

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16171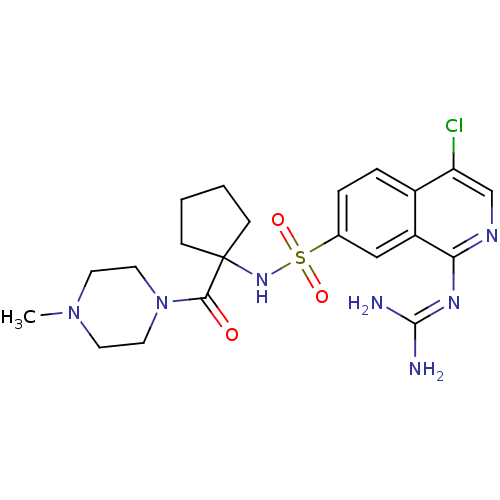 (2-[4-chloro-7-({1-[(4-methylpiperazin-1-yl)carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16170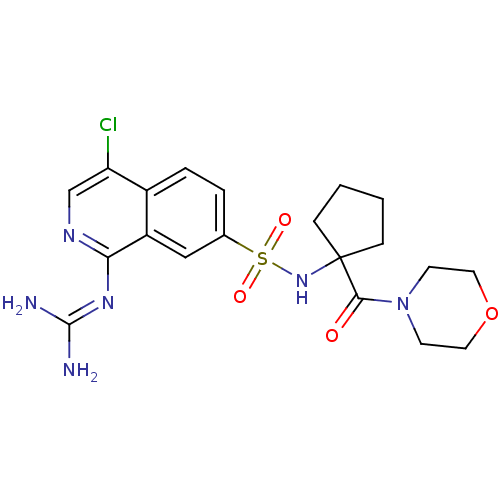 (2-(4-chloro-7-{[1-(morpholin-4-ylcarbonyl)cyclopen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16165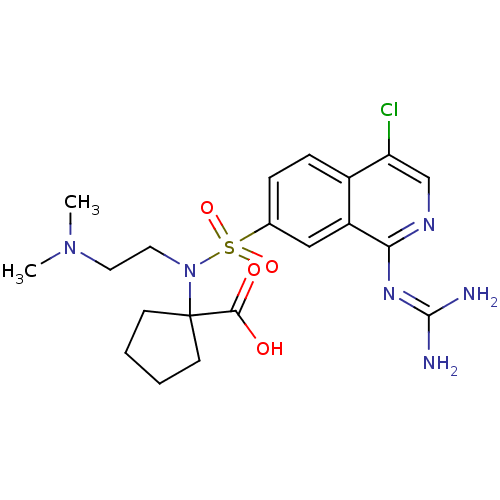 (1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16154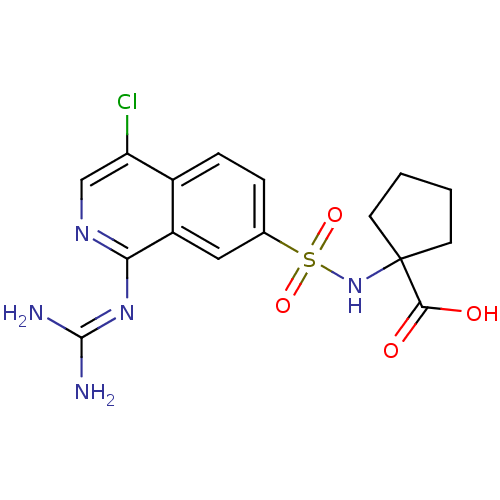 (1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16156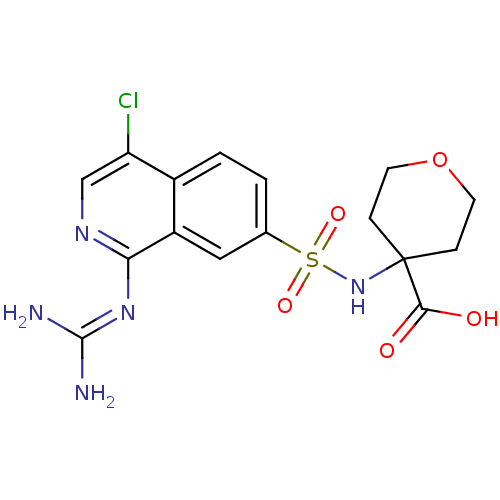 (4-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16153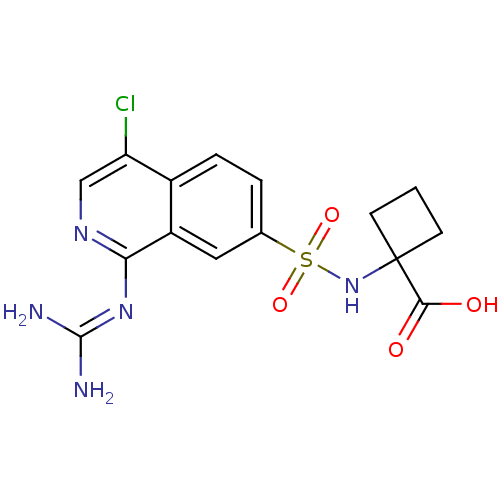 (1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16155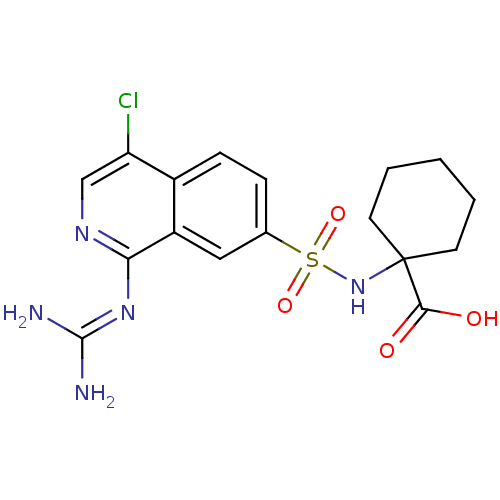 (1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16159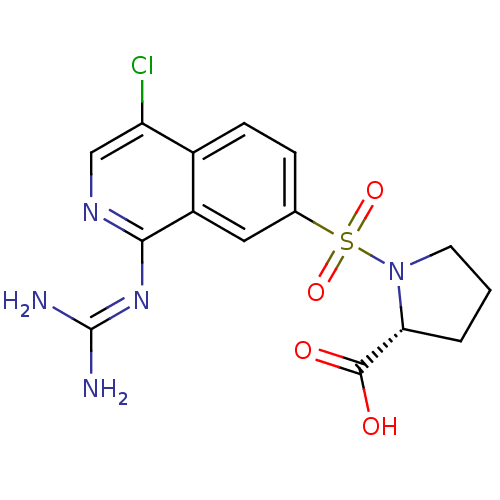 ((2R)-1-({4-chloro-1-[(diaminomethylidene)amino]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16169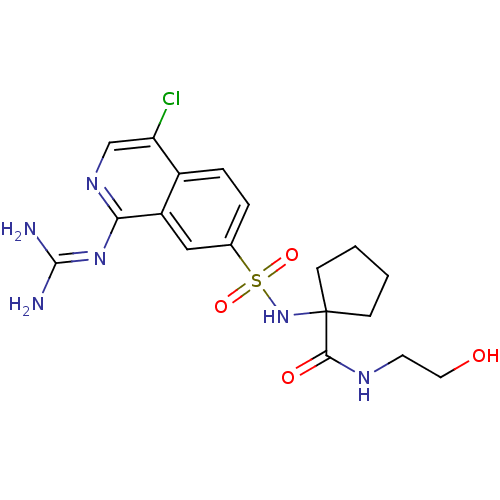 (1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16152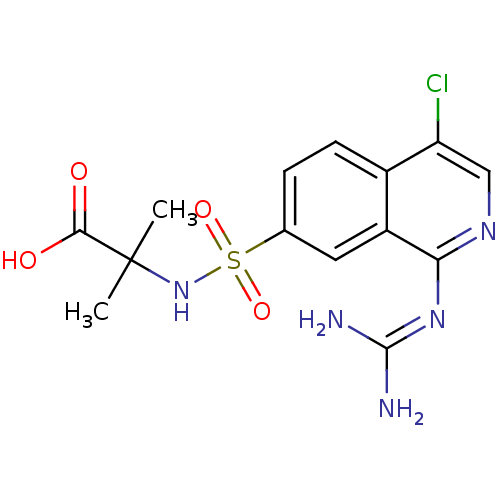 (2-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16157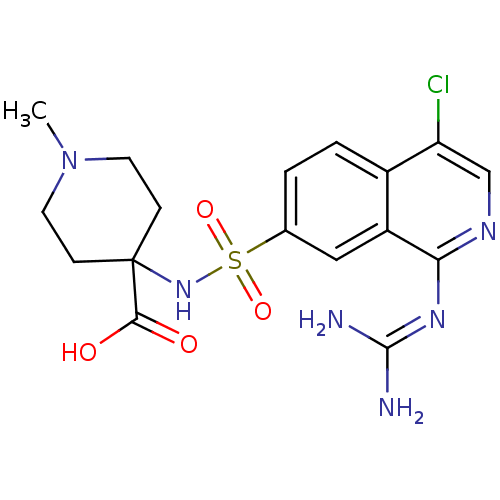 (4-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16164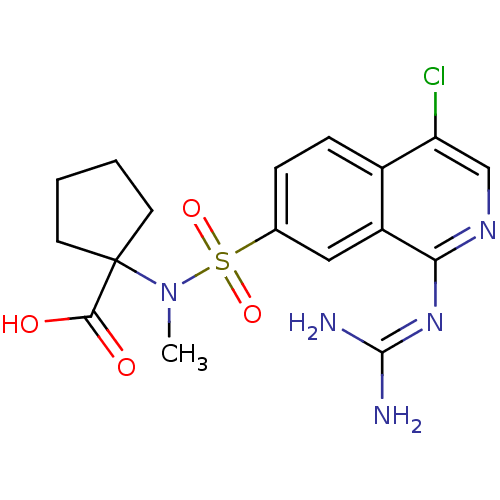 (1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16166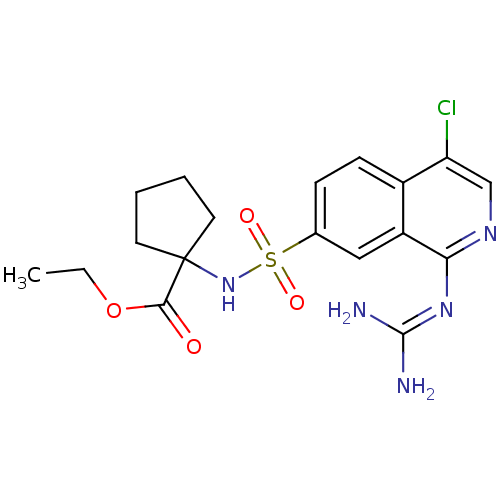 (cycloleucine deriv. 40 | ethyl 1-({4-chloro-1-[(di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50257637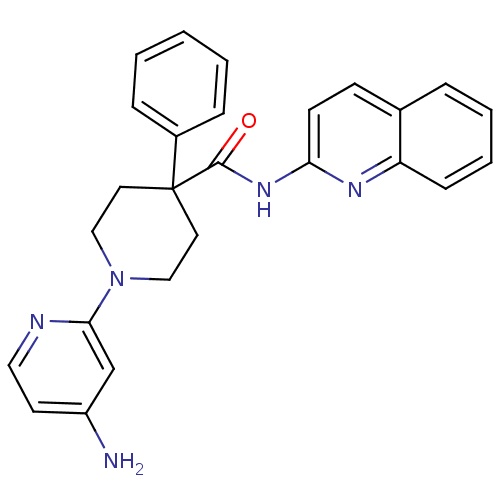 (1-(4-aminopyridin-2-yl)-4-phenyl-N-(quinolin-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 1702-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.106 BindingDB Entry DOI: 10.7270/Q21G0M40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16129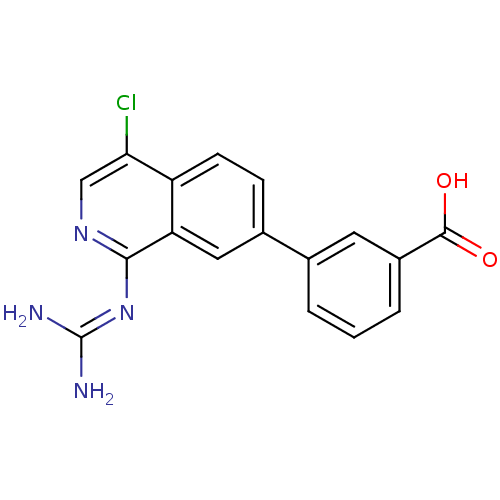 (1-isoquinolinylguanidine 3 | 3-{4-chloro-1-[(diami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | -46.1 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50257723 (2-(3-phenyl-3H-spiro[isobenzofuran-1,4'-piperidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 