

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28206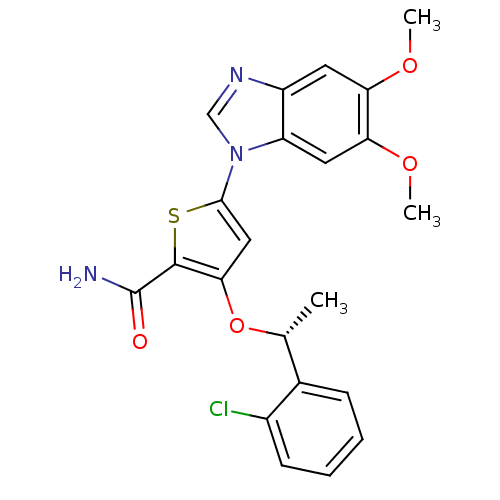 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28208 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-methoxy-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28210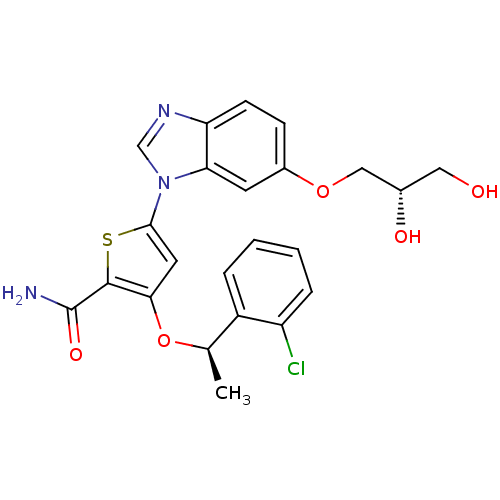 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28216 (5-{6-[(1-methylpiperidin-4-yl)oxy]-1H-1,3-benzodia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28178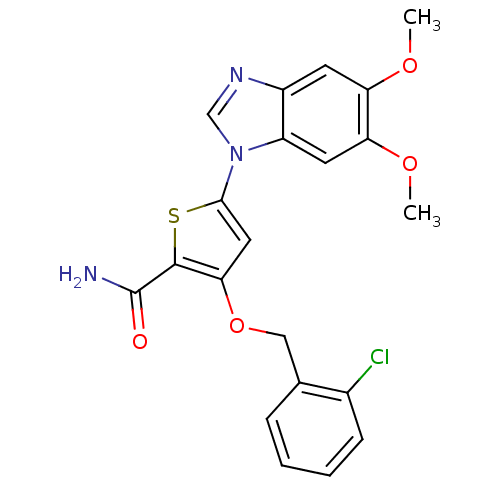 (3-[(2-chlorophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28217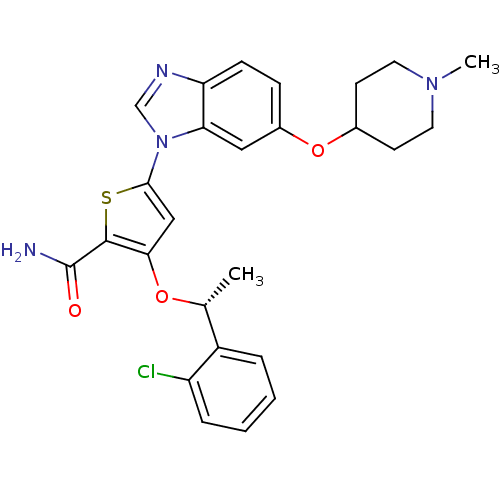 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28209 (5-(6-methoxy-1H-1,3-benzodiazol-1-yl)-3-[(1R)-1-[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28212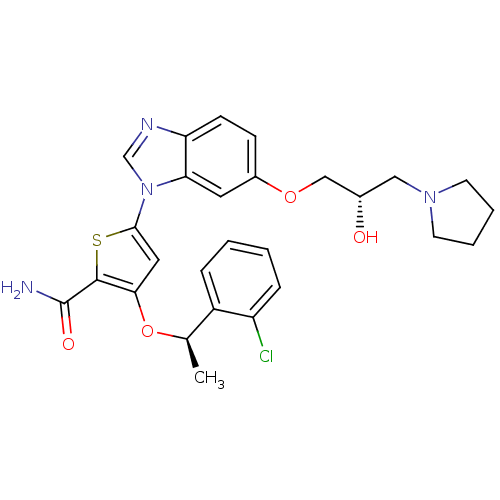 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28215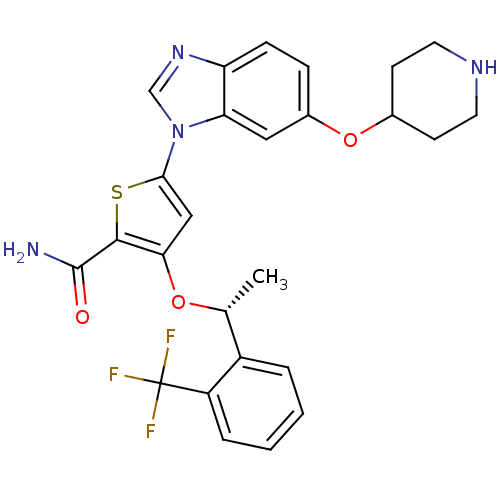 (5-[6-(piperidin-4-yloxy)-1H-1,3-benzodiazol-1-yl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28218 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4S)-1-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336943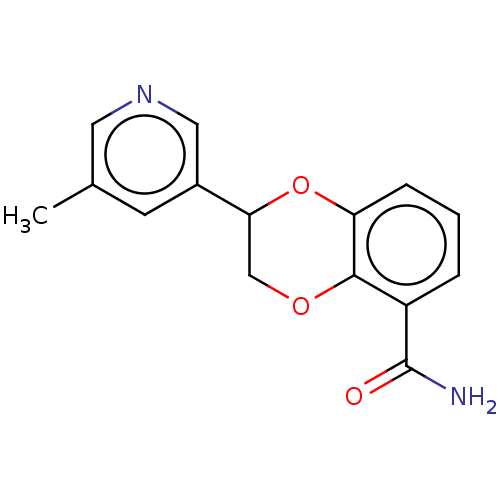 (US9745289, Compound 59A | US9745289, Compound 59B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28219 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4R)-1-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28211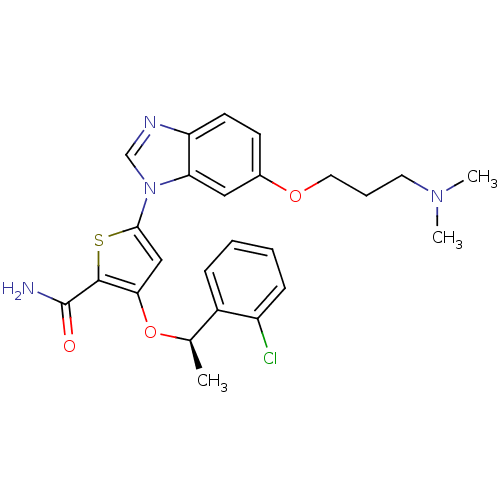 