 Found 8741 hits with Last Name = 'hou' and Initial = 'x'
Found 8741 hits with Last Name = 'hou' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50582801
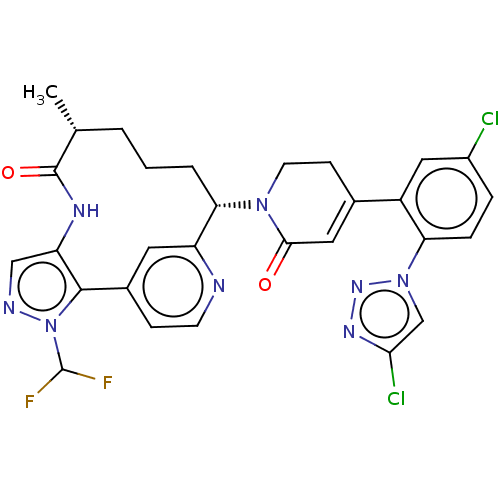
(CHEMBL5076656)Show SMILES C[C@@H]1CCC[C@H](N2CCC(=CC2=O)c2cc(Cl)ccc2-n2cc(Cl)nn2)c2cc(ccn2)-c2c(NC1=O)cnn2C(F)F |r,c:9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50250493
(CHEMBL4068445)Show SMILES CN1CCC2(CCN(CC2)c2cccc3[C@H](N(CCc23)C(=O)\C=C\c2c(F)c(Cl)ccc2-n2cnnn2)C(=O)Nc2ccc(cc2)C(O)=O)C1=O |r| Show InChI InChI=1S/C36H34ClFN8O5/c1-43-18-14-36(35(43)51)15-19-44(20-16-36)28-4-2-3-25-24(28)13-17-45(32(25)33(48)40-23-7-5-22(6-8-23)34(49)50)30(47)12-9-26-29(46-21-39-41-42-46)11-10-27(37)31(26)38/h2-12,21,32H,13-20H2,1H3,(H,40,48)(H,49,50)/b12-9+/t32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method |
J Med Chem 60: 9703-9723 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01171
BindingDB Entry DOI: 10.7270/Q2K64MHN |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50040861
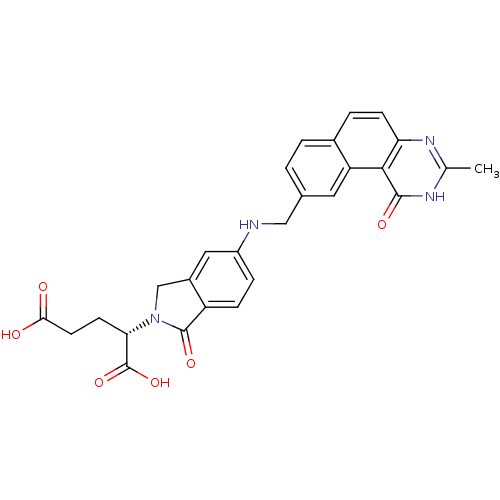
((S)-2-(5-(((1,2-DIHYDRO-3-METHYL-1-OXOBENZO(F)QUIN...)Show SMILES Cc1nc2ccc3ccc(CNc4ccc5C(=O)N(Cc5c4)[C@@H](CCC(O)=O)C(O)=O)cc3c2c(=O)[nH]1 Show InChI InChI=1S/C27H24N4O6/c1-14-29-21-7-4-16-3-2-15(10-20(16)24(21)25(34)30-14)12-28-18-5-6-19-17(11-18)13-31(26(19)35)22(27(36)37)8-9-23(32)33/h2-7,10-11,22,28H,8-9,12-13H2,1H3,(H,32,33)(H,36,37)(H,29,30,34)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of TS by spectrophotometry |
Bioorg Med Chem 19: 3585-94 (2011)
Article DOI: 10.1016/j.bmc.2011.03.067
BindingDB Entry DOI: 10.7270/Q22V2GGP |
More data for this
Ligand-Target Pair | |
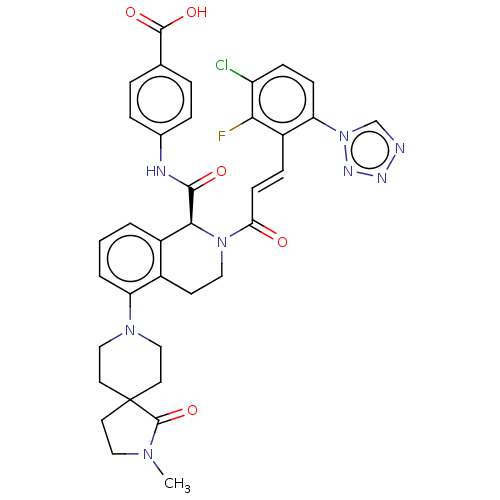
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50250492
(CHEMBL4097304)Show SMILES CN(C)C1CCN(CC1)c1cccc2[C@H](N(CCc12)C(=O)\C=C\c1c(F)c(Cl)ccc1-n1cnnn1)C(=O)Nc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C34H34ClFN8O4/c1-41(2)23-14-17-42(18-15-23)28-5-3-4-25-24(28)16-19-43(32(25)33(46)38-22-8-6-21(7-9-22)34(47)48)30(45)13-10-26-29(44-20-37-39-40-44)12-11-27(35)31(26)36/h3-13,20,23,32H,14-19H2,1-2H3,(H,38,46)(H,47,48)/b13-10+/t32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method |
J Med Chem 60: 9703-9723 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01171
BindingDB Entry DOI: 10.7270/Q2K64MHN |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398003
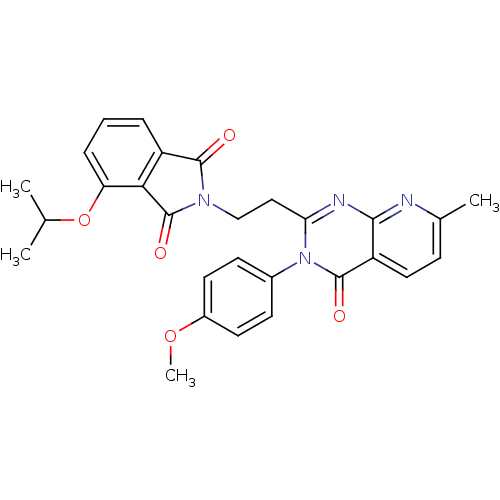
(CHEMBL2180402)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2nc(C)ccc2c1=O Show InChI InChI=1S/C28H26N4O5/c1-16(2)37-22-7-5-6-20-24(22)28(35)31(26(20)33)15-14-23-30-25-21(13-8-17(3)29-25)27(34)32(23)18-9-11-19(36-4)12-10-18/h5-13,16H,14-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398003

(CHEMBL2180402)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2nc(C)ccc2c1=O Show InChI InChI=1S/C28H26N4O5/c1-16(2)37-22-7-5-6-20-24(22)28(35)31(26(20)33)15-14-23-30-25-21(13-8-17(3)29-25)27(34)32(23)18-9-11-19(36-4)12-10-18/h5-13,16H,14-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
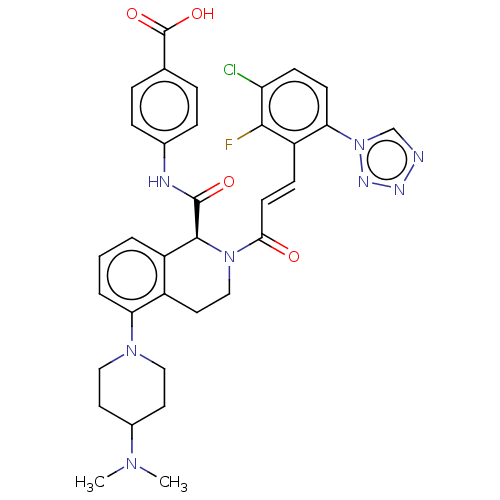
Coagulation factor XI
(Homo sapiens (Human)) | BDBM247411

