 Found 915 hits with Last Name = 'horwell' and Initial = 'd'
Found 915 hits with Last Name = 'horwell' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neurotensin receptor type 1
(MOUSE) | BDBM50281791
(Ac-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-OH | CHEMBL...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CC(N)=O)NC(C)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C55H91N17O13/c1-6-31(4)44(50(81)69-40(53(84)85)27-30(2)3)70-47(78)38(28-33-18-20-34(74)21-19-33)68-49(80)42-17-12-26-72(42)52(83)37(15-10-24-63-55(60)61)66-45(76)35(14-9-23-62-54(58)59)65-48(79)41-16-11-25-71(41)51(82)36(13-7-8-22-56)67-46(77)39(29-43(57)75)64-32(5)73/h18-21,30-31,35-42,44,74H,6-17,22-29,56H2,1-5H3,(H2,57,75)(H,64,73)(H,65,79)(H,66,76)(H,67,77)(H,68,80)(H,69,81)(H,70,78)(H,84,85)(H4,58,59,62)(H4,60,61,63)/t31-,35+,36+,37+,38+,39+,40+,41+,42+,44+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50240845
((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C38H64N12O8/c1-5-22(4)30(34(55)48-28(36(57)58)19-21(2)3)49-32(53)27(20-23-12-14-24(51)15-13-23)47-33(54)29-11-8-18-50(29)35(56)26(10-7-17-45-38(42)43)46-31(52)25(39)9-6-16-44-37(40)41/h12-15,21-22,25-30,51H,5-11,16-20,39H2,1-4H3,(H,46,52)(H,47,54)(H,48,55)(H,49,53)(H,57,58)(H4,40,41,44)(H4,42,43,45)/t22-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50281781

(Ac-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-OH | CHEMBL2645...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(C)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C51H85N15O11/c1-6-30(4)41(46(73)63-38(49(76)77)27-29(2)3)64-43(70)37(28-32-18-20-33(68)21-19-32)62-45(72)40-17-12-26-66(40)48(75)36(15-10-24-58-51(55)56)61-42(69)34(14-9-23-57-50(53)54)60-44(71)39-16-11-25-65(39)47(74)35(59-31(5)67)13-7-8-22-52/h18-21,29-30,34-41,68H,6-17,22-28,52H2,1-5H3,(H,59,67)(H,60,71)(H,61,69)(H,62,72)(H,63,73)(H,64,70)(H,76,77)(H4,53,54,57)(H4,55,56,58)/t30-,34+,35+,36+,37+,38+,39+,40+,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50071733
(CHEMBL413196 | Compound GRP)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C)[C@@H](C)O)C(C)C)[C@@H](C)O)C(C)C)C(O)=O Show InChI InChI=1S/C130H203N37O32S2/c1-65(2)47-86(114(183)154-85(129(198)199)39-46-201-18)155-115(184)89(52-77-56-136-63-145-77)149-101(175)62-144-122(191)104(69(9)10)162-109(178)72(14)147-113(182)88(51-76-55-139-81-28-20-19-27-80(76)81)156-116(185)90(53-78-57-137-64-146-78)157-117(186)91(54-97(132)171)150-100(174)61-143-110(179)82(30-23-41-138-130(134)135)152-120(189)95-32-25-43-166(95)127(196)93(50-75-34-36-79(170)37-35-75)159-112(181)84(38-45-200-17)151-111(180)83(29-21-22-40-131)153-124(193)107(74(16)169)164-118(187)87(48-66(3)4)158-123(192)105(70(11)12)163-125(194)106(73(15)168)161-102(176)60-141-98(172)58-140-99(173)59-142-108(177)71(13)148-119(188)94-31-24-42-165(94)126(195)92(49-67(5)6)160-121(190)96-33-26-44-167(96)128(197)103(133)68(7)8/h19-20,27-28,34-37,55-57,63-74,82-96,103-107,139,168-170H,21-26,29-33,38-54,58-62,131,133H2,1-18H3,(H2,132,171)(H,136,145)(H,137,146)(H,140,173)(H,141,172)(H,142,177)(H,143,179)(H,144,191)(H,147,182)(H,148,188)(H,149,175)(H,150,174)(H,151,180)(H,152,189)(H,153,193)(H,154,183)(H,155,184)(H,156,185)(H,157,186)(H,158,192)(H,159,181)(H,160,190)(H,161,176)(H,162,178)(H,163,194)(H,164,187)(H,198,199)(H4,134,135,138)/t71-,72-,73+,74+,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,103-,104-,105-,106-,107-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Bombesin BB2 receptor in the presence of [125I]-[Tyr] bombesin. |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071745
(CHEMBL403317 | Compound NMB | Gly-Asn-Leu-Trp-Ala-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)CN)[C@@H](C)O)C(N)=O Show InChI InChI=1S/C52H73N15O12S/c1-27(2)17-36(64-51(78)40(21-41(54)69)61-42(70)22-53)48(75)66-38(19-31-23-57-34-14-10-9-13-33(31)34)47(74)60-28(3)46(73)67-44(29(4)68)52(79)58-25-43(71)62-39(20-32-24-56-26-59-32)50(77)65-37(18-30-11-7-6-8-12-30)49(76)63-35(45(55)72)15-16-80-5/h6-14,23-24,26-29,35-40,44,57,68H,15-22,25,53H2,1-5H3,(H2,54,69)(H2,55,72)(H,56,59)(H,58,79)(H,60,74)(H,61,70)(H,62,71)(H,63,76)(H,64,78)(H,65,77)(H,66,75)(H,67,73)/t28-,29+,35-,36-,37-,38-,39-,40-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50281785
((S)-2-{2-[(S)-2-{[(S)-1-((S)-2-{(S)-2-[((S)-1-Acet...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C45H73N13O10/c1-6-26(4)36(41(65)55-33(43(67)68)23-25(2)3)56-38(62)32(24-28-15-17-29(60)18-16-28)54-40(64)35-14-10-22-58(35)42(66)31(12-8-20-51-45(48)49)53-37(61)30(11-7-19-50-44(46)47)52-39(63)34-13-9-21-57(34)27(5)59/h15-18,25-26,30-36,60H,6-14,19-24H2,1-5H3,(H,52,63)(H,53,61)(H,54,64)(H,55,65)(H,56,62)(H,67,68)(H4,46,47,50)(H4,48,49,51)/t26-,30+,31+,32+,33+,34+,35+,36+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50290967
