 Found 10570 hits with Last Name = 'hou' and Initial = 'd'
Found 10570 hits with Last Name = 'hou' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293089
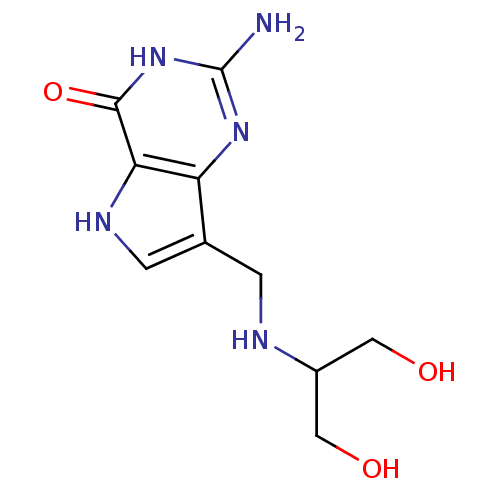
(2-Amino-7-{[(1,3-dihydroxypropan-2-yl)amino]methyl...)Show InChI InChI=1S/C10H15N5O3/c11-10-14-7-5(1-12-6(3-16)4-17)2-13-8(7)9(18)15-10/h2,6,12-13,16-17H,1,3-4H2,(H3,11,14,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293090
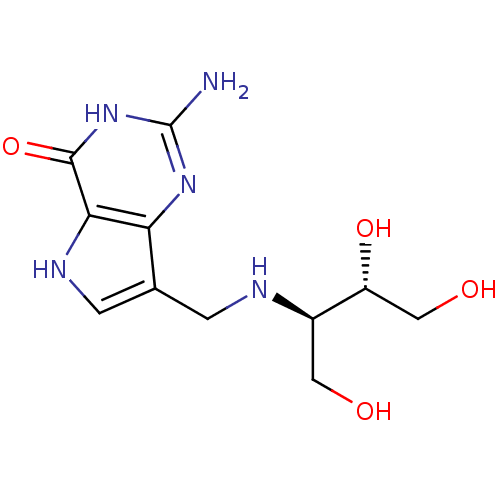
(2-Amino-7-({[(2R,3S)-1,3,4-trihydroxybutan-2-yl]am...)Show SMILES Nc1nc2c(CN[C@H](CO)[C@H](O)CO)c[nH]c2c(=O)[nH]1 |r| Show InChI InChI=1S/C11H17N5O4/c12-11-15-8-5(2-14-9(8)10(20)16-11)1-13-6(3-17)7(19)4-18/h2,6-7,13-14,17-19H,1,3-4H2,(H3,12,15,16,20)/t6-,7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50246593
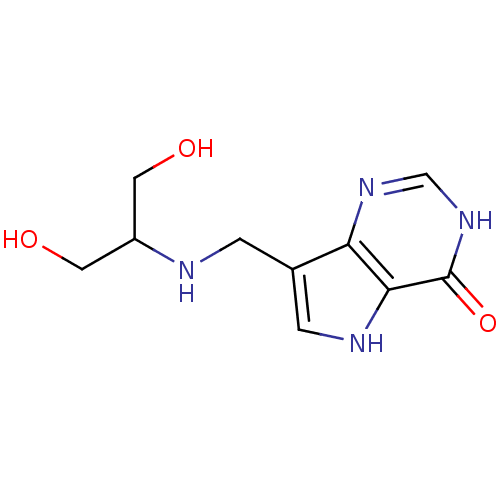
(7-((1,3-dihydroxypropan-2-ylamino)methyl)-3H-pyrro...)Show InChI InChI=1S/C10H14N4O3/c15-3-7(4-16)11-1-6-2-12-9-8(6)13-5-14-10(9)17/h2,5,7,11-12,15-16H,1,3-4H2,(H,13,14,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293087
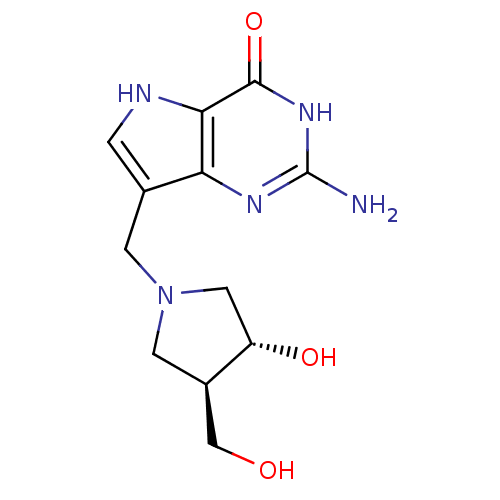
(2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...)Show SMILES Nc1nc2c(CN3C[C@H](O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 |r| Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293091

