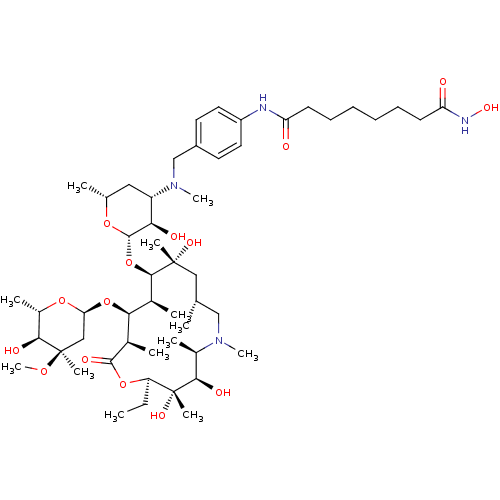 (Azithromycinarylalkylhydroxamic Acid, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27176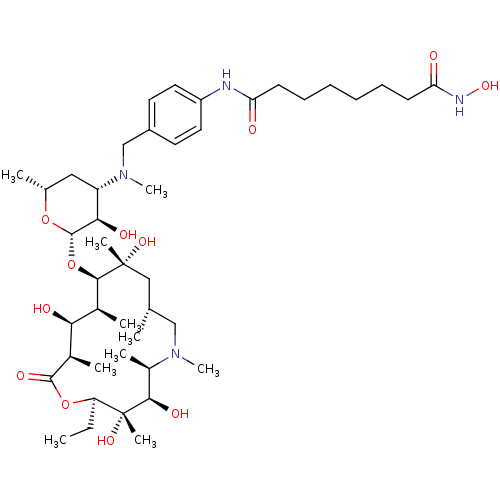 (Desclasinose Azithromycinarylalkyl Hydroxamate, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50491823 (CHEMBL2386911) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50491821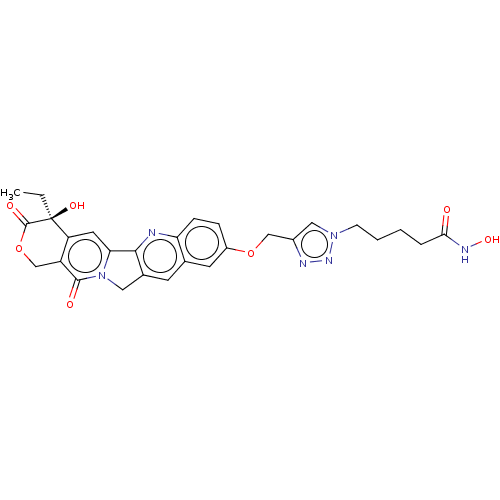 (CHEMBL2386910) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50491820 (CHEMBL2386907) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27190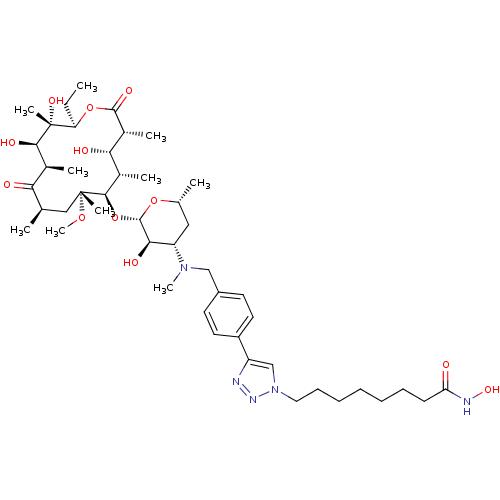 (triazole-linked clarithromycin-based compound, 24f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50491820 (CHEMBL2386907) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50491821 (CHEMBL2386910) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27183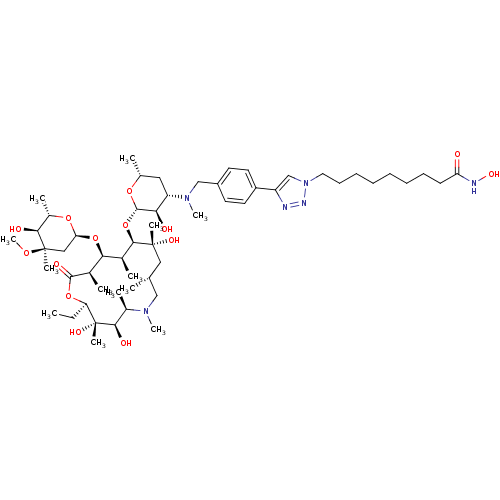 (triazole-linked azithromycin-based compound, 16g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50491824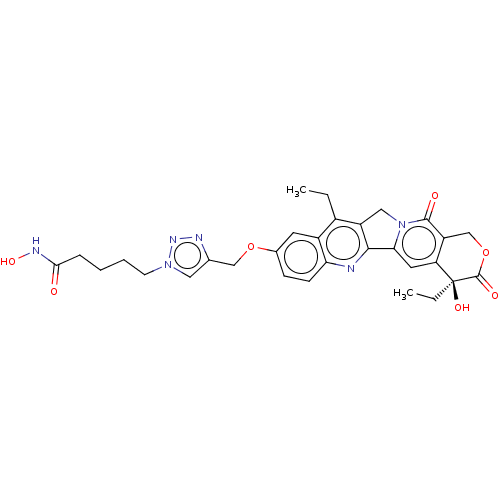 (CHEMBL2386906) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27191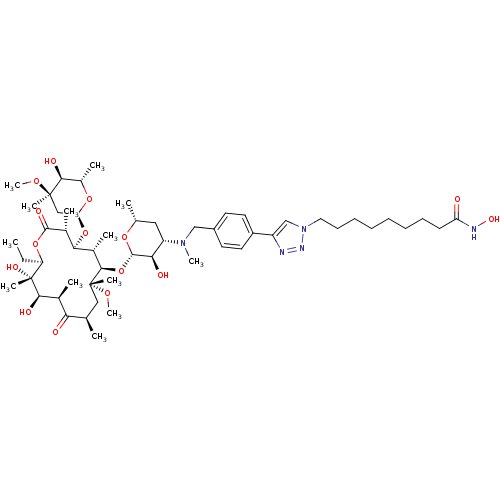 (triazole-linked clarithromycin-based compound, 24g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50491822 (CHEMBL2386909) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27192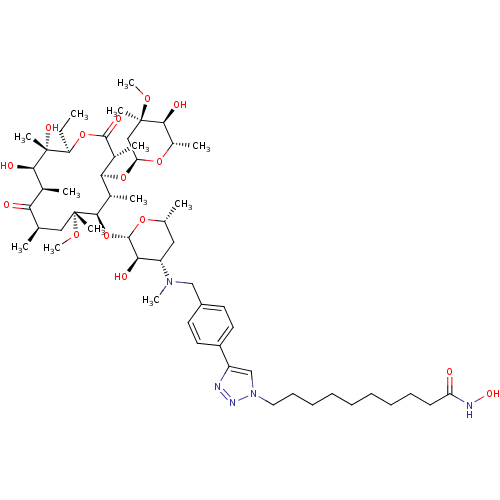 (triazole-linked clarithromycin-based compound, 24h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27184 (triazole-linked azithromycin-based compound, 16h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 227 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50491821 (CHEMBL2386910) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50491822 (CHEMBL2386909) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM27179 (triazole-linked azithromycin-based compound, 16c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 994 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM27180 (7-{4-[4-({[(2S,3R,4S,6R)-2-{[(2R,3S,4R,5R,8R,10R,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50491817 (CHEMBL2386912) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC8 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM27188 (triazole-linked clarithromycin-based compound, 24d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50491818 (CHEMBL2386905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC8 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM27187 (triazole-linked clarithromycin-based compound, 24c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC8 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM27176 (Desclasinose Azithromycinarylalkyl Hydroxamate, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50491822 (CHEMBL2386909) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC8 in human HeLa cells using Fluor-de-Lys as substrate after 15 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3283-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.108 BindingDB Entry DOI: 10.7270/Q2B56NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 61 total ) | Next | Last >> |