(1-{(5R,7S,8S)-8-[(2-Benzofuran-4-yl-acetyl)-methyl...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1cccc2occc12 Show InChI InChI=1S/C24H32N2O3/c1-25(23(27)16-18-6-4-7-22-19(18)9-15-28-22)20-8-11-24(10-5-14-29-24)17-21(20)26-12-2-3-13-26/h4,6-7,9,15,20-21H,2-3,5,8,10-14,16-17H2,1H3/t20-,21-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of binding of [3H]-U-69,593 to cloned rat Opioid receptor kappa 1 expressed in CHO cell line |
Bioorg Med Chem Lett 7: 291-296 (1997)
Article DOI: 10.1016/S0960-894X(96)00615-4
BindingDB Entry DOI: 10.7270/Q2G160VS |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50281793
(Ac-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(C)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C75H118N20O19/c1-8-42(6)61(70(110)92-56(73(113)114)36-41(4)5)93-67(107)54(38-45-22-26-47(98)27-23-45)91-69(109)58-19-14-34-95(58)72(112)51(17-12-32-83-75(80)81)87-62(102)48(16-11-31-82-74(78)79)86-68(108)57-18-13-33-94(57)71(111)50(15-9-10-30-76)88-66(106)55(39-59(77)99)90-63(103)49(28-29-60(100)101)85-65(105)53(37-44-20-24-46(97)25-21-44)89-64(104)52(35-40(2)3)84-43(7)96/h20-27,40-42,48-58,61,97-98H,8-19,28-39,76H2,1-7H3,(H2,77,99)(H,84,96)(H,85,105)(H,86,108)(H,87,102)(H,88,106)(H,89,104)(H,90,103)(H,91,109)(H,92,110)(H,93,107)(H,100,101)(H,113,114)(H4,78,79,82)(H4,80,81,83)/t42-,48+,49+,50+,51+,52+,53+,54+,55+,56+,57+,58+,61+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50281795

(Ac-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-OH | CH...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C60H98N18O16/c1-6-33(4)48(55(90)75-43(58(93)94)29-32(2)3)76-52(87)41(30-35-18-20-36(80)21-19-35)74-54(89)45-17-12-28-78(45)57(92)40(15-10-26-68-60(65)66)71-49(84)37(14-9-25-67-59(63)64)70-53(88)44-16-11-27-77(44)56(91)39(13-7-8-24-61)72-51(86)42(31-46(62)81)73-50(85)38(69-34(5)79)22-23-47(82)83/h18-21,32-33,37-45,48,80H,6-17,22-31,61H2,1-5H3,(H2,62,81)(H,69,79)(H,70,88)(H,71,84)(H,72,86)(H,73,85)(H,74,89)(H,75,90)(H,76,87)(H,82,83)(H,93,94)(H4,63,64,67)(H4,65,66,68)/t33-,37+,38+,39+,40+,41+,42+,43+,44+,45+,48+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50240844
((S)-2-((2S,3R)-2-((S)-2-((S)-1-((S)-2-((S)-2-aceta...)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C40H66N12O9/c1-6-23(4)32(36(58)50-30(38(60)61)20-22(2)3)51-34(56)29(21-25-13-15-26(54)16-14-25)49-35(57)31-12-9-19-52(31)37(59)28(11-8-18-46-40(43)44)48-33(55)27(47-24(5)53)10-7-17-45-39(41)42/h13-16,22-23,27-32,54H,6-12,17-21H2,1-5H3,(H,47,53)(H,48,55)(H,49,57)(H,50,58)(H,51,56)(H,60,61)(H4,41,42,45)(H4,43,44,46)/t23-,27+,28+,29+,30+,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071735
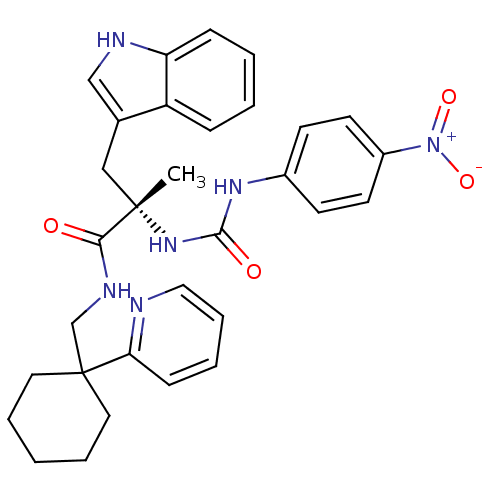
((S)-3-(1H-Indol-3-yl)-2-methyl-2-[3-(4-nitro-pheny...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)C(=O)NCC1(CCCCC1)c1ccccn1 Show InChI InChI=1S/C31H34N6O4/c1-30(19-22-20-33-26-10-4-3-9-25(22)26,36-29(39)35-23-12-14-24(15-13-23)37(40)41)28(38)34-21-31(16-6-2-7-17-31)27-11-5-8-18-32-27/h3-5,8-15,18,20,33H,2,6-7,16-17,19,21H2,1H3,(H,34,38)(H2,35,36,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM85484
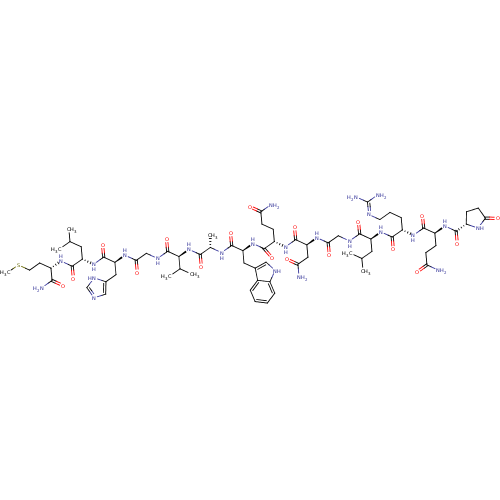
(Bombesin)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |wU:82.92,74.81,8.12,4.4,30.31,53.61,16.23,wD:93.101,39.52,62.69,102.104,34.35,(24.84,-22.59,;25.32,-21.13,;24.29,-19.98,;24.77,-18.52,;23.74,-17.37,;24.22,-15.91,;25.72,-15.59,;26.75,-16.74,;26.2,-14.13,;27.71,-13.81,;28.74,-14.96,;30.24,-14.64,;28.26,-16.42,;25.17,-12.98,;25.65,-11.52,;27.16,-11.2,;24.62,-10.37,;25.1,-8.91,;26.61,-8.59,;27.75,-9.62,;29.09,-8.86,;28.77,-7.35,;27.24,-7.19,;23.12,-10.69,;22.09,-9.54,;22.57,-8.08,;20.58,-9.86,;19.55,-8.72,;18.04,-9.03,;17.57,-10.5,;17.02,-7.89,;15.51,-8.2,;14.48,-7.06,;14.96,-5.59,;12.97,-7.38,;12.5,-8.84,;11.95,-6.23,;10.44,-6.55,;9.96,-8.01,;9.41,-5.4,;7.9,-5.72,;7.42,-7.18,;8.32,-8.42,;7.42,-9.66,;5.97,-9.19,;4.63,-9.96,;3.3,-9.19,;3.3,-7.65,;4.63,-6.88,;5.97,-7.65,;9.89,-3.94,;8.86,-2.79,;7.35,-3.11,;9.34,-1.33,;10.85,-1.01,;11.32,.45,;12.83,.77,;13.31,2.24,;13.86,-.37,;8.31,-.18,;8.79,1.28,;10.3,1.6,;7.76,2.43,;6.25,2.11,;5.22,3.26,;3.72,2.94,;5.7,4.72,;8.24,3.89,;9.75,4.21,;10.77,3.06,;10.22,5.67,;11.73,5.99,;12.21,7.46,;11.18,8.6,;13.72,7.77,;14.74,6.63,;16.25,6.94,;17.28,5.8,;16.73,8.41,;14.19,9.24,;13.17,10.38,;11.66,10.07,;13.64,11.85,;15.15,12.17,;15.63,13.63,;17.14,13.95,;17.61,15.41,;19.12,15.73,;19.6,17.19,;20.15,14.58,;12.62,12.99,;13.09,14.46,;14.6,14.78,;12.07,15.6,;10.56,15.29,;9.53,16.43,;8.02,16.11,;7,17.26,;7.55,14.65,;12.54,17.07,;11.52,18.21,;10.01,17.9,;11.99,19.68,;11.09,20.93,;12,22.17,;13.46,21.69,;14.71,22.59,;13.46,20.15,;17.49,-6.42,;19,-6.11,;16.47,-5.28,;22.23,-17.69,;21.75,-19.16,;21.2,-16.55,)| Show