(US10336754, Example 353 | US11053247, Example 353 ...)Show SMILES C[C@@H]1CCC[C@@H](c2cc(ccn2)-c2c(NC1=O)cnn2C(F)F)n1cnc(cc1=O)-c1cc(Cl)ccc1-n1cc(Cl)nn1 |r| Show InChI InChI=1S/C28H23Cl2F2N9O2/c1-15-3-2-4-23(20-9-16(7-8-33-20)26-21(36-27(15)43)12-35-41(26)28(31)32)39-14-34-19(11-25(39)42)18-10-17(29)5-6-22(18)40-13-24(30)37-38-40/h5-15,23,28H,2-4H2,1H3,(H,36,43)/t15-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50582799
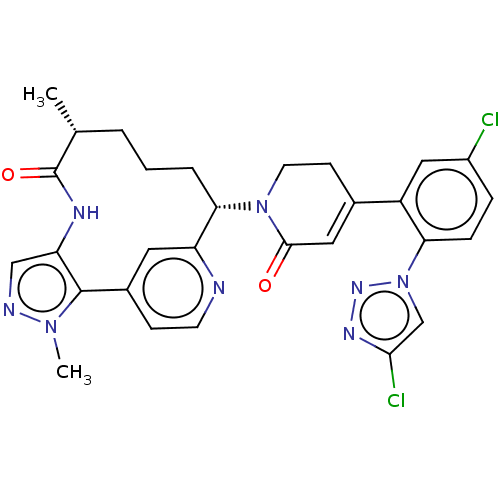
(CHEMBL5094166)Show SMILES C[C@@H]1CCC[C@H](N2CCC(=CC2=O)c2cc(Cl)ccc2-n2cc(Cl)nn2)c2cc(ccn2)-c2c(NC1=O)cnn2C |r,c:9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50582800
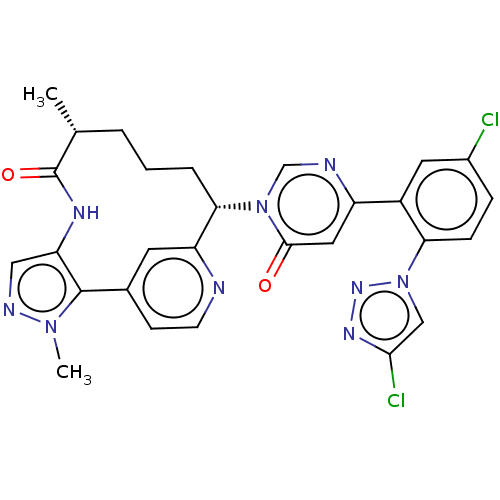
(CHEMBL5093567)Show SMILES C[C@@H]1CCC[C@@H](c2cc(ccn2)-c2c(NC1=O)cnn2C)n1cnc(cc1=O)-c1cc(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Opioid growth factor receptor-like protein 1
(Homo sapiens (Human)) | BDBM220380
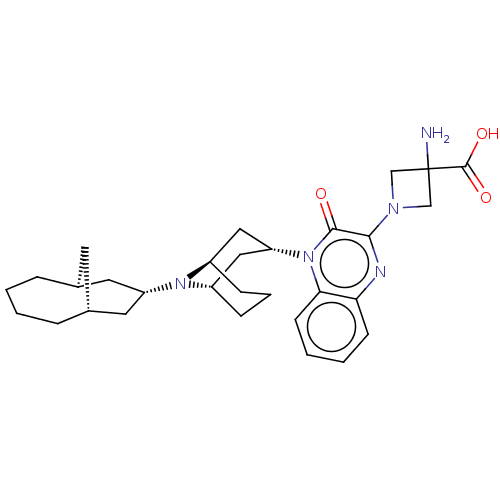
(US9290488, ZA01)Show SMILES NC1(CN(C1)c1nc2ccccc2n([C@@H]2C[C@@H]3CCC[C@H](C2)N3[C@H]2C[C@@H]3C[C@H](C2)CCCC3)c1=O)C(O)=O |r,THB:23:22:14.15.21:17.19.18| Show InChI InChI=1S/C30H41N5O3/c31-30(29(37)38)17-33(18-30)27-28(36)35(26-11-4-3-10-25(26)32-27)24-15-21-8-5-9-22(16-24)34(21)23-13-19-6-1-2-7-20(12-19)14-23/h3-4,10-11,19-24H,1-2,5-9,12-18,31H2,(H,37,38)/t19-,20+,21-,22+,23-,24+ | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... |
US Patent US9290488 (2016)
BindingDB Entry DOI: 10.7270/Q2GF0SCW |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50252829
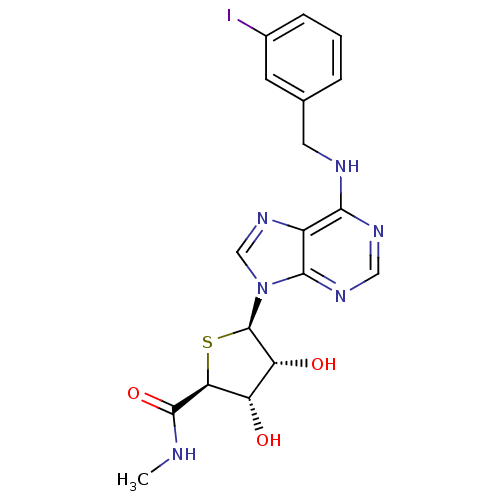
((2S,3S,4R,5R)-5-(6-(3-iodobenzylamino)-9H-purin-9-...)Show SMILES CNC(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O3S/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells |
J Med Chem 51: 6609-13 (2008)
Article DOI: 10.1021/jm8008647
BindingDB Entry DOI: 10.7270/Q2XG9QZG |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541586
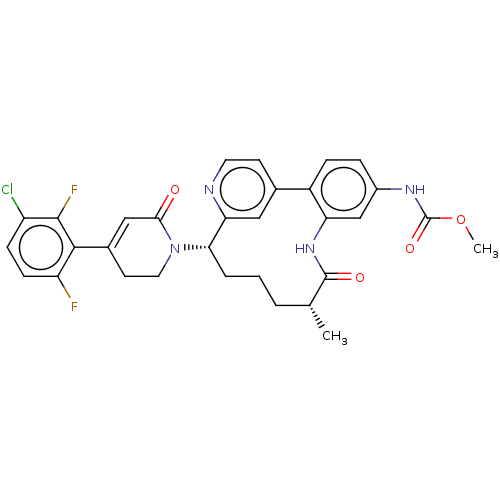
(CHEMBL4638245)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CCC(=CC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r,c:22| Show InChI InChI=1S/C31H29ClF2N4O4/c1-17-4-3-5-26(38-13-11-19(15-27(38)39)28-23(33)9-8-22(32)29(28)34)25-14-18(10-12-35-25)21-7-6-20(36-31(41)42-2)16-24(21)37-30(17)40/h6-10,12,14-17,26H,3-5,11,13H2,1-2H3,(H,36,41)(H,37,40)/t17-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50594944
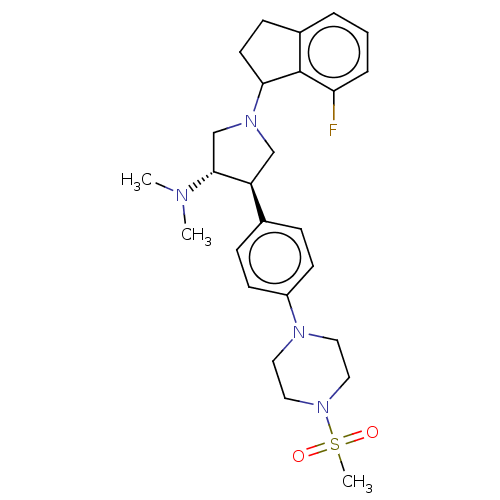
(CHEMBL5181703)Show SMILES CN(C)[C@@H]1CN(C[C@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide
(RAT) | BDBM50250019
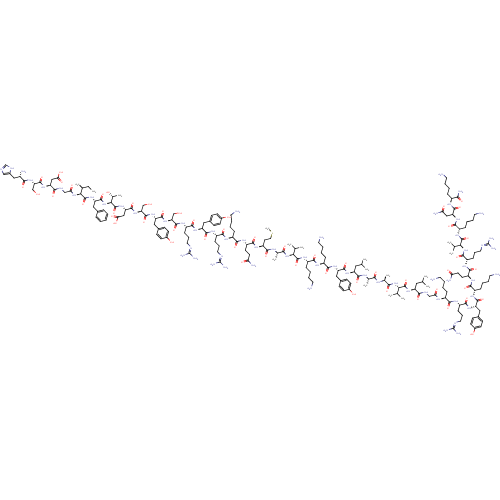
(CHEMBL524658 | PACAP | PACAP(1-38) | PACAP-38 | PA...)Show SMILES CC[C@H](C)[C@H](NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C203H331N63O53S/c1-18-109(12)162(262-156(279)99-230-170(290)147(95-157(280)281)255-193(313)149(100-267)259-168(288)124(211)93-119-97-224-103-231-119)198(318)257-145(88-114-40-20-19-21-41-114)191(311)266-163(113(16)270)199(319)258-148(96-158(282)283)190(310)261-151(102-269)194(314)253-144(92-118-59-67-123(274)68-60-118)188(308)260-150(101-268)192(312)243-134(51-38-83-227-202(220)221)180(300)251-142(90-116-55-63-121(272)64-56-116)186(306)242-132(49-36-81-225-200(216)217)176(296)237-127(44-24-31-76-206)173(293)245-137(70-72-153(213)276)182(302)246-138(73-85-320-17)171(291)233-112(15)167(287)263-159(106(6)7)195(315)247-130(47-27-34-79-209)175(295)238-129(46-26-33-78-208)177(297)252-143(91-117-57-65-122(273)66-58-117)187(307