1702-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.106 BindingDB Entry DOI: 10.7270/Q21G0M40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16148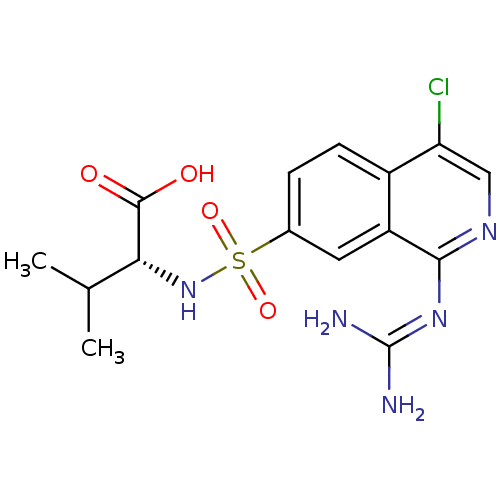 ((2R)-2-({4-chloro-1-[(diaminomethylidene)amino]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16147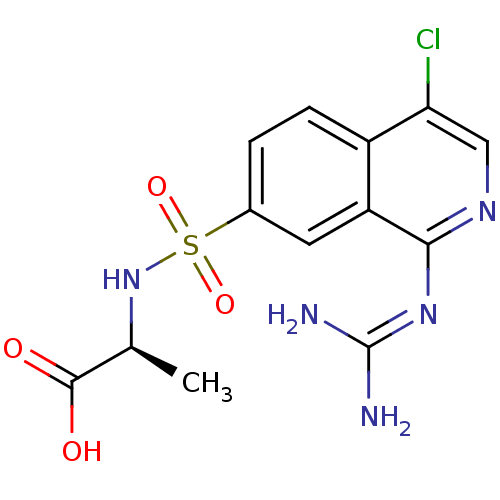 ((2S)-2-({4-chloro-1-[(diaminomethylidene)amino]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16150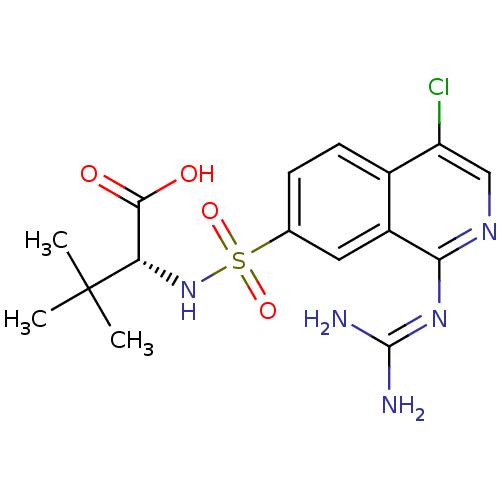 ((2R)-2-({4-chloro-1-[(diaminomethylidene)amino]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16161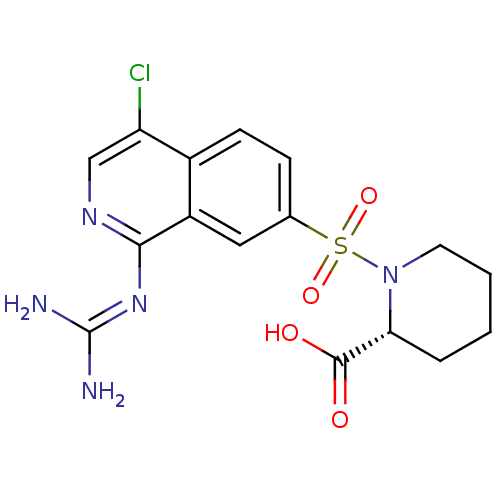 ((2R)-1-({4-chloro-1-[(diaminomethylidene)amino]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16151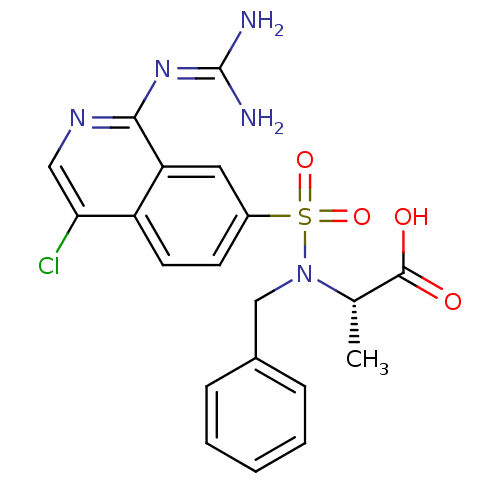 ((2S)-2-[benzyl({4-chloro-1-[(diaminomethylidene)am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16146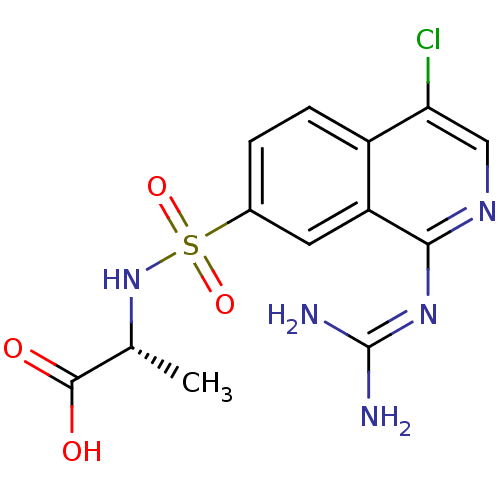 ((2R)-2-({4-chloro-1-[(diaminomethylidene)amino]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16143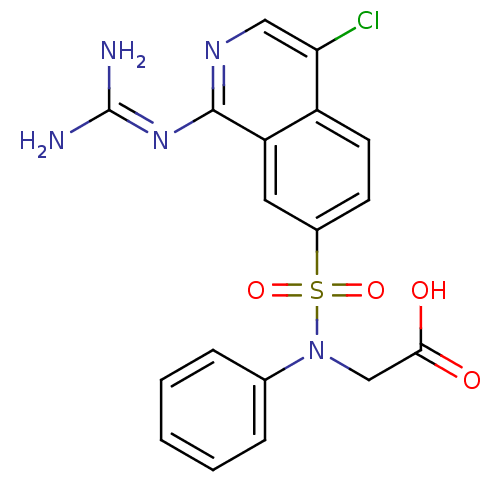 (2-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16168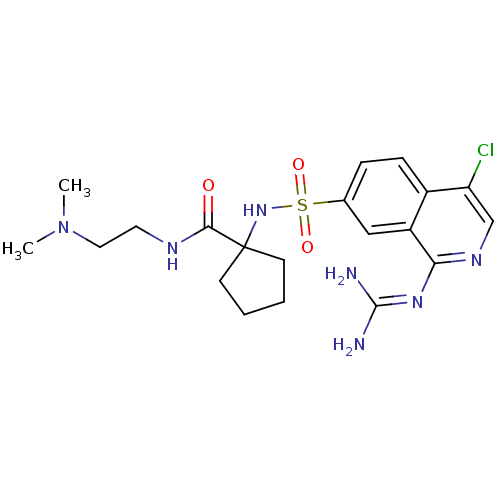 (1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16145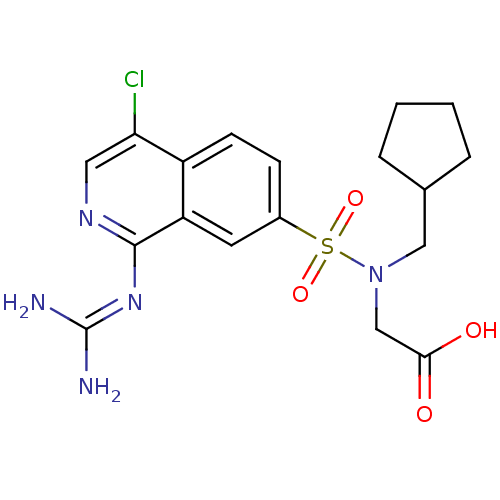 (2-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16144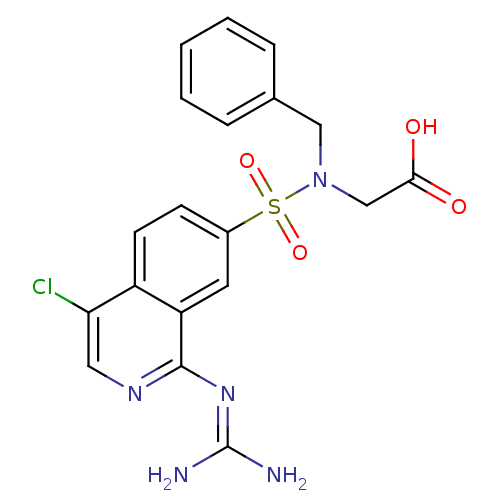 (2-[benzyl({4-chloro-1-[(diaminomethylidene)amino]i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50257639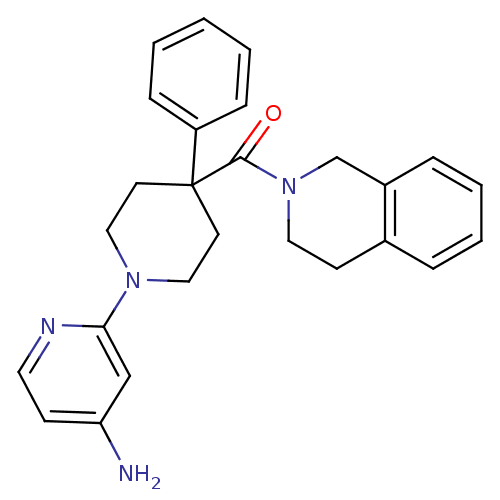 ((1-(4-aminopyridin-2-yl)-4-phenylpiperidin-4-yl)(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 1702-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.106 BindingDB Entry DOI: 10.7270/Q21G0M40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16141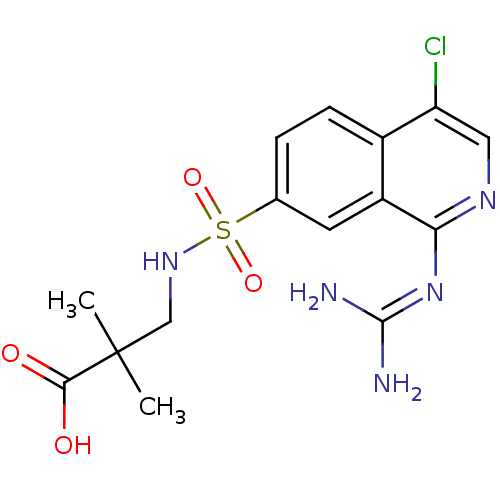 (1-guanidino-7-sulfonamidoisoquinoline 15 | 3-({4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | -43.7 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16139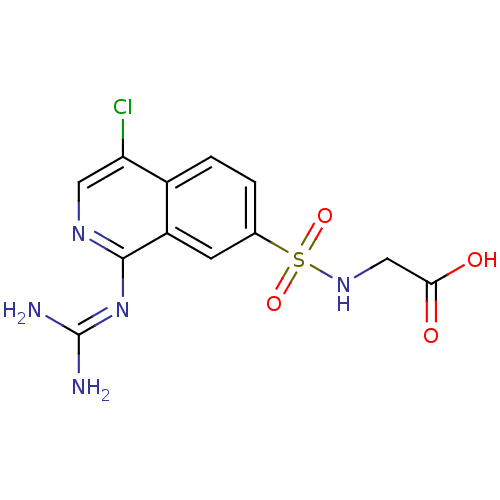 (1-guanidino-7-sulfonamidoisoquinoline 13 | 2-({4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 48 | -43.5 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16142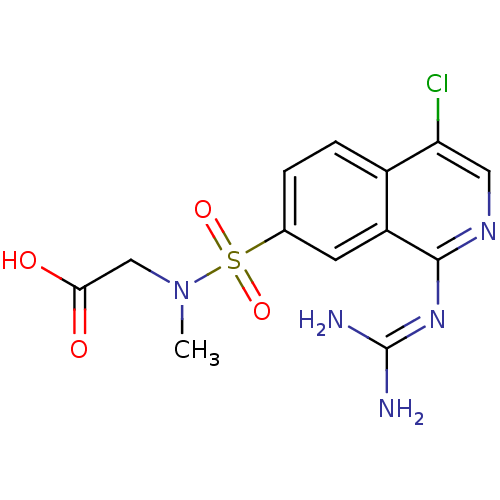 (2-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16163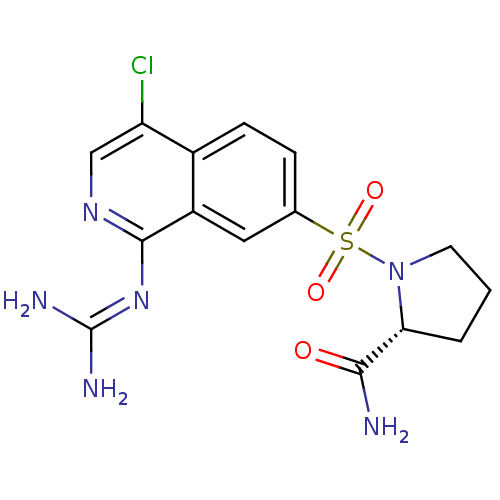 ((2R)-1-({4-chloro-1-[(diaminomethylidene)amino]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16140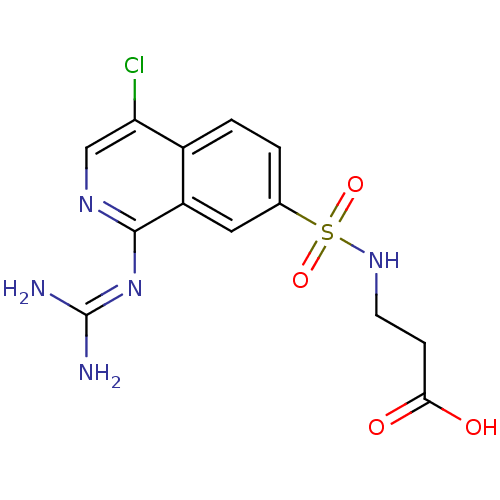 (1-guanidino-7-sulfonamidoisoquinoline 14 | 3-({4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -43.0 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16138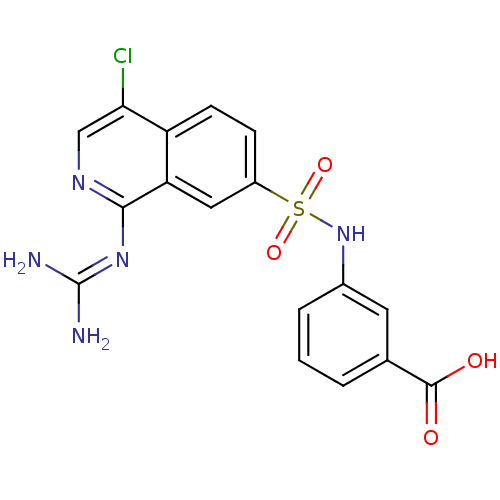 (1-guanidino-7-sulfonamidoisoquinoline 12 | 3-({4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 59 | -42.9 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16149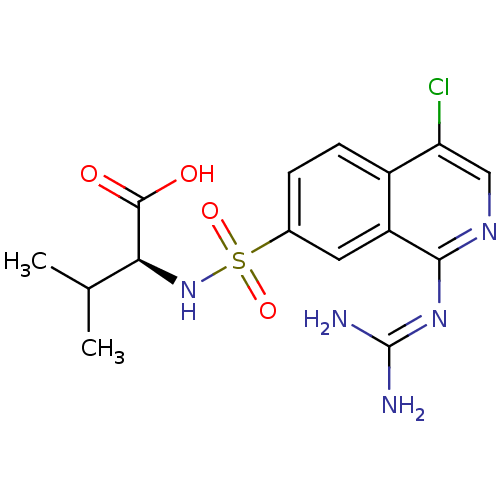 ((2S)-2-({4-chloro-1-[(diaminomethylidene)amino]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16167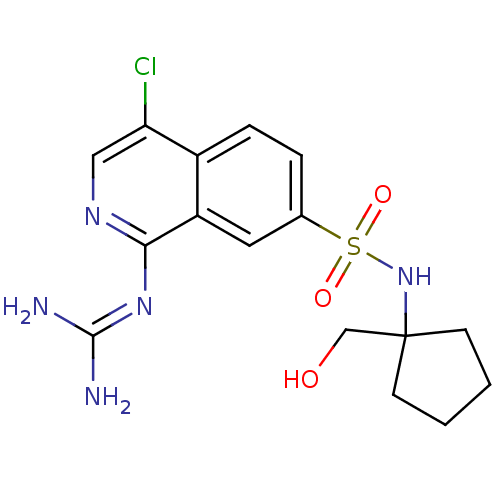 (2-(4-chloro-7-{[1-(hydroxymethyl)cyclopentyl]sulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16133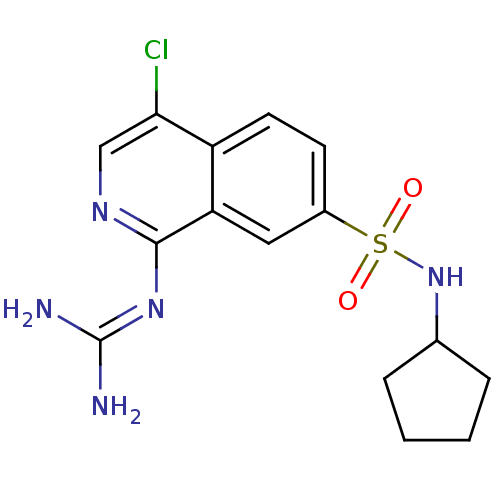 (1-guanidino-7-sulfonamidoisoquinoline 7 | 2-[4-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | -42.4 