InChI InChI=1S/C71H110N24O18S/c1-34(2)24-47(92-62(105)43(14-11-22-79-71(76)77)89-64(107)45(15-18-52(72)96)90-63(106)44-17-20-55(99)85-44)61(104)81-31-56(100)87-51(28-54(74)98)69(112)91-46(16-19-53(73)97)65(108)94-49(26-38-29-80-41-13-10-9-12-40(38)41)66(109)84-37(7)60(103)95-58(36(5)6)70(113)82-32-57(101)86-50(27-39-30-78-33-83-39)68(111)93-48(25-35(3)4)67(110)88-42(59(75)102)21-23-114-8/h9-10,12-13,29-30,33-37,42-51,58,80H,11,14-28,31-32H2,1-8H3,(H2,72,96)(H2,73,97)(H2,74,98)(H2,75,102)(H,78,83)(H,81,104)(H,82,113)(H,84,109)(H,85,99)(H,86,101)(H,87,100)(H,88,110)(H,89,107)(H,90,106)(H,91,112)(H,92,105)(H,93,111)(H,94,108)(H,95,103)(H4,76,77,79)/t37-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,58-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Bombesin BB2 receptor in the presence of [125I]-[Tyr] bombesin. |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM85484

(Bombesin)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |wU:82.92,74.81,8.12,4.4,30.31,53.61,16.23,wD:93.101,39.52,62.69,102.104,34.35,(24.84,-22.59,;25.32,-21.13,;24.29,-19.98,;24.77,-18.52,;23.74,-17.37,;24.22,-15.91,;25.72,-15.59,;26.75,-16.74,;26.2,-14.13,;27.71,-13.81,;28.74,-14.96,;30.24,-14.64,;28.26,-16.42,;25.17,-12.98,;25.65,-11.52,;27.16,-11.2,;24.62,-10.37,;25.1,-8.91,;26.61,-8.59,;27.75,-9.62,;29.09,-8.86,;28.77,-7.35,;27.24,-7.19,;23.12,-10.69,;22.09,-9.54,;22.57,-8.08,;20.58,-9.86,;19.55,-8.72,;18.04,-9.03,;17.57,-10.5,;17.02,-7.89,;15.51,-8.2,;14.48,-7.06,;14.96,-5.59,;12.97,-7.38,;12.5,-8.84,;11.95,-6.23,;10.44,-6.55,;9.96,-8.01,;9.41,-5.4,;7.9,-5.72,;7.42,-7.18,;8.32,-8.42,;7.42,-9.66,;5.97,-9.19,;4.63,-9.96,;3.3,-9.19,;3.3,-7.65,;4.63,-6.88,;5.97,-7.65,;9.89,-3.94,;8.86,-2.79,;7.35,-3.11,;9.34,-1.33,;10.85,-1.01,;11.32,.45,;12.83,.77,;13.31,2.24,;13.86,-.37,;8.31,-.18,;8.79,1.28,;10.3,1.6,;7.76,2.43,;6.25,2.11,;5.22,3.26,;3.72,2.94,;5.7,4.72,;8.24,3.89,;9.75,4.21,;10.77,3.06,;10.22,5.67,;11.73,5.99,;12.21,7.46,;11.18,8.6,;13.72,7.77,;14.74,6.63,;16.25,6.94,;17.28,5.8,;16.73,8.41,;14.19,9.24,;13.17,10.38,;11.66,10.07,;13.64,11.85,;15.15,12.17,;15.63,13.63,;17.14,13.95,;17.61,15.41,;19.12,15.73,;19.6,17.19,;20.15,14.58,;12.62,12.99,;13.09,14.46,;14.6,14.78,;12.07,15.6,;10.56,15.29,;9.53,16.43,;8.02,16.11,;7,17.26,;7.55,14.65,;12.54,17.07,;11.52,18.21,;10.01,17.9,;11.99,19.68,;11.09,20.93,;12,22.17,;13.46,21.69,;14.71,22.59,;13.46,20.15,;17.49,-6.42,;19,-6.11,;16.47,-5.28,;22.23,-17.69,;21.75,-19.16,;21.2,-16.55,)| Show InChI InChI=1S/C71H110N24O18S/c1-34(2)24-47(92-62(105)43(14-11-22-79-71(76)77)89-64(107)45(15-18-52(72)96)90-63(106)44-17-20-55(99)85-44)61(104)81-31-56(100)87-51(28-54(74)98)69(112)91-46(16-19-53(73)97)65(108)94-49(26-38-29-80-41-13-10-9-12-40(38)41)66(109)84-37(7)60(103)95-58(36(5)6)70(113)82-32-57(101)86-50(27-39-30-78-33-83-39)68(111)93-48(25-35(3)4)67(110)88-42(59(75)102)21-23-114-8/h9-10,12-13,29-30,33-37,42-51,58,80H,11,14-28,31-32H2,1-8H3,(H2,72,96)(H2,73,97)(H2,74,98)(H2,75,102)(H,78,83)(H,81,104)(H,82,113)(H,84,109)(H,85,99)(H,86,101)(H,87,100)(H,88,110)(H,89,107)(H,90,106)(H,91,112)(H,92,105)(H,93,111)(H,94,108)(H,95,103)(H4,76,77,79)/t37-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,58-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against [125 I][4Tyr]-bombesin labeled cloned human GRP(gastrin releasing peptide) receptors stably expressed in CHO cells |
Bioorg Med Chem Lett 6: 2617-2622 (1996)
Article DOI: 10.1016/0960-894X(96)00481-7
BindingDB Entry DOI: 10.7270/Q2NC61QD |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071739
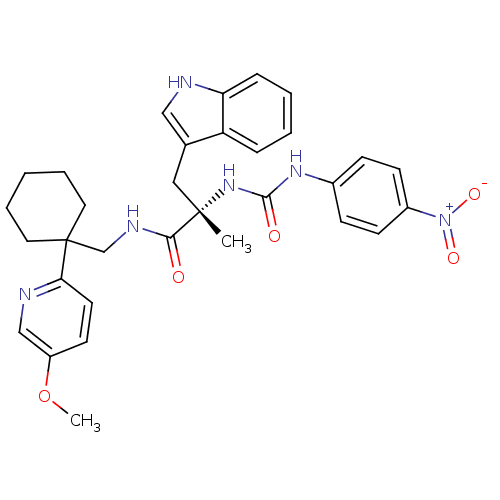
((S)-3-(1H-Indol-3-yl)-N-[1-(5-methoxy-pyridin-2-yl...)Show SMILES COc1ccc(nc1)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C32H36N6O5/c1-31(18-22-19-33-27-9-5-4-8-26(22)27,37-30(40)36-23-10-12-24(13-11-23)38(41)42)29(39)35-21-32(16-6-3-7-17-32)28-15-14-25(43-2)20-34-28/h4-5,8-15,19-20,33H,3,6-7,16-18,21H2,1-2H3,(H,35,39)(H2,36,37,40)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half maximal inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptor in neonatal mouse whole brain (minus cereb... |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281737
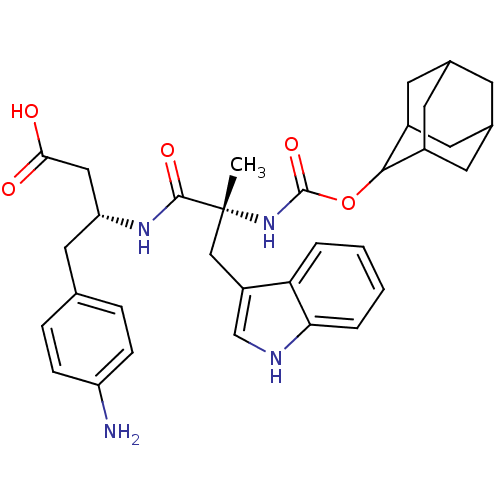