)249-140(87-105(4)5)184(304)234-110(13)165(285)232-111(14)166(286)264-160(107(8)9)197(317)256-139(86-104(2)3)169(289)229-98-155(278)235-126(43-23-30-75-205)172(292)239-133(50-37-82-226-201(218)219)179(299)250-141(89-115-53-61-120(271)62-54-115)185(305)241-128(45-25-32-77-207)174(294)244-136(69-71-152(212)275)181(301)240-135(52-39-84-228-203(222)223)183(303)265-161(108(10)11)196(316)248-131(48-28-35-80-210)178(298)254-146(94-154(214)277)189(309)236-125(164(215)284)42-22-29-74-204/h19-21,40-41,53-68,97,103-113,124-151,159-163,267-274H,18,22-39,42-52,69-96,98-102,204-211H2,1-17H3,(H2,212,275)(H2,213,276)(H2,214,277)(H2,215,284)(H,224,231)(H,229,289)(H,230,290)(H,232,285)(H,233,291)(H,234,304)(H,235,278)(H,236,309)(H,237,296)(H,238,295)(H,239,292)(H,240,301)(H,241,305)(H,242,306)(H,243,312)(H,244,294)(H,245,293)(H,246,302)(H,247,315)(H,248,316)(H,249,307)(H,250,299)(H,251,300)(H,252,297)(H,253,314)(H,254,298)(H,255,313)(H,256,317)(H,257,318)(H,258,319)(H,259,288)(H,260,308)(H,261,310)(H,262,279)(H,263,287)(H,264,286)(H,265,303)(H,266,311)(H,280,281)(H,282,283)(H4,216,217,225)(H4,218,219,226)(H4,220,221,227)(H4,222,223,228)/t109-,110-,111-,112-,113+,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,142-,143-,144-,145-,146-,147-,148-,149-,150-,151-,159-,160-,161-,162-,163-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 1189-95 (1994)
Article DOI: 10.1016/s0028-3908(05)80009-7
BindingDB Entry DOI: 10.7270/Q2251GP3 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide
(RAT) | BDBM82448

([Ala2]PACAP(1-27))Show SMILES CCC(C)[C@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O |r,wU:48.49,63.64,81.83,98.100,121.124,139.142,152.155,168.171,189.193,202.207,214.218,49.52,4.4,12.17,26.26,wD:37.37,55.56,69.70,87.89,110.113,147.151,177.180,197.202,207.211,130.133,159.162,21.21,(-14.46,-50.6,;-14.44,-52.33,;-15.67,-53.24,;-17,-52.47,;-15.67,-54.78,;-17,-55.55,;-18.34,-54.78,;-18.34,-53.24,;-19.43,-55.87,;-20.76,-55.1,;-22.14,-55.94,;-22.14,-57.48,;-23.47,-55.17,;-23.47,-53.63,;-24.56,-52.54,;-24.56,-51,;-25.89,-50.23,;-23.23,-50.23,;-24.56,-56.25,;-25.89,-55.48,;-25.96,-54.17,;-27.23,-56.25,;-27.23,-57.79,;-28.56,-55.48,;-29.89,-56.25,;-29.89,-57.79,;-31.23,-55.48,;-32.54,-56.66,;-31.23,-53.94,;-32,-52.61,;-32,-51.07,;-34.67,-51.07,;-34.67,-52.61,;-33.33,-53.38,;-14.34,-55.55,;-14.41,-57.12,;-13,-54.78,;-11.67,-55.55,;-11.67,-57.09,;-10.34,-57.86,;-9,-57.09,;-7.66,-57.86,;-7.66,-59.41,;-9,-60.18,;-10.34,-59.41,;-10.34,-54.78,;-10.34,-53.24,;-9,-55.55,;-7.67,-54.78,;-7.67,-53.24,;-9,-52.47,;-6.33,-52.47,;-6.33,-55.55,;-6.33,-57.09,;-5,-54.78,;-3.67,-55.55,;-3.67,-57.09,;-2.33,-57.86,;-1,-57.09,;-2.33,-59.4,;-2.33,-54.78,;-2.33,-53.24,;-1,-55.55,;.33,-54.78,;.33,-53.24,;1.67,-52.47,;1.67,-55.55,;1.67,-57.09,;3,-54.78,;4.34,-55.55,;4.34,-57.09,;5.67,-57.86,;7.01,-57.09,;8.35,-57.86,;8.35,-59.41,;9.68,-60.18,;7.01,-60.18,;5.67,-59.41,;5.67,-54.78,;5.67,-53.24,;7,-55.55,;8.34,-54.78,;8.34,-53.24,;9.67,-52.47,;9.67,-55.55,;9.67,-57.09,;11,-54.78,;12.34,-55.55,;12.34,-57.09,;13.67,-57.86,;13.67,-59.4,;15,-60.17,;15,-61.71,;13.67,-62.48,;16.34,-62.48,;13.67,-54.78,;13.67,-53.24,;15,-55.55,;16.34,-54.78,;16.34,-53.24,;17.67,-52.47,;19.01,-53.25,;20.35,-52.47,;20.35,-50.93,;21.68,-50.16,;19.01,-50.16,;17.67,-50.93,;17.67,-55.55,;17.67,-57.