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50257684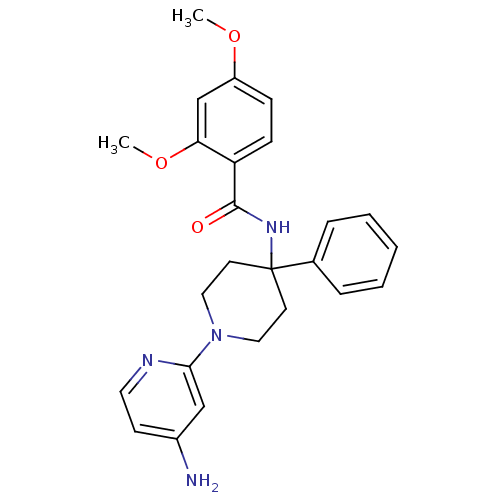 (CHEMBL495346 | N-(1-(4-aminopyridin-2-yl)-4-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 1702-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.106 BindingDB Entry DOI: 10.7270/Q21G0M40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16137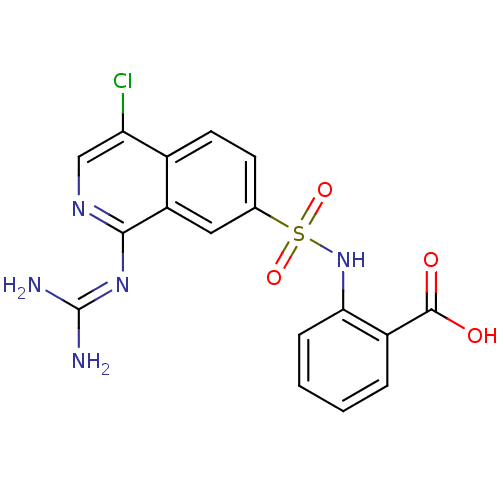 (1-guanidino-7-sulfonamidoisoquinoline 11 | 2-({4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 86 | -42.0 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16160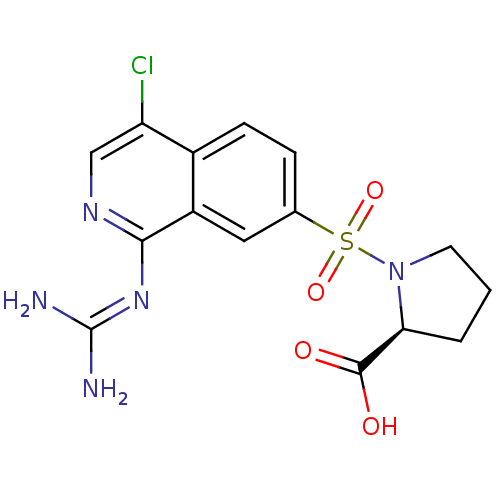 ((2S)-1-({4-chloro-1-[(diaminomethylidene)amino]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16134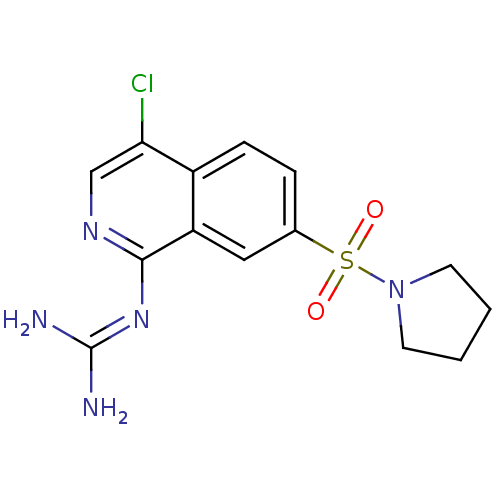 (1-guanidino-7-sulfonamidoisoquinoline 8 | 2-[4-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | -40.9 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16132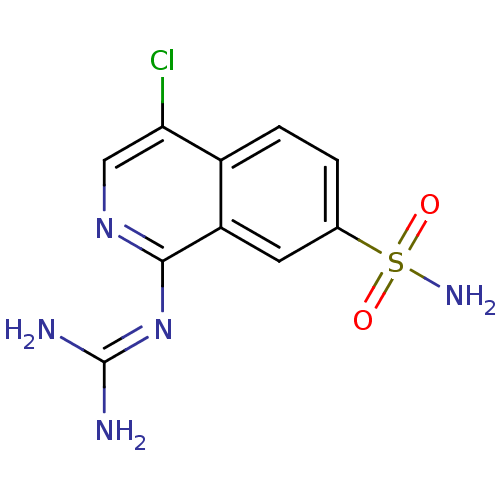 (1-guanidino-7-sulfonamidoisoquinoline 6 | 2-(4-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 140 | -40.7 