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N)cc1 |wU:1.0,wD:1.13,29.33,TLB:22:17:25:21.23.20,22:21:16.17.18:25,15:16:21.23.22:19.18.25,THB:20:21:16:19.18.25,20:19:16:21.23.22,15:16:25:21.23.20,(3.41,-9.92,;4.74,-10.71,;6.07,-9.95,;6.09,-8.41,;5.19,-7.17,;6.1,-5.93,;7.57,-6.4,;8.89,-5.63,;10.23,-6.4,;10.23,-7.94,;8.89,-8.71,;7.56,-7.94,;3.39,-11.46,;2.06,-10.69,;2.08,-9.15,;.73,-11.44,;-.81,-11.44,;-.62,-13,;-2,-13.53,;-3.42,-13.21,;-4.47,-14.66,;-3,-14.03,;-1.49,-14.43,;-3.21,-12.42,;-2.3,-11.04,;-3.61,-11.69,;6.07,-11.48,;6.05,-13.02,;7.4,-10.71,;8.73,-11.48,;8.73,-13.02,;10.06,-13.79,;10.06,-15.33,;8.73,-14.54,;10.06,-10.71,;11.39,-11.48,;11.39,-13.02,;12.72,-13.79,;14.05,-13.02,;15.38,-13.79,;14.05,-11.46,;12.72,-10.71,)| Show InChI InChI=1S/C33H40N4O5/c1-33(17-24-18-35-28-5-3-2-4-27(24)28,31(40)36-26(16-29(38)39)15-19-6-8-25(34)9-7-19)37-32(41)42-30-22-11-20-10-21(13-22)14-23(30)12-20/h2-9,18,20-23,26,30,35H,10-17,34H2,1H3,(H,36,40)(H,37,41)(H,38,39)/t20?,21?,22?,23?,26-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of binding of [3H]-PD 140376 to Cholecystokinin type B receptor in mouse cortex. |
Bioorg Med Chem Lett 3: 885-888 (1993)
Article DOI: 10.1016/S0960-894X(00)80686-1
BindingDB Entry DOI: 10.7270/Q2D79BWZ |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50281784
(Ac-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-OH ...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C69H107N19O18/c1-6-38(4)56(64(102)85-51(67(105)106)33-37(2)3)86-61(99)49(35-41-20-24-43(91)25-21-41)84-63(101)53-17-12-32-88(53)66(104)47(15-10-30-77-69(74)75)81-57(95)44(14-9-29-76-68(72)73)80-62(100)52-16-11-31-87(52)65(103)46(13-7-8-28-70)82-60(98)50(36-54(71)92)83-58(96)45(26-27-55(93)94)79-59(97)48(78-39(5)89)34-40-18-22-42(90)23-19-40/h18-25,37-38,44-53,56,90-91H,6-17,26-36,70H2,1-5H3,(H2,71,92)(H,78,89)(H,79,97)(H,80,100)(H,81,95)(H,82,98)(H,83,96)(H,84,101)(H,85,102)(H,86,99)(H,93,94)(H,105,106)(H4,72,73,76)(H4,74,75,77)/t38-,44+,45+,46+,47+,48+,49+,50+,51+,52+,53+,56+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50281787
((1S,4R)-2-{2-[2-({(S)-1-[(S)-2-((S)-2-Amino-propio...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)N)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C35H57N9O8/c1-6-20(4)28(32(49)42-26(34(51)52)17-19(2)3)43-30(47)25(18-22-11-13-23(45)14-12-22)41-31(48)27-10-8-16-44(27)33(50)24(40-29(46)21(5)36)9-7-15-39-35(37)38/h11-14,19-21,24-28,45H,6-10,15-18,36H2,1-5H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)(H,51,52)(H4,37,38,39)/t20-,21+,24+,25+,26+,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Cholecystokinin type B receptor |
Bioorg Med Chem Lett 3: 799-802 (1993)
Article DOI: 10.1016/S0960-894X(00)80669-1
BindingDB Entry DOI: 10.7270/Q29S1QZZ |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071750
((S)-3-(1H-Indol-3-yl)-2-methyl-N-[1-(4-nitro-pheny...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)C(=O)NCC1(CCCCC1)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C32H34N6O6/c1-31(19-22-20-33-28-8-4-3-7-27(22)28,36-30(40)35-24-11-15-26(16-12-24)38(43)44)29(39)34-21-32(17-5-2-6-18-32)23-9-13-25(14-10-23)37(41)42/h3-4,7-16,20,33H,2,5-6,17-19,21H2,1H3,(H,34,39)(H2,35,36,40)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071746
((S)-2-[3-(4-Cyano-phenyl)-ureido]-3-(1H-indol-3-yl...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)C#N)C(=O)NCC1(CCCCC1)c1ccccn1 Show InChI InChI=1S/C32H34N6O2/c1-31(19-24-21-35-27-10-4-3-9-26(24)27,38-30(40)37-25-14-12-23(20-33)13-15-25)29(39)36-22-32(16-6-2-7-17-32)28-11-5-8-18-34-28/h3-5,8-15,18,21,35H,2,6-7,16-17,19,22H2,1H3,(H,36,39)(H2,37,38,40)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50008856
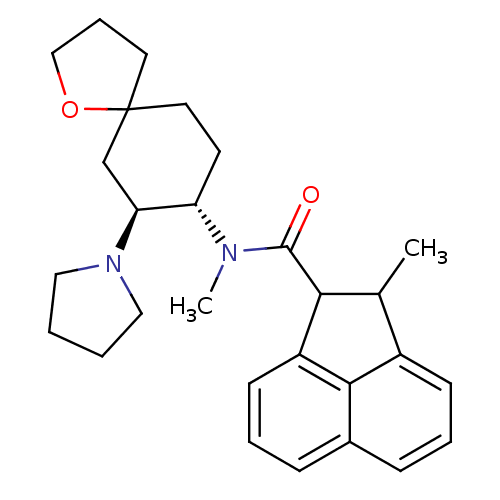
(2-Methyl-acenaphthene-1-carboxylic acid methyl-(7-...)Show SMILES CC1C(C(=O)N(C)[C@H]2CCC3(CCCO3)C[C@@H]2N2CCCC2)c2cccc3cccc1c23 Show InChI InChI=1S/C28H36N2O2/c1-19-21-10-5-8-20-9-6-11-22(26(20)21)25(19)27(31)29(2)23-12-14-28(13-7-17-32-28)18-24(23)30-15-3-4-16-30/h5-6,8-11,19,23-25H,3-4,7,12-18H2,1-2H3/t19?,23-,24-,25?,28?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Opioid receptor kappa affinity, determined with [3H]etorphine in the presence of excess unlabeled [D-Ala2-MePhe4-Glyol5]-enkephalin |
J Med Chem 34: 190-4 (1991)
BindingDB Entry DOI: 10.7270/Q2J103SG |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071748
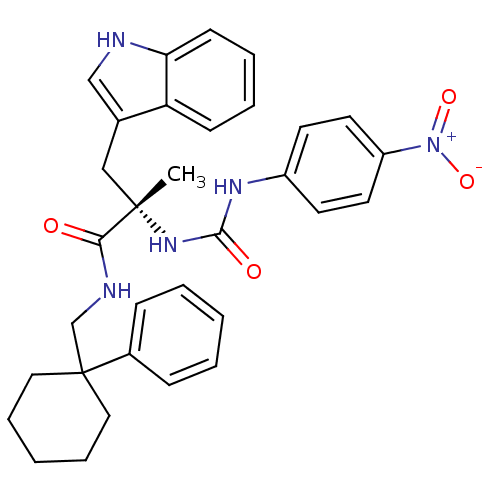
((S)-3-(1H-Indol-3-yl)-2-methyl-2-[3-(4-nitro-pheny...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)C(=O)NCC1(CCCCC1)c1ccccc1 Show InChI InChI=1S/C32H35N5O4/c1-31(20-23-21-33-28-13-7-6-12-27(23)28,36-30(39)35-25-14-16-26(17-15-25)37(40)41)29(38)34-22-32(18-8-3-9-19-32)24-10-4-2-5-11-24/h2,4-7,10-17,21,33H,3,8-9,18-20,22H2,1H3,(H,34,38)(H2,35,36,39)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50285623