09,;19.01,-54.78,;20.34,-55.55,;20.34,-57.09,;21.67,-57.86,;21.67,-59.4,;23.01,-60.17,;23.01,-61.71,;21.67,-62.48,;24.34,-62.48,;21.67,-54.78,;21.67,-53.24,;23.01,-55.55,;24.34,-54.78,;24.34,-53.24,;25.67,-52.47,;25.67,-50.93,;27.01,-50.16,;27.01,-48.62,;25.67,-55.55,;25.67,-57.09,;27.01,-54.78,;28.34,-55.55,;28.34,-57.09,;29.67,-57.86,;29.67,-59.4,;28.34,-60.17,;31.01,-60.17,;29.67,-54.78,;29.67,-53.24,;31.01,-55.55,;32.34,-54.78,;32.34,-53.24,;33.68,-52.47,;33.68,-50.93,;35.01,-50.16,;33.68,-55.55,;33.68,-57.09,;35.01,-54.78,;36.34,-55.55,;36.34,-57.09,;37.68,-54.78,;37.68,-53.24,;39.01,-55.55,;40.34,-54.78,;40.34,-53.24,;41.68,-52.47,;39.01,-52.47,;41.68,-55.55,;41.68,-57.09,;43.01,-54.78,;44.34,-55.55,;44.34,-57.09,;45.68,-57.86,;45.68,-59.4,;47.01,-60.17,;47.01,-61.71,;45.68,-54.78,;45.68,-53.24,;47.01,-55.55,;48.35,-54.78,;48.35,-53.24,;49.68,-52.47,;49.68,-50.93,;51.01,-50.16,;51.01,-48.62,;49.68,-55.55,;49.68,-57.09,;51.01,-54.78,;52.35,-55.55,;52.35,-57.09,;53.68,-57.86,;55.02,-57.09,;56.36,-57.86,;56.36,-59.41,;57.69,-60.18,;55.02,-60.18,;53.68,-59.41,;53.68,-54.78,;53.68,-53.24,;55.01,-55.55,;56.35,-54.78,;56.35,-53.24,;57.68,-52.47,;57.68,-50.93,;59.01,-53.24,;57.68,-55.55,;57.68,-57.09,;59.01,-54.78,;60.35,-55.55,;60.35,-57.09,;61.68,-54.78,;61.68,-53.24,;63.02,-55.55,;64.35,-54.78,;64.35,-53.24,;65.68,-55.55,;65.68,-57.09,;67.02,-54.78,;68.35,-55.55,;68.35,-57.09,;69.68,-57.86,;67.02,-57.86,;69.68,-54.78,;69.68,-53.24,;71.02,-55.55,;72.35,-54.78,;72.35,-53.24,;73.68,-52.47,;73.68,-50.93,;75.02,-53.24,;73.68,-55.55,;75.02,-54.78,;73.68,-57.09,)| Show InChI InChI=1S/C143H226N40O38S/c1-17-76(10)114(180-109(191)68-157-122(202)96(49-51-110(192)193)163-118(198)78(12)160-121(201)90(147)65-86-67-154-71-158-86)140(220)176-104(61-82-29-19-18-20-30-82)135(215)183-115(81(15)186)141(221)177-105(66-111(194)195)134(214)179-107(70-185)137(217)175-103(64-85-40-46-89(189)47-41-85)133(213)178-106(69-184)136(216)167-95(35-28-57-156-143(152)153)128(208)173-101(62-83-36-42-87(187)43-37-83)131(211)166-94(34-27-56-155-142(150)151)126(206)164-91(31-21-24-53-144)124(204)168-97(48-50-108(148)190)129(209)169-98(52-58-222-16)123(203)161-80(14)120(200)181-112(74(6)7)138(218)170-93(33-23-26-55-146)125(205)165-92(32-22-25-54-145)127(207)174-102(63-84-38-44-88(188)45-39-84)132(212)172-100(60-73(4)5)130(210)162-77(11)117(197)159-79(13)119(199)182-113(75(8)9)139(219)171-99(116(149)196)59-72(2)3/h18-20,29-30,36-47,67,71-81,90-107,112-115,184-189H,17,21-28,31-35,48-66,68-70,144-147H2,1-16H3,(H2,148,190)(H2,149,196)(H,154,158)(H,157,202)(H,159,197)(H,160,201)(H,161,203)(H,162,210)(H,163,198)(H,164,206)(H,165,205)(H,166,211)(H,167,216)(H,168,204)(H,169,209)(H,170,218)(H,171,219)(H,172,212)(H,173,208)(H,174,207)(H,175,217)(H,176,220)(H,177,221)(H,178,213)(H,179,214)(H,180,191)(H,181,200)(H,182,199)(H,183,215)(H,192,193)(H,194,195)(H4,150,151,155)(H4,152,153,156)/t76?,77-,78-,79-,80-,81+,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,112-,113-,114-,115-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 1189-95 (1994)
Article DOI: 10.1016/s0028-3908(05)80009-7
BindingDB Entry DOI: 10.7270/Q2251GP3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Rattus norvegicus) | BDBM21221
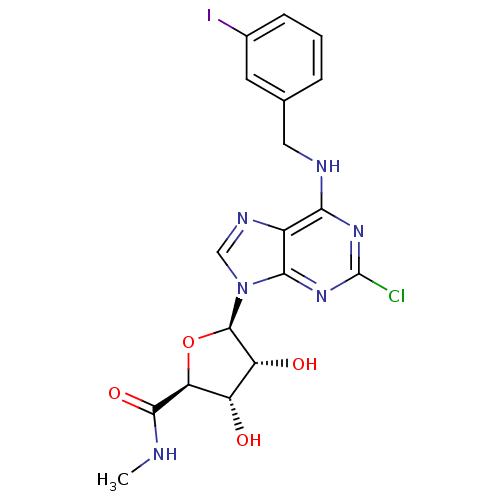