(7-({[(1R,2S)-2,3-DIHYDROXY-1-(HYDROXYMETHYL)PROPYL...)Show SMILES OC[C@@H](O)[C@@H](CO)NCc1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H16N4O4/c16-3-7(8(18)4-17)12-1-6-2-13-10-9(6)14-5-15-11(10)19/h2,5,7-8,12-13,16-18H,1,3-4H2,(H,14,15,19)/t7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109
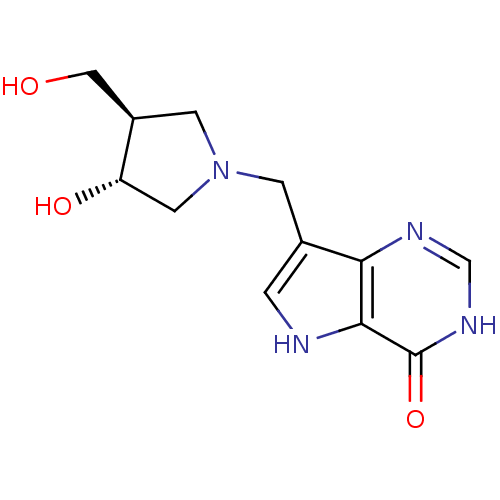
(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50109062
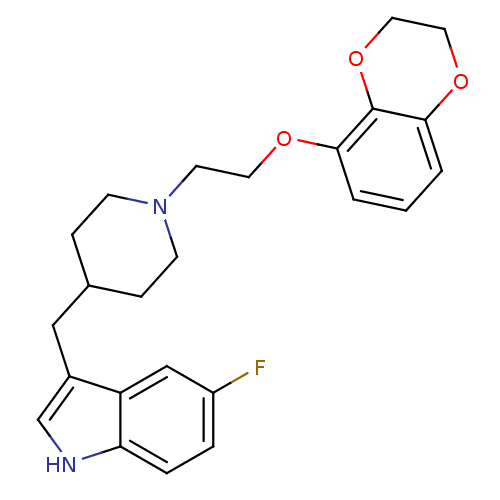
(3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCOc4cccc5OCCOc45)CC3)c2c1 Show InChI InChI=1S/C24H27FN2O3/c25-19-4-5-21-20(15-19)18(16-26-21)14-17-6-8-27(9-7-17)10-11-28-22-2-1-3-23-24(22)30-13-12-29-23/h1-5,15-17,26H,6-14H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound |
Bioorg Med Chem Lett 12: 307-10 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G2M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50109058