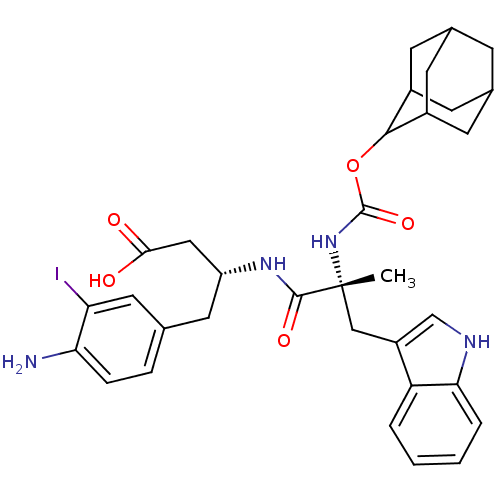
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N)c(I)c1 |wU:1.0,wD:29.33,1.13,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(8.02,-5.46,;9.37,-6.2,;10.7,-5.42,;10.68,-3.88,;11.96,-4.76,;13.17,-3.83,;12.66,-2.38,;13.41,-1.04,;12.62,.28,;11.08,.25,;10.33,-1.09,;11.13,-2.4,;9.4,-7.74,;8.09,-8.54,;8.12,-10.08,;6.74,-7.8,;5.46,-8.64,;5.45,-10.17,;4.05,-10.52,;2.72,-10.03,;1.53,-11.3,;3.03,-10.88,;4.43,-11.45,;3.02,-9.29,;4.06,-8.06,;2.72,-8.54,;10.47,-7.28,;12.01,-7.27,;10.51,-8.82,;11.86,-9.56,;11.89,-11.1,;10.57,-11.9,;10.61,-13.44,;9.22,-11.17,;13.17,-8.76,;14.52,-9.5,;14.55,-11.04,;15.9,-11.78,;17.22,-10.99,;18.57,-11.73,;17.18,-9.44,;18.5,-8.64,;15.83,-8.7,)| Show InChI InChI=1S/C33H39IN4O5/c1-33(16-23-17-36-28-5-3-2-4-25(23)28,38-32(42)43-30-21-9-19-8-20(11-21)12-22(30)10-19)31(41)37-24(15-29(39)40)13-18-6-7-27(35)26(34)14-18/h2-7,14,17,19-22,24,30,36H,8-13,15-16,35H2,1H3,(H,37,41)(H,38,42)(H,39,40)/t19?,20?,21?,22?,24-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]-PD 142308 to CCK-B receptor was determined |
Bioorg Med Chem Lett 5: 2501-2506 (1995)
Article DOI: 10.1016/0960-894X(95)00435-V
BindingDB Entry DOI: 10.7270/Q2DZ08SM |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071736
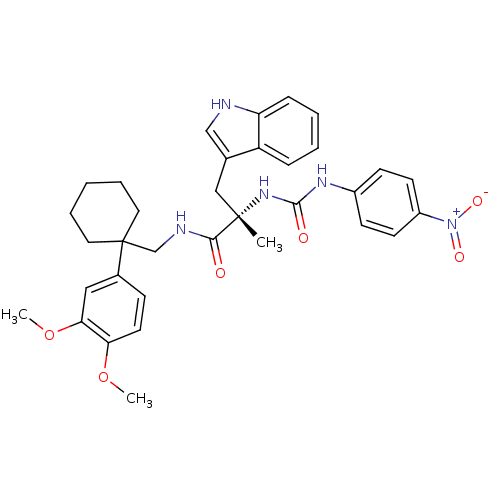
((S)-N-[1-(3,4-Dimethoxy-phenyl)-cyclohexylmethyl]-...)Show SMILES COc1ccc(cc1OC)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C34H39N5O6/c1-33(20-23-21-35-28-10-6-5-9-27(23)28,38-32(41)37-25-12-14-26(15-13-25)39(42)43)31(40)36-22-34(17-7-4-8-18-34)24-11-16-29(44-2)30(19-24)45-3/h5-6,9-16,19,21,35H,4,7-8,17-18,20,22H2,1-3H3,(H,36,40)(H2,37,38,41)/t33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071743
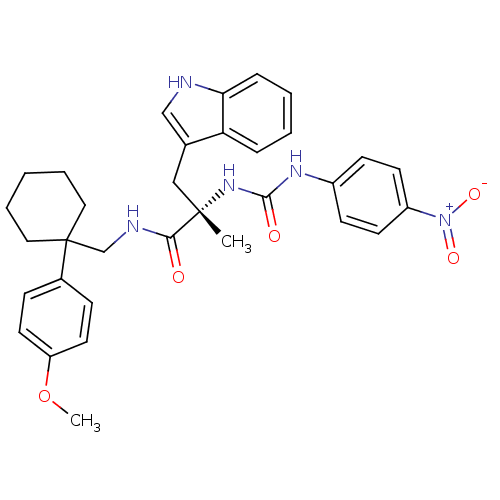
((S)-3-(1H-Indol-3-yl)-N-[1-(4-methoxy-phenyl)-cycl...)Show SMILES COc1ccc(cc1)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C33H37N5O5/c1-32(20-23-21-34-29-9-5-4-8-28(23)29,37-31(40)36-25-12-14-26(15-13-25)38(41)42)30(39)35-22-33(18-6-3-7-19-33)24-10-16-27(43-2)17-11-24/h4-5,8-17,21,34H,3,6-7,18-20,22H2,1-2H3,(H,35,39)(H2,36,37,40)/t32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50285623

((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N)c(I)c1 |wU:1.0,wD:29.33,1.13,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(8.02,-5.46,;9.37,-6.2,;10.7,-5.42,;10.68,-3.88,;11.96,-4.76,;13.17,-3.83,;12.66,-2.38,;13.41,-1.04,;12.62,.28,;11.08,.25,;10.33,-1.09,;11.13,-2.4,;9.4,-7.74,;8.09,-8.54,;8.12,-10.08,;6.74,-7.8,;5.46,-8.64,;5.45,-10.17,;4.05,-10.52,;2.72,-10.03,;1.53,-11.3,;3.03,-10.88,;4.43,-11.45,;3.02,-9.29,;4.06,-8.06,;2.72,-8.54,;10.47,-7.28,;12.01,-7.27,;10.51,-8.82,;11.86,-9.56,;11.89,-11.1,;10.57,-11.9,;10.61,-13.44,;9.22,-11.17,;13.17,-8.76,;14.52,-9.5,;14.55,-11.04,;15.9,-11.78,;17.22,-10.99,;18.57,-11.73,;17.18,-9.44,;18.5,-8.64,;15.83,-8.7,)| Show InChI InChI=1S/C33H39IN4O5/c1-33(16-23-17-36-28-5-3-2-4-25(23)28,38-32(42)43-30-21-9-19-8-20(11-21)12-22(30)10-19)31(41)37-24(15-29(39)40)13-18-6-7-27(35)26(34)14-18/h2-7,14,17,19-22,24,30,36H,8-13,15-16,35H2,1H3,(H,37,41)(H,38,42)(H,39,40)/t19?,20?,21?,22?,24-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards CCK-B receptor in mouse cerebral cortex membrane using [125I]bolton assay |
Bioorg Med Chem Lett 5: 2501-2506 (1995)
Article DOI: 10.1016/0960-894X(95)00435-V
BindingDB Entry DOI: 10.7270/Q2DZ08SM |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071741
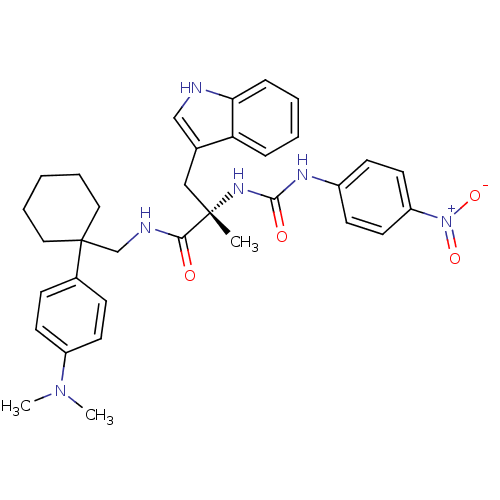
((S)-N-[1-(4-Dimethylamino-phenyl)-cyclohexylmethyl...)Show SMILES CN(C)c1ccc(cc1)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C34H40N6O4/c1-33(21-24-22-35-30-10-6-5-9-29(24)30,38-32(42)37-26-13-17-28(18-14-26)40(43)44)31(41)36-23-34(19-7-4-8-20-34)25-11-15-27(16-12-25)39(2)3/h5-6,9-18,22,35H,4,7-8,19-21,23H2,1-3H3,(H,36,41)(H2,37,38,42)/t33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50000091
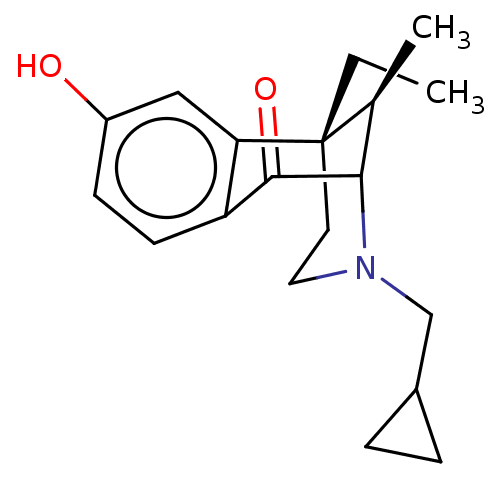
((6S,11R)-3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-...)Show SMILES CC[C@]12CCN(CC3CC3)C([C@@H]1C)C(=O)c1ccc(O)cc21 |TLB:6:5:13.21.15:11,14:13:11:5.3.4,THB:20:21:11:5.3.4| Show InChI InChI=1S/C19H25NO2/c1-3-19-8-9-20(11-13-4-5-13)17(12(19)2)18(22)15-7-6-14(21)10-16(15)19/h6-7,10,12-13,17,21H,3-5,8-9,11H2,1-2H3/t12-,17?,19-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity for the Opioid receptor kappa 1 |
J Med Chem 31: 831-6 (1988)