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16162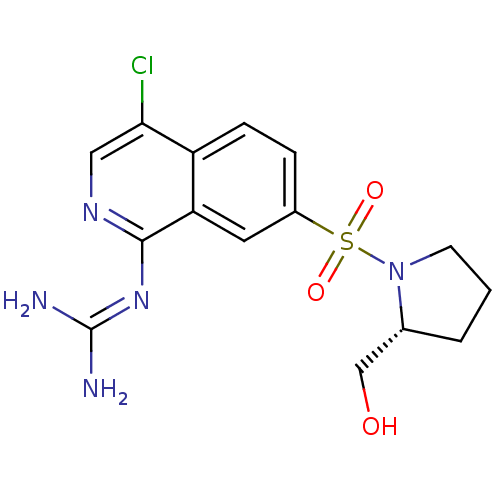 (2-(4-chloro-7-{[(2R)-2-(hydroxymethyl)pyrrolidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16131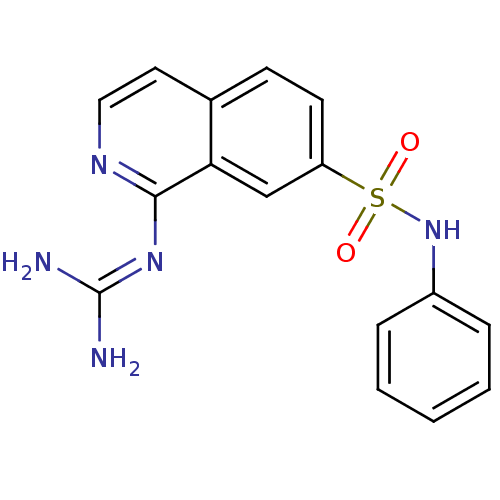 (1-guanidino-7-sulfonamidoisoquinoline 5 | 2-[7-(ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 160 | -40.3 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50257638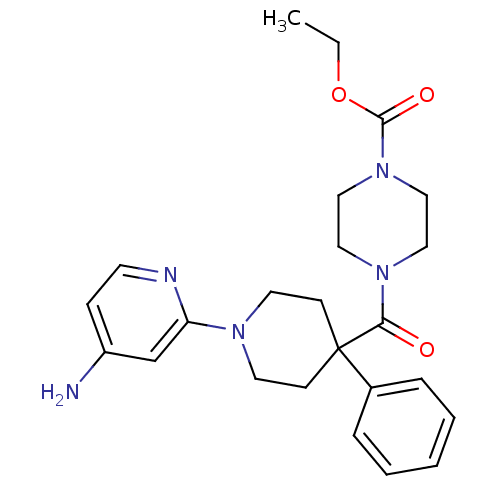 (CHEMBL493743 | ethyl 4-(1-(4-aminopyridin-2-yl)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 1702-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.106 BindingDB Entry DOI: 10.7270/Q21G0M40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50257683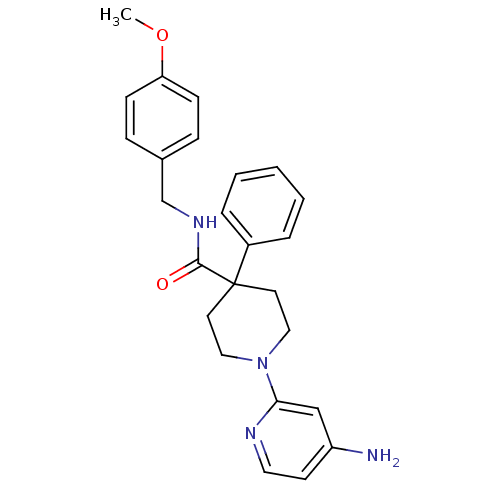 (1-(4-aminopyridin-2-yl)-N-(4-methoxybenzyl)-4-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 1702-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.106 BindingDB Entry DOI: 10.7270/Q21G0M40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16130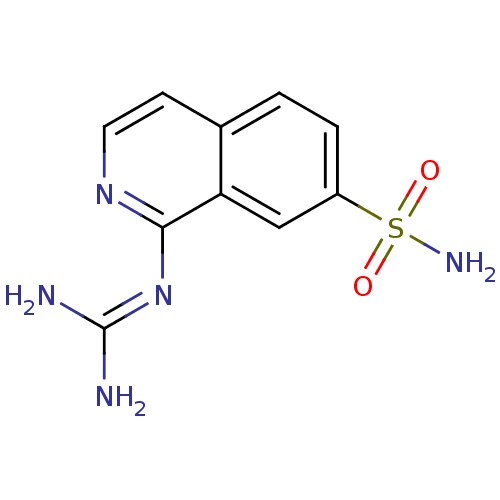 (1-guanidino-7-sulfonamidoisoquinoline 