(1H-4-indolyl 2-[4-(1H-3-indolyl)-1,2,3,6-tetrahydr...)Show SMILES C(CN1CCC(=CC1)c1c[nH]c2ccccc12)Oc1cccc2[nH]ccc12 |c:5| Show InChI InChI=1S/C23H23N3O/c1-2-5-21-18(4-1)20(16-25-21)17-9-12-26(13-10-17)14-15-27-23-7-3-6-22-19(23)8-11-24-22/h1-9,11,16,24-25H,10,12-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound |
Bioorg Med Chem Lett 12: 307-10 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G2M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519706
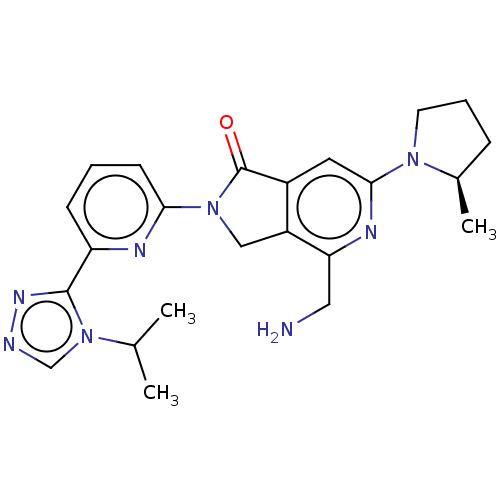
(US11142525, Example 107)Show SMILES CC(C)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CN)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086
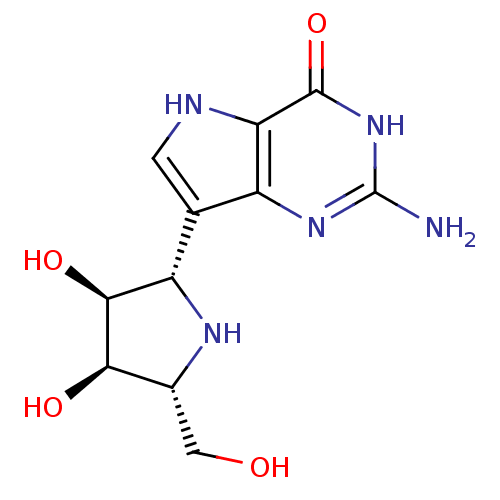
((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606126
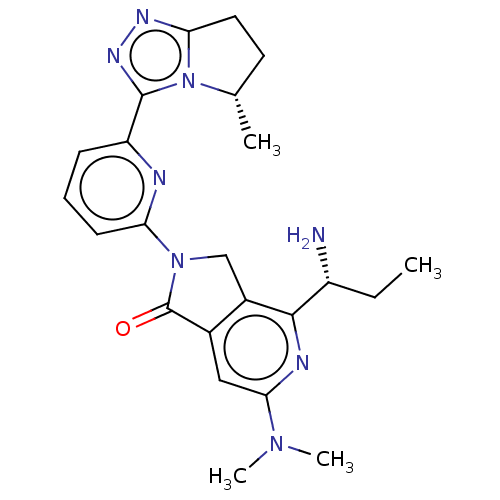
(4-[(1R)-1- aminopropyl]- 6-(dimethyl- amino)-2-{6-...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606125

(4-[(1$#958;)-1- aminoethyl]-2- {6-[(5$#958;)-5- me...)Show SMILES C[C@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606205

(2-{6-[(5S,7S)- 5,7-dimethyl-6,7- dihydro-5H- pyrro...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2[C@@H](C)C[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519601

(US11142525, Example 17)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1[C@@H](C)CC(F)(F)F)N(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519602

(US11142525, Example 18)Show SMILES CC[C@@H](CC(F)(F)F)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N(C)C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519590

(US11142525, Example 6 | US11142525, Example 79)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1C(C)C)N(C)C(C)C | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519699

(US11142525, Example 100)Show SMILES CC(C)N(C)c1cc2C(=O)N(Cc2c(CN)n1)c1cccc(n1)N1[C@@H](C)COC1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606115

(4-(2- aminopropan- 2-yl)-2-{6- [(5S)-5-methyl- 6,7...)Show SMILES C[C@@H]1CCCN1c1cc2C(=O)N(Cc2c(n1)C(C)(C)N)c1cccc(n1)-c1nnc2CC[C@H](C)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606114

(4-[(1S)-1- aminopropyl]- 2-{6-[(5$#958;)-5- ethyl-...)Show SMILES CC[C@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](CC)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606118

(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (hydroxy- m...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606123