BindingDB Entry DOI: 10.7270/Q2R78FSK |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071742
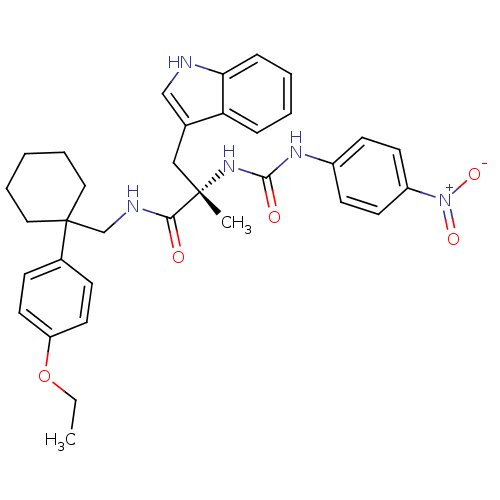
((S)-N-[1-(4-Ethoxy-phenyl)-cyclohexylmethyl]-3-(1H...)Show SMILES CCOc1ccc(cc1)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C34H39N5O5/c1-3-44-28-17-11-25(12-18-28)34(19-7-4-8-20-34)23-36-31(40)33(2,21-24-22-35-30-10-6-5-9-29(24)30)38-32(41)37-26-13-15-27(16-14-26)39(42)43/h5-6,9-18,22,35H,3-4,7-8,19-21,23H2,1-2H3,(H,36,40)(H2,37,38,41)/t33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071738
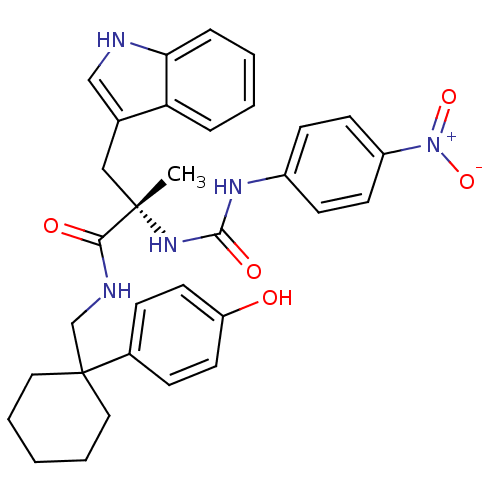
((S)-N-[1-(4-Hydroxy-phenyl)-cyclohexylmethyl]-3-(1...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)C(=O)NCC1(CCCCC1)c1ccc(O)cc1 Show InChI InChI=1S/C32H35N5O5/c1-31(19-22-20-33-28-8-4-3-7-27(22)28,36-30(40)35-24-11-13-25(14-12-24)37(41)42)29(39)34-21-32(17-5-2-6-18-32)23-9-15-26(38)16-10-23/h3-4,7-16,20,33,38H,2,5-6,17-19,21H2,1H3,(H,34,39)(H2,35,36,40)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50088375
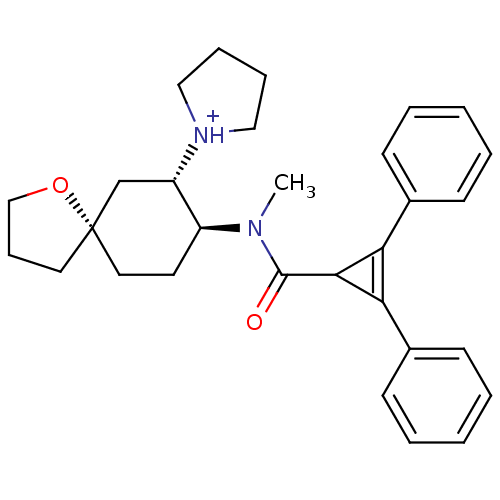
(1-{8-[(2,3-Diphenyl-cycloprop-2-enecarbonyl)-methy...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1[NH+]1CCCC1)C(=O)C1C(=C1c1ccccc1)c1ccccc1 |c:23| Show InChI InChI=1S/C30H36N2O2/c1-31(24-15-17-30(16-10-20-34-30)21-25(24)32-18-8-9-19-32)29(33)28-26(22-11-4-2-5-12-22)27(28)23-13-6-3-7-14-23/h2-7,11-14,24-25,28H,8-10,15-21H2,1H3/p+1/t24-,25-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of binding of [3H]-U-69,593 to cloned rat Opioid receptor kappa 1 expressed in CHO cell line |
Bioorg Med Chem Lett 7: 291-296 (1997)
Article DOI: 10.1016/S0960-894X(96)00615-4
BindingDB Entry DOI: 10.7270/Q2G160VS |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of binding of [3H]-PD 140376 to Cholecystokinin type B receptor in guinea pig cortex. |
Bioorg Med Chem Lett 3: 885-888 (1993)
Article DOI: 10.1016/S0960-894X(00)80686-1
BindingDB Entry DOI: 10.7270/Q2D79BWZ |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50071744
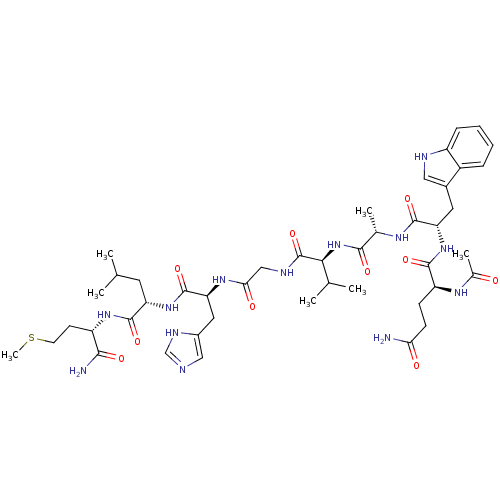
(AcBB(7-14) | CHEMBL314375)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(C)=O)C(C)C)C(N)=O Show InChI InChI=1S/C45H67N13O10S/c1-23(2)16-33(43(66)55-31(39(47)62)14-15-69-7)56-44(67)35(18-28-20-48-22-51-28)54-37(61)21-50-45(68)38(24(3)4)58-40(63)25(5)52-42(65)34(17-27-19-49-30-11-9-8-10-29(27)30)57-41(64)32(53-26(6)59)12-13-36(46)60/h8-11,19-20,22-25,31-35,38,49H,12-18,21H2,1-7H3,(H2,46,60)(H2,47,62)(H,48,51)(H,50,68)(H,52,65)(H,53,59)(H,54,61)(H,55,66)(H,56,67)(H,57,64)(H,58,63)/t25-,31-,32-,33-,34-,35-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Bombesin BB2 receptor in the presence of [125I]-[Tyr] bombesin. |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50288252
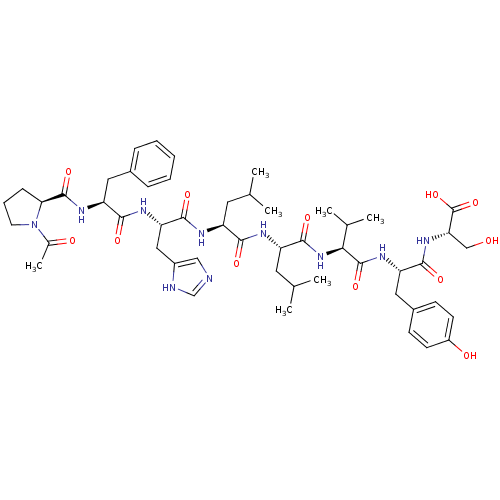
(Bombesin analogue | CHEMBL269432)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(O)=O Show InChI InChI=1S/C51H72N10O12/c1-28(2)20-36(54-47(68)40(24-34-25-52-27-53-34)56-45(66)38(22-32-12-9-8-10-13-32)57-49(70)42-14-11-19-61(42)31(7)63)44(65)55-37(21-29(3)4)48(69)60-43(30(5)6)50(71)58-39(23-33-15-17-35(64)18-16-33)46(67)59-41(26-62)51(72)73/h8-10,12-13,15-18,25,27-30,36-43,62,64H,11,14,19-24,26H2,1-7H3,(H,52,53)(H,54,68)(H,55,65)(H,56,66)(H,57,70)(H,58,71)(H,59,67)(H,60,69)(H,72,73)/t36-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against [125 I][4Tyr]-bombesin labeled cloned human GRP(gastrin releasing peptide) receptors stably expressed in CHO cells |
Bioorg Med Chem Lett 6: 2617-2622 (1996)
Article DOI: 10.1016/0960-894X(96)00481-7
BindingDB Entry DOI: 10.7270/Q2NC61QD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50008858
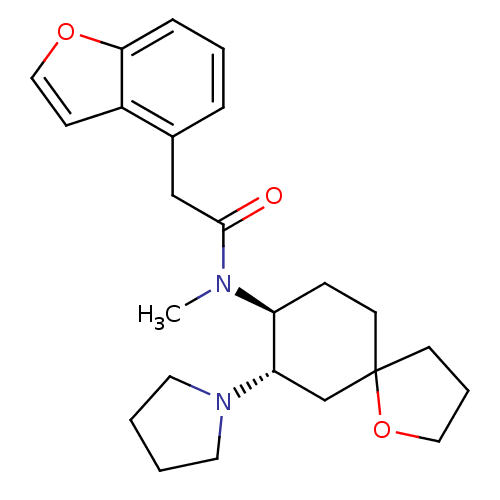
(2-Benzofuran-4-yl-N-methyl-N-(7-pyrrolidin-1-yl-1-...)Show SMILES CN([C@H]1CCC2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1cccc2occc12 Show InChI InChI=1S/C24H32N2O3/c1-25(23(27)16-18-6-4-7-22-19(18)9-15-28-22)20-8-11-24(10-5-14-29-24)17-21(20)26-12-2-3-13-26/h4,6-7,9,15,20-21H,2-3,5,8,10-14,16-17H2,1H3/t20-,21-,24?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Compound was evaluated for Opioid receptor kappa 1 affinity,determined with [3H]etorphine in the presence of excess unlabeled [D-Ala2-MePhe4-Glyol5]-... |
J Med Chem 34: 190-4 (1991)
BindingDB Entry DOI: 10.7270/Q2J103SG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50367937

(CHEMBL1202931)Show SMILES CN([C@@H]1CC[C@]2(CCCO2)C[C@H]1N1CCCC1)C(=O)Cc1cccc2occc12 |r| Show InChI InChI=1S/C24H32N2O3/c1-25(23(27)16-18-6-4-7-22-19(18)9-15-28-22)20-8-11-24(10-5-14-29-24)17-21(20)26-12-2-3-13-26/h4,6-7,9,15,20-21H,2-3,5,8,10-14,16-17H2,1H3/t20-,21-,24-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 |