4 | 2-(7-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 280 | -38.9 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16135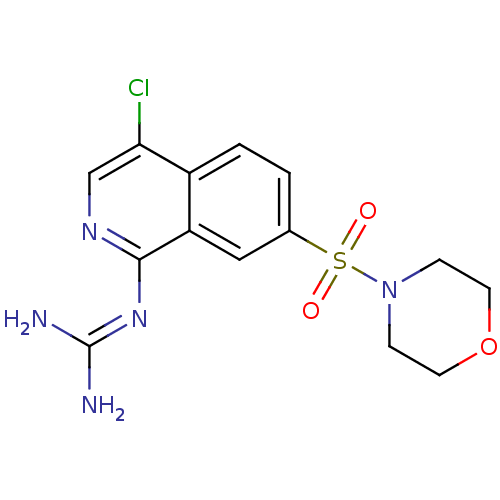 (1-guanidino-7-sulfonamidoisoquinoline 9 | 2-[4-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 330 | -38.5 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16158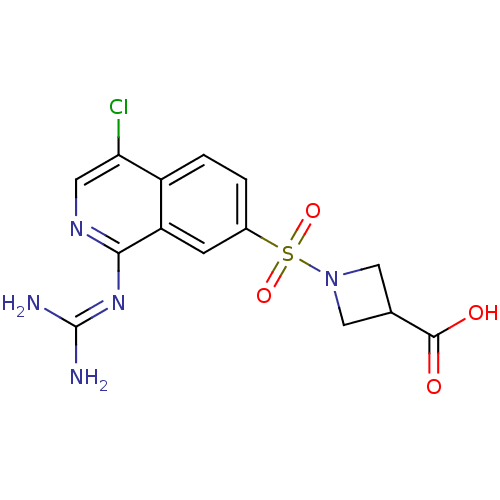 (1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50257685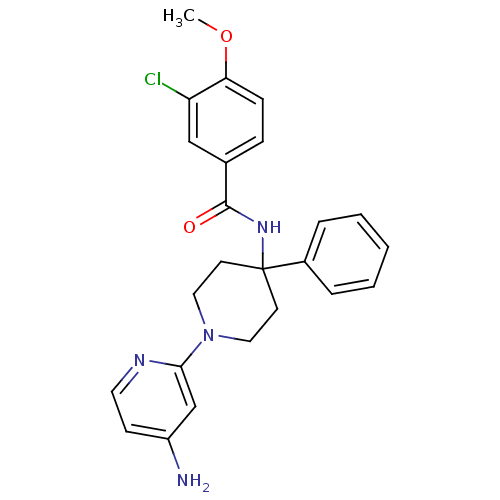 (CHEMBL492330 | N-(1-(4-aminopyridin-2-yl)-4-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 1702-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.106 BindingDB Entry DOI: 10.7270/Q21G0M40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50257833 (3-(1-(4-aminopyridin-2-yl)piperidin-4-yl)-1-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 518 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 1702-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.106 BindingDB Entry DOI: 10.7270/Q21G0M40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 201 total ) | Next | Last >> |