(4-[(1$#958;)-1- aminoethyl]-2- {6-[(5$#958;)-5- me...)Show SMILES C[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606098
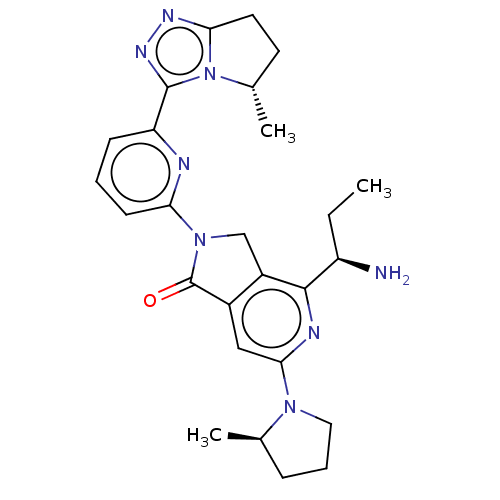
(US11684616, Example 1)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606099

(US11684616, Example 2)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@@H](C)N)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606100
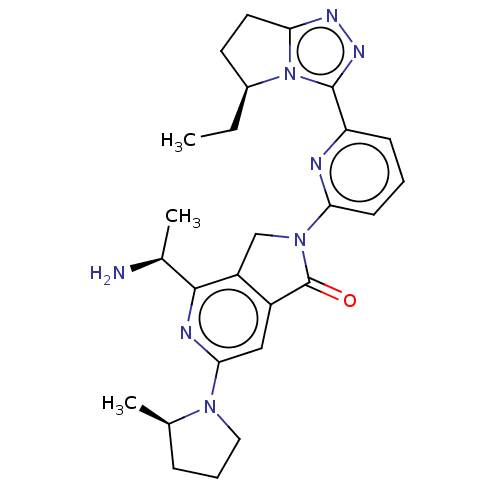
(US11684616, Example 3)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@H](C)N)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606101

(US11684616, Example 4)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@@H](C)N)N(C)C(C)C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606102

(US11684616, Example 5)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519743

(US11142525, Example 144)Show SMILES CCC(CC)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606128
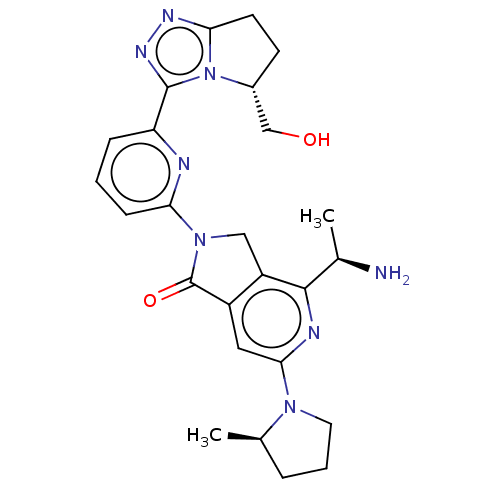
(4-[(1R)-1- aminoethyl]-2- {6-[(5R)-5- (hydroxy- me...)Show SMILES C[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606129

(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (hydroxy- m...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606135
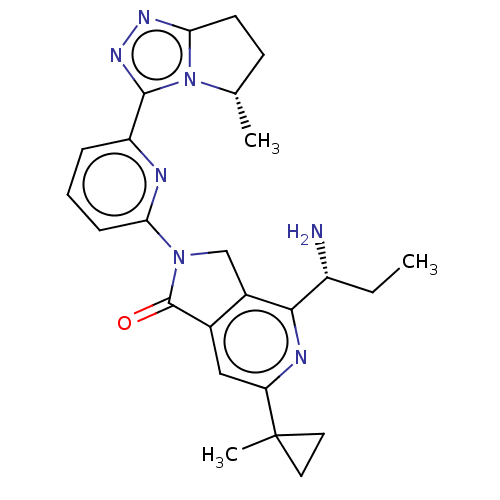
(4-[(1$#958;)-1- aminopropyl]- 6-(1- methylcyclo- p...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)C1(C)CC1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606137
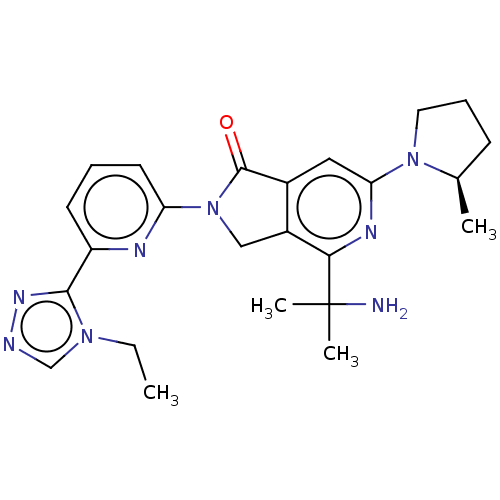
(US11684616, Example 100)Show SMILES CCn1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2C(C)(C)N)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606194