J Med Chem 33: 286-91 (1990)
BindingDB Entry DOI: 10.7270/Q2DJ5G7P |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50285625
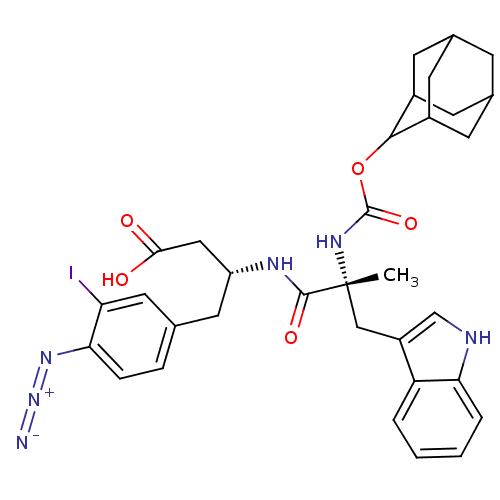
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N=[N+]=[N-])c(I)c1 |wU:1.0,wD:29.33,1.13,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(7.11,-5.46,;8.46,-6.2,;9.79,-5.42,;9.77,-3.88,;11.05,-4.76,;12.26,-3.83,;11.76,-2.38,;12.5,-1.04,;11.71,.28,;10.17,.25,;9.42,-1.09,;10.22,-2.4,;8.5,-7.74,;7.18,-8.54,;7.21,-10.08,;5.83,-7.8,;4.55,-8.64,;4.54,-10.17,;3.14,-10.52,;1.81,-10.03,;.62,-11.31,;2.12,-10.89,;3.52,-11.45,;2.11,-9.29,;3.15,-8.07,;1.81,-8.54,;9.57,-7.28,;11.1,-7.28,;9.6,-8.82,;10.95,-9.56,;10.98,-11.1,;9.67,-11.9,;9.7,-13.44,;8.31,-11.17,;12.27,-8.76,;13.62,-9.5,;13.64,-11.04,;14.99,-11.79,;16.31,-10.99,;17.66,-11.73,;18.98,-10.93,;20.3,-10.13,;16.28,-9.44,;17.59,-8.64,;14.93,-8.7,)| Show InChI InChI=1S/C33H37IN6O5/c1-33(16-23-17-36-27-5-3-2-4-25(23)27,38-32(44)45-30-21-9-19-8-20(11-21)12-22(30)10-19)31(43)37-24(15-29(41)42)13-18-6-7-28(39-40-35)26(34)14-18/h2-7,14,17,19-22,24,30,36H,8-13,15-16H2,1H3,(H,37,43)(H,38,44)(H,41,42)/t19?,20?,21?,22?,24-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards CCK-B receptor in mouse cerebral cortex membrane using [125I]bolton assay |
Bioorg Med Chem Lett 5: 2501-2506 (1995)
Article DOI: 10.1016/0960-894X(95)00435-V
BindingDB Entry DOI: 10.7270/Q2DZ08SM |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50281794

((S)-2-{(2S,3R)-2-[(S)-2-{2-[2-((S)-2-(S)-Amino-5-g...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C36H62N12O8/c1-6-20(4)28(33(54)47-27(34(55)56)17-19(2)3)48-32(53)26(18-22-11-13-23(49)14-12-22)46-29(50)21(5)44-31(52)25(10-8-16-43-36(40)41)45-30(51)24(37)9-7-15-42-35(38)39/h11-14,19-21,24-28,49H,6-10,15-18,37H2,1-5H3,(H,44,52)(H,45,51)(H,46,50)(H,47,54)(H,48,53)(H,55,56)(H4,38,39,42)(H4,40,41,43)/t20-,21+,24+,25+,26+,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071737
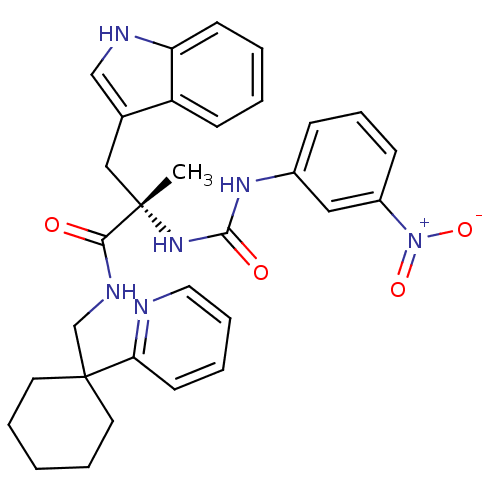
((S)-3-(1H-Indol-3-yl)-2-methyl-2-[3-(3-nitro-pheny...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)Nc1cccc(c1)[N+]([O-])=O)C(=O)NCC1(CCCCC1)c1ccccn1 Show InChI InChI=1S/C31H34N6O4/c1-30(19-22-20-33-26-13-4-3-12-25(22)26,36-29(39)35-23-10-9-11-24(18-23)37(40)41)28(38)34-21-31(15-6-2-7-16-31)27-14-5-8-17-32-27/h3-5,8-14,17-18,20,33H,2,6-7,15-16,19,21H2,1H3,(H,34,38)(H2,35,36,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50449787
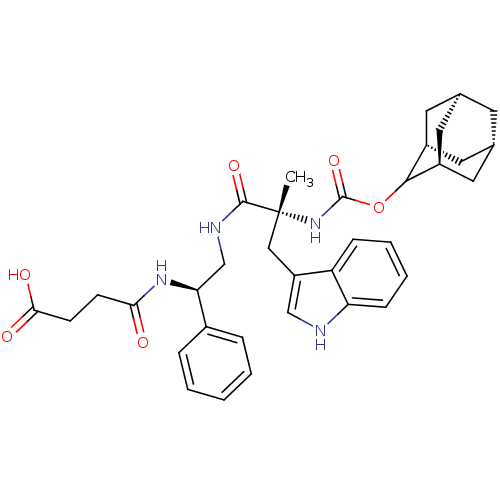
(CHEMBL2062154 | PD-134308)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)N[C@](C)(Cc1c[nH]c4ccccc14)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1)[C@@]([H])(C2)C3 |wU:30.41,14.15,45.49,3.3,wD:6.6,1.0,TLB:5:3:47:9.6.8,10:9:47:3.48.2,THB:5:6:47:3.48.2,10:9:1.47.8:3.5.48,2:3:9:1.47.8,2:1:9:3.5.48,(-14.99,-2.1,;-13.56,-2.66,;-14.77,-3.94,;-13.26,-3.53,;-13.35,-5.06,;-11.86,-4.09,;-10.83,-2.82,;-9.38,-3.33,;-12.24,-3.16,;-10.83,-1.28,;-9.29,-1.31,;-8.5,.01,;-9.25,1.36,;-6.96,-.01,;-6.19,1.3,;-5.42,-.02,;-7.44,2.2,;-7.28,3.74,;-8.44,4.76,;-7.81,6.18,;-6.29,6.03,;-5.14,7.07,;-3.69,6.6,;-3.34,5.08,;-4.49,4.06,;-5.94,4.52,;-4.66,1.42,;-3.99,2.8,;-3.79,.15,;-2.27,.27,;-1.4,-1.02,;-2.08,-2.4,;-1.22,-3.69,;.32,-3.58,;-1.9,-5.07,;-3.43,-5.16,;-4.1,-6.57,;-5.64,-6.67,;-3.25,-7.83,;.14,-.91,;.99,-2.2,;2.51,-2.08,;3.19,-.7,;2.32,.59,;.8,.47,;-12.23,-.7,;-12.2,.82,;-13.58,-1.18,;-13.27,-1.94,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/t21-,22+,24-,25+,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of binding of [3H]-PD 140376 to Cholecystokinin type B receptor in guinea pig cortex. |
Bioorg Med Chem Lett 3: 885-888 (1993)
Article DOI: 10.1016/S0960-894X(00)80686-1
BindingDB Entry DOI: 10.7270/Q2D79BWZ |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50071739

((S)-3-(1H-Indol-3-yl)-N-[1-(5-methoxy-pyridin-2-yl...)Show SMILES COc1ccc(nc1)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C32H36N6O5/c1-31(18-22-19-33-27-9-5-4-8-26(22)27,37-30(40)36-23-10-12-24(13-11-23)38(41)42)29(39)35-21-32(16-6-3-7-17-32)28-15-14-25(43-2)20-34-28/h4-5,8-15,19-20,33H,3,6-7,16-18,21H2,1-2H3,(H,35,39)(H2,36,37,40)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonistic activity against cloned human Bombesin receptor bb2 labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells; 0.66-1.3 |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50008852
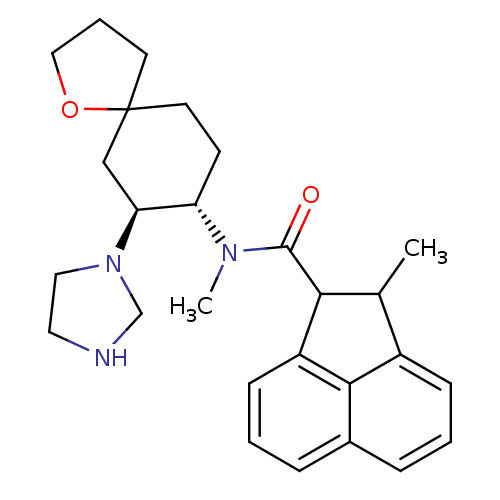