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[3-(dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50438224 (CHEMBL2407759) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP | Bioorg Med Chem Lett 23: 4517-22 (2013) Article DOI: 10.1016/j.bmcl.2013.06.053 BindingDB Entry DOI: 10.7270/Q2ZK5J2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336866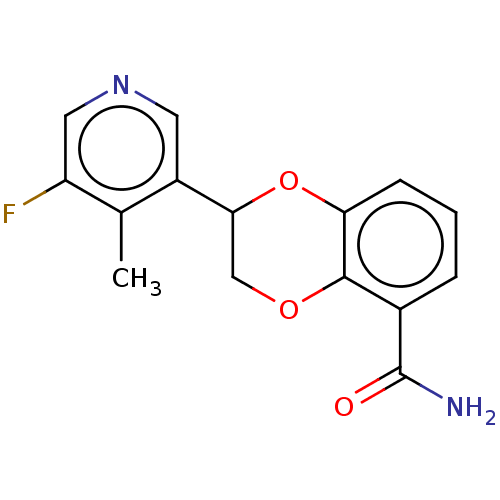 (US9745289, Compound 21A | US9745289, Compound 21B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336914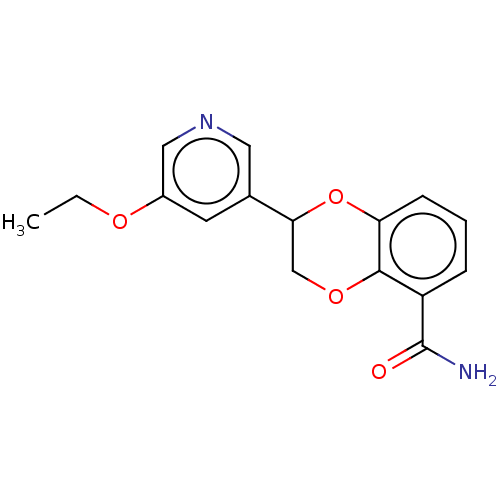 (US9745289, Compound 45A | US9745289, Compound 45B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336900 (US9745289, Compound 38A | US9745289, Compound 38B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28214 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28206 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM27973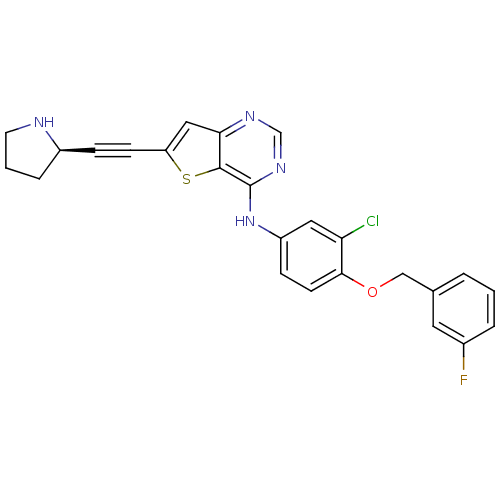 (6-Ethynylthieno[3,2-d]pyrimidine, 8 | N-{3-chloro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of EGFR kinase | Bioorg Med Chem Lett 18: 5738-40 (2009) Article DOI: 10.1016/j.bmcl.2008.09.090 BindingDB Entry DOI: 10.7270/Q2H70FTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28213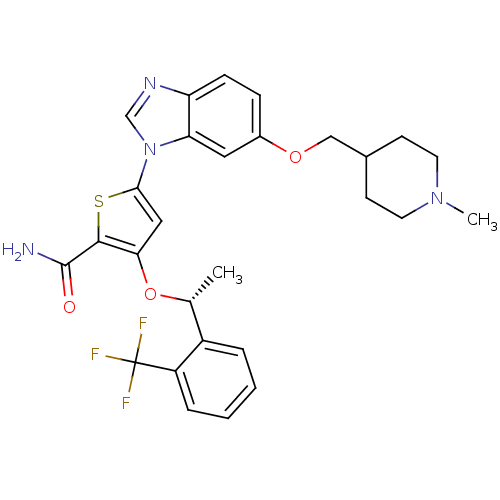 (5-{6-[(1-methylpiperidin-4-yl)methoxy]-1H-1,3-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336870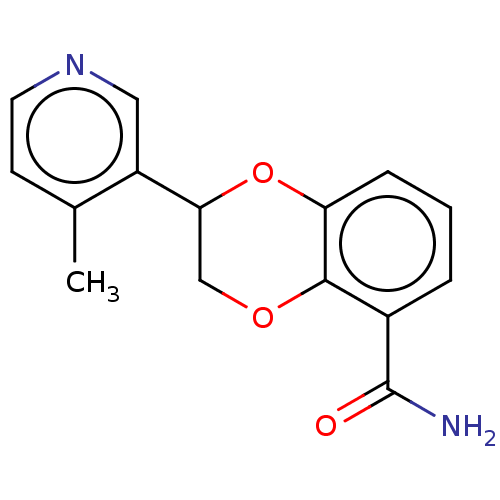 (US9745289, Compound 23A | US9745289, Compound 23B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336894 (US9745289, Compound 35A | US9745289, Compound 35B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336906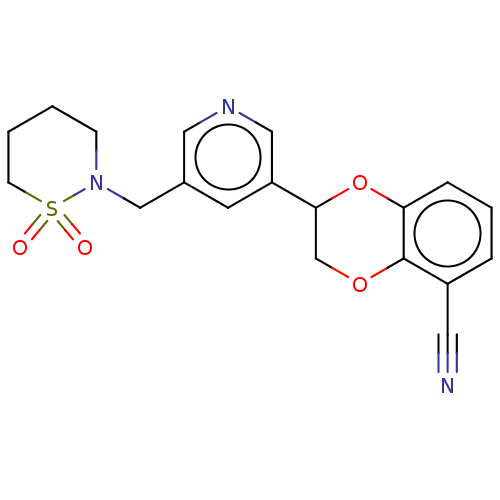 (US9745289, Compound 41A | US9745289, Compound 41B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336925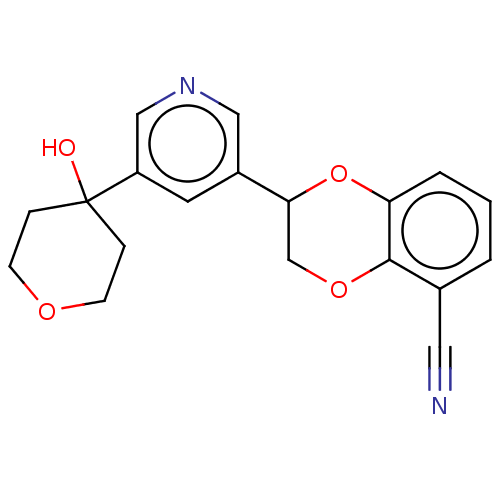 (US9745289, Compound 50B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50438335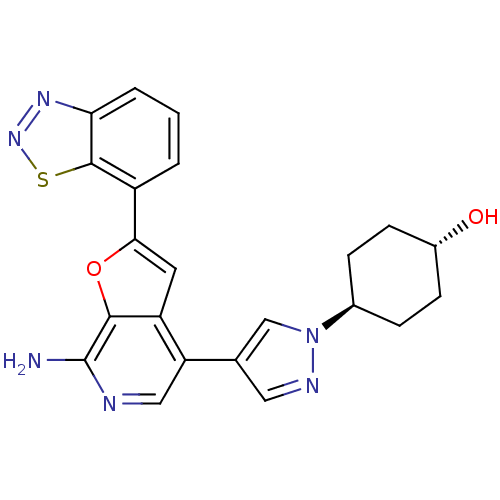 (CHEMBL2408610) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of TAK1-TAB1 (unknown origin) by alphascreen assay in presence of ATP | Bioorg Med Chem Lett 23: 4511-6 (2013) Article DOI: 10.1016/j.bmcl.2013.06.054 BindingDB Entry DOI: 10.7270/Q2FN17MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336912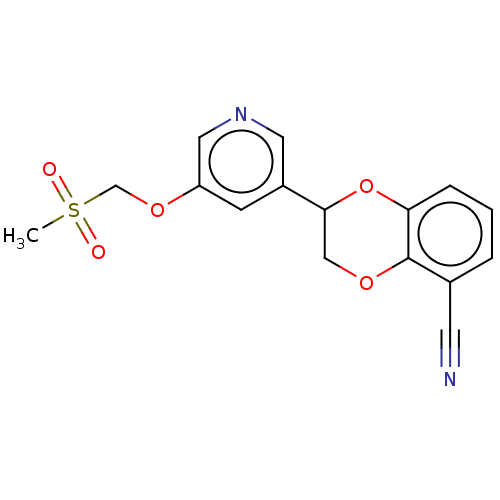 (US9745289, Compound 44A | US9745289, Compound 44B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28178 (3-[(2-chlorophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336892 (US9745289, Compound 34A | US9745289, Compound 34B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336848 (US9745289, Compound 12A | US9745289, Compound 12B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336842 (US9745289, Compound 9A | US9745289, Compound 9B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7 (Homo sapiens (Human)) | BDBM50438223 (CHEMBL2407758) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation | Bioorg Med Chem Lett 23: 4517-22 (2013) Article DOI: 10.1016/j.bmcl.2013.06.053 BindingDB Entry DOI: 10.7270/Q2ZK5J2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336918 (US9745289, Compound 47A | US9745289, Compound 47B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor coactivator 1 (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of Src1 | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336908 (US9745289, Compound 42A | US9745289, Compound 42B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of Lyn | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-2B (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of ACTR-2B | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of Btk | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of ErbB4 | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50293255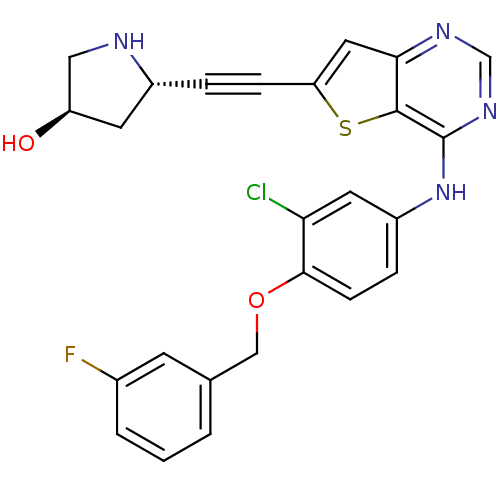 ((3R,5S)-5-((4-(3-chloro-4-(3-fluorobenzyloxy)pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of ErbB2 kinase | Bioorg Med Chem Lett 18: 5738-40 (2009) Article DOI: 10.1016/j.bmcl.2008.09.090 BindingDB Entry DOI: 10.7270/Q2H70FTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336868 (US9745289, Compound 22A | US9745289, Compound 22B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336902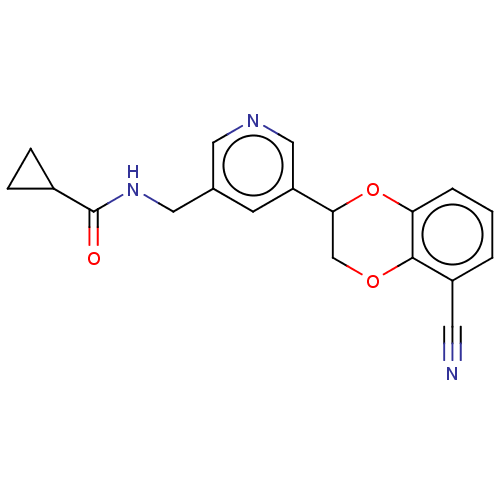 (US9745289, Compound 39A | US9745289, Compound 39B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336866 (US9745289, Compound 21A | US9745289, Compound 21B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336898 (US9745289, Compound 37A | US9745289, Compound 37B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7 (Homo sapiens (Human)) | BDBM50438224 (CHEMBL2407759) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation | Bioorg Med Chem Lett 23: 4517-22 (2013) Article DOI: 10.1016/j.bmcl.2013.06.053 BindingDB Entry DOI: 10.7270/Q2ZK5J2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM27973 (6-Ethynylthieno[3,2-d]pyrimidine, 8 | N-{3-chloro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of ErbB2 kinase | Bioorg Med Chem Lett 18: 5738-40 (2009) Article DOI: 10.1016/j.bmcl.2008.09.090 BindingDB Entry DOI: 10.7270/Q2H70FTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50438223 (CHEMBL2407758) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP | Bioorg Med Chem Lett 23: 4517-22 (2013) Article DOI: 10.1016/j.bmcl.2013.06.053 BindingDB Entry DOI: 10.7270/Q2ZK5J2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336870 (US9745289, Compound 23A | US9745289, Compound 23B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi... | US Patent US9745289 (2017) BindingDB Entry DOI: 10.7270/Q2NS0X12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 545 total ) | Next | Last >> |