(US11684616, Example 200)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4CNC)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606195

(US11684616, Example 201)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4CNC)N(C)C(C)C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606199

(2-{6-[(5R)-5- (fluoromethyl)- 6,7-dihydro-5H- pyrr...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CF)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606204

(4-[(methyl- amino)methyl]- 2-{6-[(5S)- 5-methyl-6,...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519673

(US11142525, Example 74)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)N1[C@@H](C)COC1=O)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519653
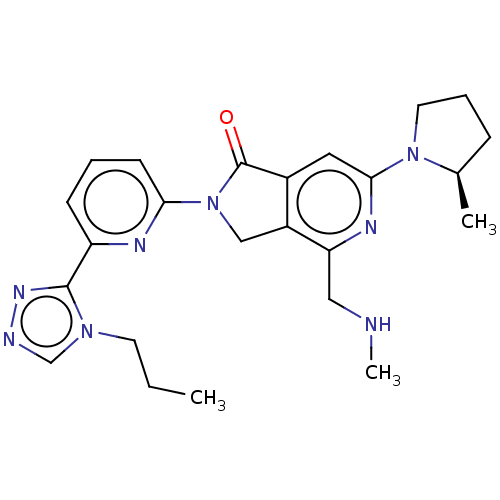
(US11142525, Example 55)Show SMILES CCCn1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519746

(US11142525, Example 150)Show SMILES CCC(CC)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CN)N(C)C(C)C)C1=O | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519708

(US11142525, Example 109)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1C(C)C)N1CCCC1(C)C | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606130
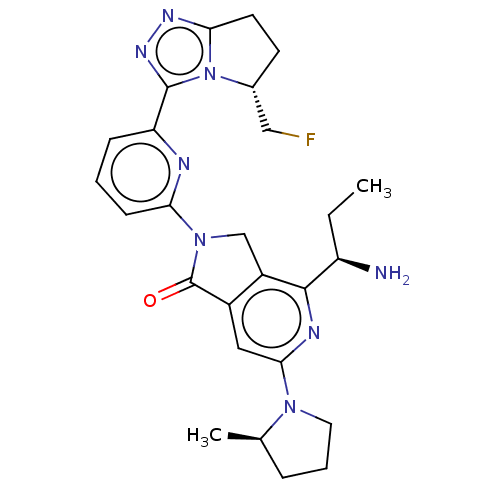
(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (fluorometh...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CF)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519770
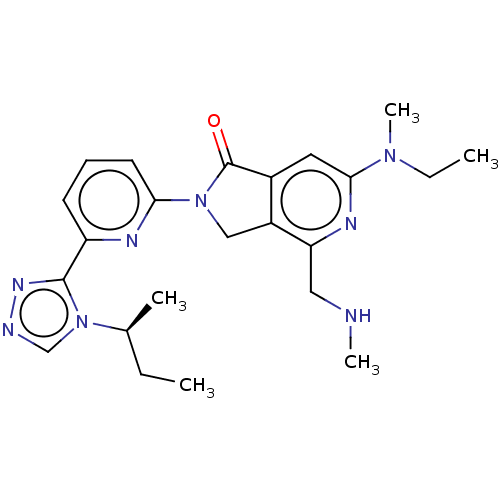
(US11142525, Example 173)Show SMILES CC[C@H](C)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N(C)CC)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606196