((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C18H18ClIN6O4/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from rat adenosine A3 receptor expressed in CHO cells |
J Med Chem 51: 6609-13 (2008)
Article DOI: 10.1021/jm8008647
BindingDB Entry DOI: 10.7270/Q2XG9QZG |
More data for this
Ligand-Target Pair | |
Opioid growth factor receptor-like protein 1
(Homo sapiens (Human)) | BDBM220382
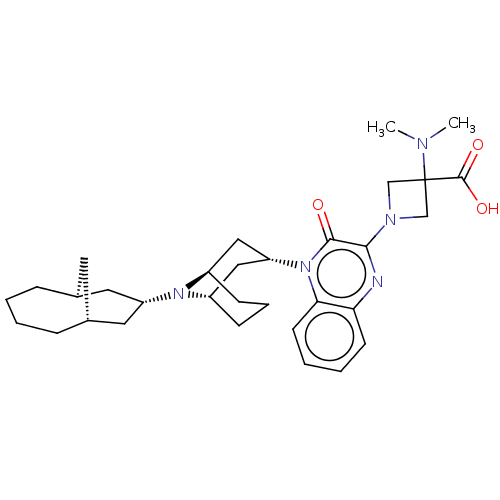
(US9290488, ZA03)Show SMILES CN(C)C1(CN(C1)c1nc2ccccc2n([C@@H]2C[C@@H]3CCC[C@H](C2)N3[C@H]2C[C@@H]3C[C@H](C2)CCCC3)c1=O)C(O)=O |r,THB:25:24:16.17.23:19.21.20| Show InChI InChI=1S/C32H45N5O3/c1-34(2)32(31(39)40)19-35(20-32)29-30(38)37(28-13-6-5-12-27(28)33-29)26-17-23-10-7-11-24(18-26)36(23)25-15-21-8-3-4-9-22(14-21)16-25/h5-6,12-13,21-26H,3-4,7-11,14-20H2,1-2H3,(H,39,40)/t21-,22+,23-,24+,25-,26+ | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.340 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... |
US Patent US9290488 (2016)
BindingDB Entry DOI: 10.7270/Q2GF0SCW |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50250501

(CHEMBL4078562)Show SMILES OC(=O)c1ccc(NC(=O)[C@H]2N(CCc3c2cccc3N2CCOCC2)C(=O)\C=C\c2c(F)c(Cl)ccc2-n2cnnn2)cc1 |r| Show InChI InChI=1S/C31H27ClFN7O5/c32-24-9-10-26(40-18-34-36-37-40)23(28(24)33)8-11-27(41)39-13-12-21-22(2-1-3-25(21)38-14-16-45-17-15-38)29(39)30(42)35-20-6-4-19(5-7-20)31(43)44/h1-11,18,29H,12-17H2,(H,35,42)(H,43,44)/b11-8+/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method |
J Med Chem 60: 9703-9723 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01171
BindingDB Entry DOI: 10.7270/Q2K64MHN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50180197
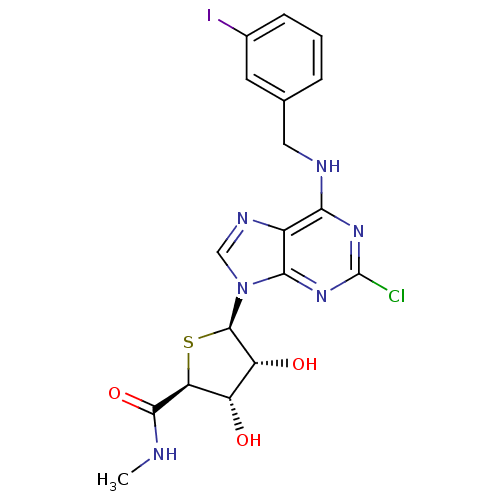
((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...)Show SMILES CNC(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C18H18ClIN6O3S/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]I-AB-MECA from human recombinant adenosine A3 receptor expressed in CHO cells by liquid scintillation counting |
Bioorg Med Chem 17: 3733-8 (2009)
Article DOI: 10.1016/j.bmc.2009.03.034
BindingDB Entry DOI: 10.7270/Q2V69JMJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50180197

((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...)Show SMILES CNC(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C18H18ClIN6O3S/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
J Med Chem 55: 342-56 (2012)
Article DOI: 10.1021/jm201229j
BindingDB Entry DOI: 10.7270/Q2VQ334S |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50180197

((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...)Show SMILES CNC(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C18H18ClIN6O3S/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells |
J Med Chem 51: 6609-13 (2008)
Article DOI: 10.1021/jm8008647
BindingDB Entry DOI: 10.7270/Q2XG9QZG |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50250490
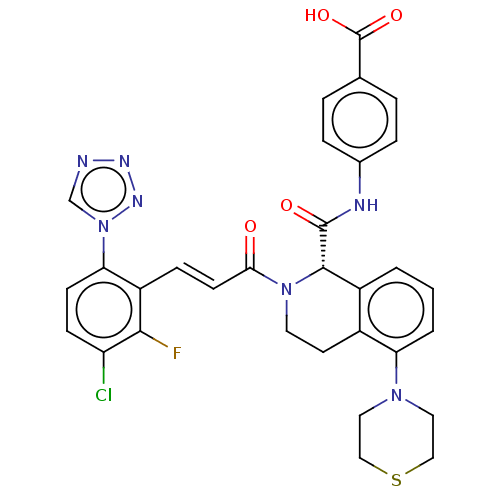
(CHEMBL4087166)Show SMILES OC(=O)c1ccc(NC(=O)[C@H]2N(CCc3c2cccc3N2CCSCC2)C(=O)\C=C\c2c(F)c(Cl)ccc2-n2cnnn2)cc1 |r| Show InChI InChI=1S/C31H27ClFN7O4S/c32-24-9-10-26(40-18-34-36-37-40)23(28(24)33)8-11-27(41)39-13-12-21-22(2-1-3-25(21)38-14-16-45-17-15-38)29(39)30(42)35-20-6-4-19(5-7-20)31(43)44/h1-11,18,29H,12-17H2,(H,35,42)(H,43,44)/b11-8+/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method |