(2-Methyl-acenaphthene-1-carboxylic acid (7-imidazo...)Show SMILES CC1C(C(=O)N(C)[C@H]2CCC3(CCCO3)C[C@@H]2N2CCNC2)c2cccc3cccc1c23 Show InChI InChI=1S/C27H35N3O2/c1-18-20-8-3-6-19-7-4-9-21(25(19)20)24(18)26(31)29(2)22-10-12-27(11-5-15-32-27)16-23(22)30-14-13-28-17-30/h3-4,6-9,18,22-24,28H,5,10-17H2,1-2H3/t18?,22-,23-,24?,27?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Opioid receptor kappa affinity, determined with [3H]etorphine in the presence of excess unlabeled [D-Ala2-MePhe4-Glyol5]-enkephalin |
J Med Chem 34: 190-4 (1991)
BindingDB Entry DOI: 10.7270/Q2J103SG |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(Homo sapiens (Human)) | BDBM50071749
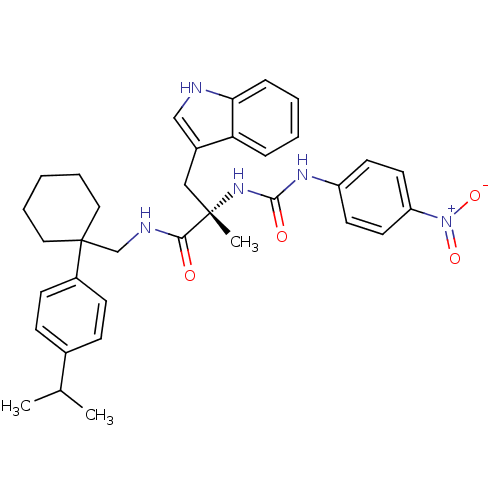
((S)-3-(1H-Indol-3-yl)-N-[1-(4-isopropyl-phenyl)-cy...)Show SMILES CC(C)c1ccc(cc1)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1 Show InChI InChI=1S/C35H41N5O4/c1-24(2)25-11-13-27(14-12-25)35(19-7-4-8-20-35)23-37-32(41)34(3,21-26-22-36-31-10-6-5-9-30(26)31)39-33(42)38-28-15-17-29(18-16-28)40(43)44/h5-6,9-18,22,24,36H,4,7-8,19-21,23H2,1-3H3,(H,37,41)(H2,38,39,42)/t34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cells |
Bioorg Med Chem Lett 8: 2589-94 (1999)
BindingDB Entry DOI: 10.7270/Q2DB82B4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50000091

((6S,11R)-3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-...)Show SMILES CC[C@]12CCN(CC3CC3)C([C@@H]1C)C(=O)c1ccc(O)cc21 |TLB:6:5:13.21.15:11,14:13:11:5.3.4,THB:20:21:11:5.3.4| Show InChI InChI=1S/C19H25NO2/c1-3-19-8-9-20(11-13-4-5-13)17(12(19)2)18(22)15-7-6-14(21)10-16(15)19/h6-7,10,12-13,17,21H,3-5,8-9,11H2,1-2H3/t12-,17?,19-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity for the Opioid receptor mu 1 |
J Med Chem 31: 831-6 (1988)
BindingDB Entry DOI: 10.7270/Q2R78FSK |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50281632
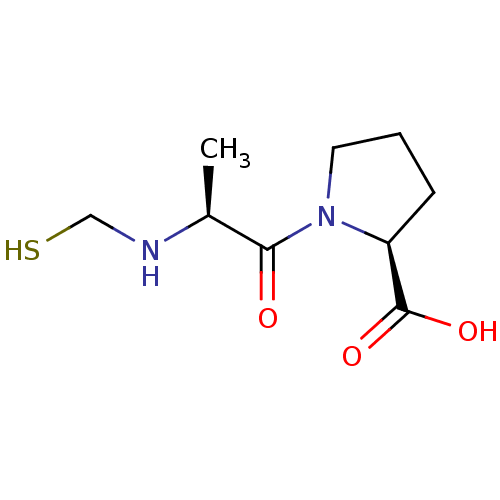
((2S)-1-{(2S)-2-[(lambda~4~-sulfanylmethyl)amino]pr...)Show InChI InChI=1S/C9H16N2O3S/c1-6(10-5-15)8(12)11-4-2-3-7(11)9(13)14/h6-7,10,15H,2-5H2,1H3,(H,13,14)/t6-,7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Angiotensin I converting enzyme |
Bioorg Med Chem Lett 3: 799-802 (1993)
Article DOI: 10.1016/S0960-894X(00)80669-1
BindingDB Entry DOI: 10.7270/Q29S1QZZ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50281634
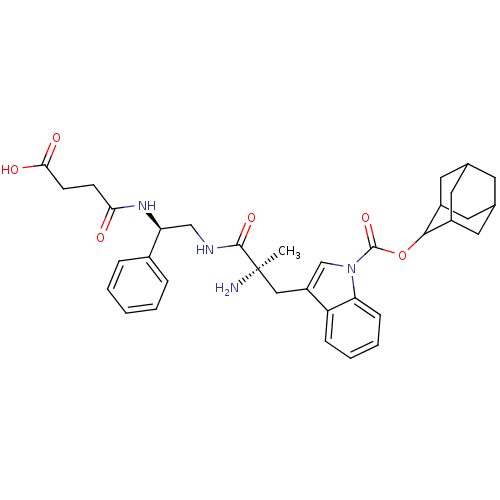
(3-{(R)-2-Amino-2-[(R)-2-(3-carboxy-propionylamino)...)Show SMILES C[C@@](N)(Cc1cn(C(=O)OC2C3CC4CC(C3)CC2C4)c2ccccc12)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1 |wU:30.35,1.0,wD:1.1,TLB:16:11:19:15.17.14,16:15:10.11.12:19,THB:9:10:19:15.17.14,14:13:10:15.17.16,14:15:10:13.12.19,(2.53,-5.38,;2.53,-3.85,;1.17,-4.57,;2.6,-2.31,;1.29,-1.47,;-.16,-2.01,;-1.11,-.84,;-2.46,-1.57,;-2.49,-3.1,;-3.77,-.78,;-5.12,-1.52,;-5.28,-2.91,;-6.8,-3.17,;-8.17,-2.48,;-9.27,-3.57,;-7.75,-3.24,;-6.4,-3.99,;-7.61,-1.7,;-6.43,-.73,;-7.94,-.96,;-.27,.47,;-.69,1.95,;.4,3.03,;1.9,2.63,;2.29,1.17,;1.2,.07,;3.83,-4.67,;3.76,-6.22,;5.21,-3.96,;6.49,-4.78,;7.86,-4.06,;9.17,-4.89,;10.52,-4.17,;10.59,-2.63,;11.82,-5,;13.18,-4.29,;14.48,-5.1,;15.86,-4.39,;14.48,-6.64,;7.91,-2.54,;9.24,-1.72,;9.19,-.17,;7.84,.54,;6.53,-.28,;6.58,-1.79,)| Show InChI InChI=1S/C35H42N4O6/c1-35(36,33(43)37-19-28(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42)18-26-20-39(29-10-6-5-9-27(26)29)34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21/h2-10,20-22,24-25,28,32H,11-19,36H2,1H3,(H,37,43)(H,38,40)(H,41,42)/t21?,22?,24?,25?,28-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Cholecystokinin type B receptor |
Bioorg Med Chem Lett 3: 799-802 (1993)
Article DOI: 10.1016/S0960-894X(00)80669-1
BindingDB Entry DOI: 10.7270/Q29S1QZZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity towards mu opioid receptor was determined |
J Med Chem 32: 1620-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GH9JJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity for the Opioid receptor mu 1 |
J Med Chem 31: 831-6 (1988)
BindingDB Entry DOI: 10.7270/Q2R78FSK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Mus musculus-MOUSE) | BDBM50002477

((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H44N6O8S/c1-34(2,3)48-33(47)40-26(17-21-19-36-23-13-9-8-12-22(21)23)31(45)37-24(14-15-49-4)30(44)39-27(18-28(41)42)32(46)38-25(29(35)43)16-20-10-6-5-7-11-20/h5-13,19,24-27,36H,14-18H2,1-4H3,(H2,35,43)(H,37,45)(H,38,46)(H,39,44)(H,40,47)(H,41,42)/t24-,25-,26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]pentagastrin from Cholecystokinin receptor of mouse cerebral cortex |
J Med Chem 30: 729-32 (1987)
BindingDB Entry DOI: 10.7270/Q2KK9F0J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















