(US11684616, Example 202)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CF)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606119
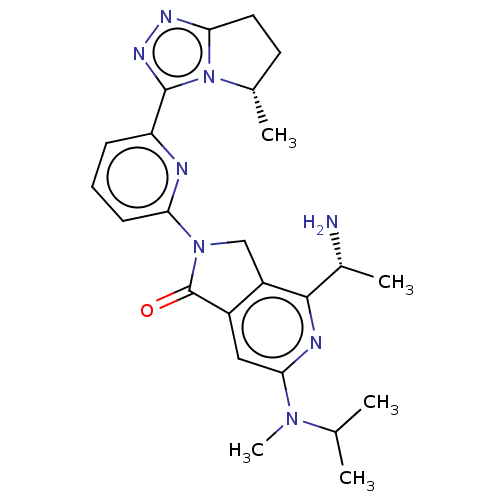
(4-[(1R)-1- aminoethyl]-2- {6-[(5S)-5- methyl-6,7- ...)Show SMILES CC(C)N(C)c1cc2C(=O)N(Cc2c(n1)[C@@H](C)N)c1cccc(n1)-c1nnc2CC[C@H](C)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606111

(4-[(1R)-1- aminopropyl]- 2-{6-[(5$#958;)-5- ethyl-...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](CC)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519599
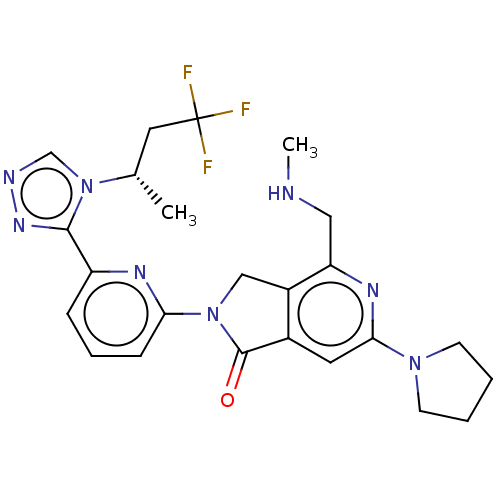
(US11142525, Example 15)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1[C@@H](C)CC(F)(F)F)N1CCCC1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606178

(4-[(1R)-1- aminopropyl]- 2-[6-(4-ethyl- 4H-1,2,4- ...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1CC)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519667

(US11142525, Example 68)Show SMILES C[C@@H]1CCCN1c1cc2C(=O)N(Cc2c(CN)n1)c1cccc(n1)N1[C@@H](CF)COC1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606134

(4-[(1$#958;)-1- aminopropyl]- 2-{6-[(5R)-5- (hydro...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)C1(C)CC1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50150101
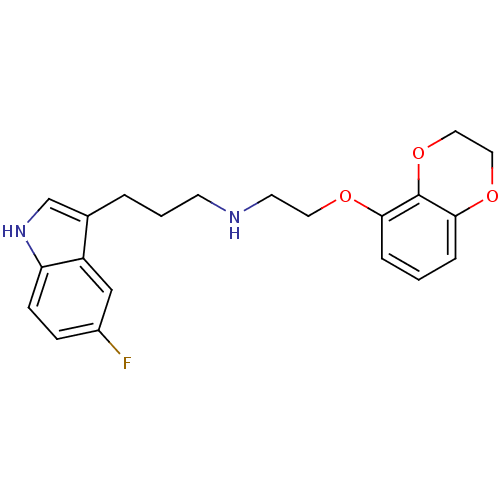
(CHEMBL419240 | [2-(2,3-Dihydro-benzo[1,4]dioxin-5-...)Show InChI InChI=1S/C21H23FN2O3/c22-16-6-7-18-17(13-16)15(14-24-18)3-2-8-23-9-10-25-19-4-1-5-20-21(19)27-12-11-26-20/h1,4-7,13-14,23-24H,2-3,8-12H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity against serotonin transporter in rat cortical tissues using radioligand [3H]-paroxetine |
J Med Chem 47: 3823-42 (2004)
Article DOI: 10.1021/jm0304010
BindingDB Entry DOI: 10.7270/Q2S46RDW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data