J Med Chem 60: 9703-9723 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01171
BindingDB Entry DOI: 10.7270/Q2K64MHN |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50512455
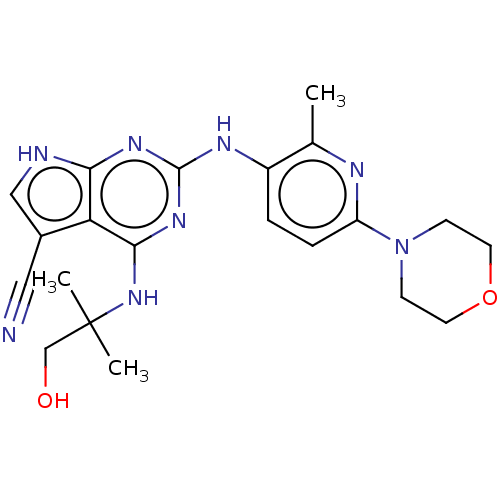
(CHEMBL4436510)Show SMILES Cc1nc(ccc1Nc1nc(NC(C)(C)CO)c2c(c[nH]c2n1)C#N)N1CCOCC1 Show InChI InChI=1S/C21H26N8O2/c1-13-15(4-5-16(24-13)29-6-8-31-9-7-29)25-20-26-18-17(14(10-22)11-23-18)19(27-20)28-21(2,3)12-30/h4-5,11,30H,6-9,12H2,1-3H3,(H3,23,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 (510 to 857 residues) catalytic domain (unknown origin) expressed in Escherichia coli after 15 mins by mass-spectrometry analysis |
Eur J Med Chem 175: 247-268 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.047
BindingDB Entry DOI: 10.7270/Q21V5J8N |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide
(RAT) | BDBM50250023
(CHEMBL500280 | [Ala2]PACAP(1-38) | [Ala2]PACAP38)Show SMILES CC[C@H](C)[C@H](NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C203H331N63O52S/c1-19-109(12)162(262-156(278)100-230-171(290)148(96-157(279)280)250-166(285)111(14)233-169(288)125(211)94-120-98-224-103-231-120)198(317)258-146(89-115-41-21-20-22-42-115)192(311)266-163(114(17)269)199(318)259-149(97-158(281)282)191(310)261-151(102-268)194(313)255-145(93-119-60-68-124(273)69-61-119)189(308)260-150(101-267)193(312)244-135(52-39-84-227-202(220)221)181(300)253-143(91-117-56-64-122(271)65-57-117)187(306)243-133(50-37-82-225-200(216)217)177(296)238-128(45-25-32-77-206)174(293)246-138(71-73-153(213)275)183(302)247-139(74-86-319-18)172(291)234-113(16)168(287)263-159(106(6)7)195(314)248-131(48-28-35-80-209)176(295)239-130(47-27-34-79-208)178(297)254-144(92-118-58-66-123(272)67-59-118)188(307)251-141(88-105(4)5)185(304)235-110(13)165(284)232-112(15)167(286)264-160(107(8)9)197(316)257-140(87-104(2)3)170(289)229-99-155(277)236-127(44-24-31-76-205)173(292)240-134(51-38-83-226-201(218)219)180(299)252-142(90-116-54-62-121(270)63-55-116)186(305)242-129(46-26-33-78-207)175(294)245-137(70-72-152(212)274)182(301)241-136(53-40-85-228-203(222)223)184(303)265-161(108(10)11)196(315)249-132(49-29-36-81-210)179(298)256-147(95-154(214)276)190(309)237-126(164(215)283)43-23-30-75-204/h20-22,41-42,54-69,98,103-114,125-151,159-163,267-273H,19,23-40,43-53,70-97,99-102,204-211H2,1-18H3,(H2,212,274)(H2,213,275)(H2,214,276)(H2,215,283)(H,224,231)(H,229,289)(H,230,290)(H,232,284)(H,233,288)(H,234,291)(H,235,304)(H,236,277)(H,237,309)(H,238,296)(H,239,295)(H,240,292)(H,241,301)(H,242,305)(H,243,306)(H,244,312)(H,245,294)(H,246,293)(H,247,302)(H,248,314)(H,249,315)(H,250,285)(H,251,307)(H,252,299)(H,253,300)(H,254,297)(H,255,313)(H,256,298)(H,257,316)(H,258,317)(H,259,318)(H,260,308)(H,261,310)(H,262,278)(H,263,287)(H,264,286)(H,265,303)(H,266,311)(H,279,280)(H,281,282)(H4,216,217,225)(H4,218,219,226)(H4,220,221,227)(H4,222,223,228)/t109-,110-,111-,112-,113-,114+,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,142-,143-,144-,145-,146-,147-,148-,149-,150-,151-,159-,160-,161-,162-,163-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 1189-95 (1994)
Article DOI: 10.1016/s0028-3908(05)80009-7
BindingDB Entry DOI: 10.7270/Q2251GP3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50512458
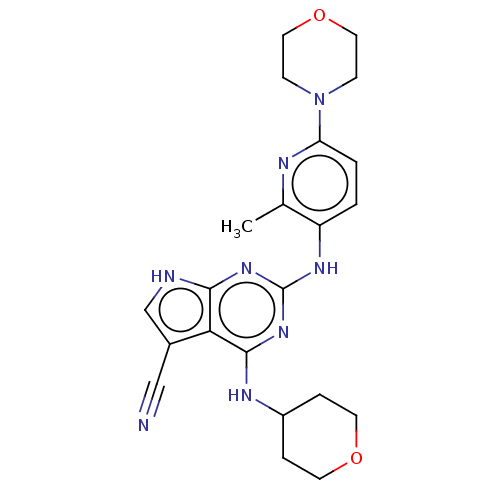
(CHEMBL4450240)Show SMILES Cc1nc(ccc1Nc1nc(NC2CCOCC2)c2c(c[nH]c2n1)C#N)N1CCOCC1 Show InChI InChI=1S/C22H26N8O2/c1-14-17(2-3-18(25-14)30-6-10-32-11-7-30)27-22-28-20-19(15(12-23)13-24-20)21(29-22)26-16-4-8-31-9-5-16/h2-3,13,16H,4-11H2,1H3,(H3,24,26,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 (510 to 857 residues) catalytic domain (unknown origin) expressed in Escherichia coli after 15 mins by mass-spectrometry analysis |
Eur J Med Chem 175: 247-268 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.047
BindingDB Entry DOI: 10.7270/Q21V5J8N |
More data for this
Ligand-Target Pair | |
Ras guanyl-releasing protein 3
(Homo sapiens (Human)) | BDBM50280750

(CHEMBL4160361)Show SMILES CC(C)(C)C(=O)OCC1(CO)C\C(=C/c2ccc(Cl)cc2)C(=O)O1 Show InChI InChI=1S/C18H21ClO5/c1-17(2,3)16(22)23-11-18(10-20)9-13(15(21)24-18)8-12-4-6-14(19)7-5-12/h4-8,20H,9-11H2,1-3H3/b13-8+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting |
J Med Chem 61: 6261-6276 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00661
BindingDB Entry DOI: 10.7270/Q28P632D |
More data for this
Ligand-Target Pair | |
RAS guanyl-releasing protein 1
(Homo sapiens (Human)) | BDBM50244449
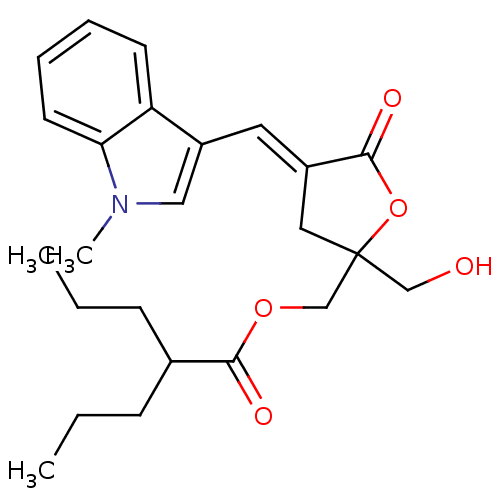
((E)-(2-(hydroxymethyl)-4-((1-methyl-1H-indol-3-yl)...)Show SMILES CCCC(CCC)C(=O)OCC1(CO)C\C(=C/c2cn(C)c3ccccc23)C(=O)O1 Show InChI InChI=1S/C24H31NO5/c1-4-8-17(9-5-2)22(27)29-16-24(15-26)13-18(23(28)30-24)12-19-14-25(3)21-11-7-6-10-20(19)21/h6-7,10-12,14,17,26H,4-5,8-9,13,15-16H2,1-3H3/b18-12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Industrial Technology
Curated by ChEMBL
| Assay Description
Displacement of [20-3H]phorbol 12,13-dibutyrate from RasGRP1 (unknown origin) in the presence of porcine brain phosphatidylserine by scintillation co... |
Bioorg Med Chem 25: 2971-2980 (2017)
Article DOI: 10.1016/j.bmc.2017.03.022
BindingDB Entry DOI: 10.7270/Q28P62P2 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398012
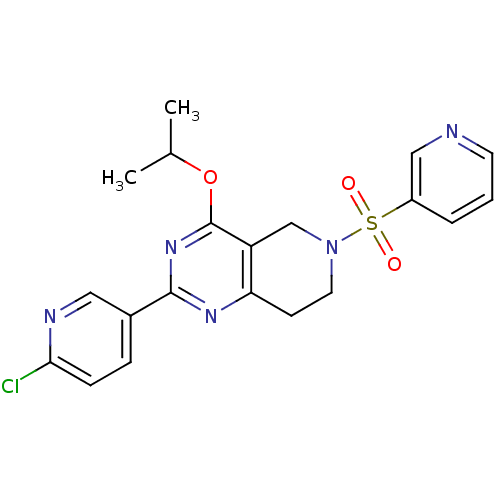
(CHEMBL2180422 | US8492392, 1-17)Show SMILES CC(C)Oc1nc(nc2CCN(Cc12)S(=O)(=O)c1cccnc1)-c1ccc(Cl)nc1 Show InChI InChI=1S/C20H20ClN5O3S/c1-13(2)29-20-16-12-26(30(27,28)15-4-3-8-22-11-15)9-7-17(16)24-19(25-20)14-5-6-18(21)23-10-14/h3-6,8,10-11,13H,7,9,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide
(RAT) | BDBM82447

([DAla2]PACAP(1-38))Show SMILES CCC(C)[C@H](NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C203H331N63O52S/c1-19-109(12)162(262-156(278)100-230-171(290)148(96-157(279)280)250-166(285)111(14)233-169(288)125(211)94-120-98-224-103-231-120)198(317)258-146(89-115-41-21-20-22-42-115)192(311)266-163(114(17)269)199(318)259-149(97-158(281)282)191(310)261-151(102-268)194(313)255-145(93-119-60-68-124(273)69-61-119)189(308)260-150(101-267)193(312)244-135(52-39-84-227-202(220)221)181(300)253-143(91-117-56-64-122(271)65-57-117)187(306)243-133(50-37-82-225-200(216)217)177(296)238-128(45-25-32-77-206)174(293)246-138(71-73-153(213)275)183(302)247-139(74-86-319-18)172(291)234-113(16)168(287)263-159(106(6)7)195(314)248-131(48-28-35-80-209)176(295)239-130(47-27-34-79-208)178(297)254-144(92-118-58-66-123(272)67-59-118)188(307)251-141(88-105(4)5)185(304)235-110(13)165(284)232-112(15)167(286)264-160(107(8)9)197(316)257-140(87-104(2)3)170(289)229-99-155(277)236-127(44-24-31-76-205)173(292)240-134(51-38-83-226-201(218)219)180(299)252-142(90-116-54-62-121(270)63-55-116)186(305)242-129(46-26-33-78-207)175(294)245-137(70-72-152(212)274)182(301)241-136(53-40-85-228-203(222)223)184(303)265-161(108(10)11)196(315)249-132(49-29-36-81-210)179(298)256-147(95-154(214)276)190(309)237-126(164(215)283)43-23-30-75-204/h20-22,41-42,54-69,98,103-114,125-151,159-163,267-273H,19,23-40,43-53,70-97,99-102,204-211H2,1-18H3,(H2,212,274)(H2,213,275)(H2,214,276)(H2,215,283)(H,224,231)(H,229,289)(H,230,290)(H,232,284)(H,233,288)(H,234,291)(H,235,304)(H,236,277)(H,237,309)(H,238,296)(H,239,295)(H,240,292)(H,241,301)(H,242,305)(H,243,306)(H,244,312)(H,245,294)(H,246,293)(H,247,302)(H,248,314)(H,249,315)(H,250,285)(H,251,307)(H,252,299)(H,253,300)(H,254,297)(H,255,313)(H,256,298)(H,257,316)(H,258,317)(H,259,318)(H,260,308)(H,261,310)(H,262,278)(H,263,287)(H,264,286)(H,265,303)(H,266,311)(H,279,280)(H,281,282)(H4,216,217,225)(H4,218,219,226)(H4,220,221,227)(H4,222,223,228)/t109?,110-,111+,112-,113-,114+,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,142-,143-,144-,145-,146-,147-,148-,149-,150-,151-,159-,160-,161-,162-,163-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 1189-95 (1994)
Article DOI: 10.1016/s0028-3908(05)80009-7
BindingDB Entry DOI: 10.7270/Q2251GP3 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM349975
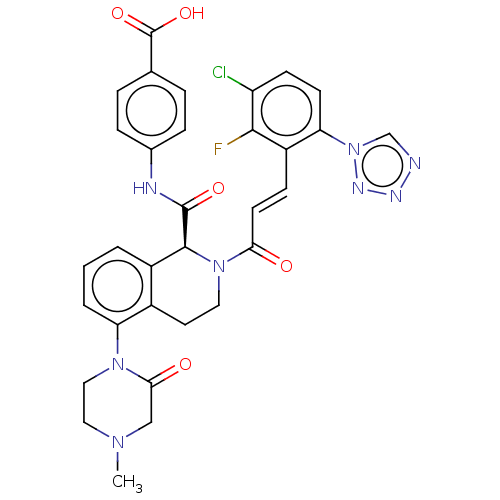
((S,E)-4-(2-(3-(3-chloro-2-fluoro-6-(1H-tetrazol-1-...)Show SMILES CN1CCN(C(=O)C1)c1cccc2[C@H](N(CCc12)C(=O)\C=C\c1c(F)c(Cl)ccc1-n1cnnn1)C(=O)Nc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C32H28ClFN8O5/c1-39-15-16-40(28(44)17-39)25-4-2-3-22-21(25)13-14-41(30(22)31(45)36-20-7-5-19(6-8-20)32(46)47)27(43)12-9-23-26(42-18-35-37-38-42)11-10-24(33)29(23)34/h2-12,18,30H,13-17H2,1H3,(H,36,45)(H,46,47)/b12-9+/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method |
J Med Chem 60: 9703-9723 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01171
BindingDB Entry DOI: 10.7270/Q2K64MHN |
More data for this
Ligand-Target Pair | |
Opioid growth factor receptor-like protein 1
(Homo sapiens (Human)) | BDBM172430

(US9090618, ZA53 | US9598411, Ref. No. ZA53)Show SMILES O=C1CC[C@@H](Cc2nc3ccccc3n2[C@@H]2C[C@@H]3CCC[C@H](C2)N3[C@H]2C[C@@H]3C[C@H](C2)CCCC3)N1 |r,THB:24:23:15.16.22:18.20.19| Show InChI InChI=1S/C30H42N4O/c35-30-13-12-22(31-30)17-29-32-27-10-3-4-11-28(27)34(29)26-18-23-8-5-9-24(19-26)33(23)25-15-20-6-1-2-7-21(14-20)16-25/h3-4,10-11,20-26H,1-2,5-9,12-19H2,(H,31,35)/t20-,21+,22-,23-,24+,25-,26+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 ug membrane protein in a fin... |
US Patent US9090618 (2015)
BindingDB Entry DOI: 10.7270/Q2G73CGB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM172430

(US9090618, ZA53 | US9598411, Ref. No. ZA53)Show SMILES O=C1CC[C@@H](Cc2nc3ccccc3n2[C@@H]2C[C@@H]3CCC[C@H](C2)N3[C@H]2C[C@@H]3C[C@H](C2)CCCC3)N1 |r,THB:24:23:15.16.22:18.20.19| Show InChI InChI=1S/C30H42N4O/c35-30-13-12-22(31-30)17-29-32-27-10-3-4-11-28(27)34(29)26-18-23-8-5-9-24(19-26)33(23)25-15-20-6-1-2-7-21(14-20)16-25/h3-4,10-11,20-26H,1-2,5-9,12-19H2,(H,31,35)/t20-,21+,22-,23-,24+,25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
US Patent
| Assay Description
δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... |
US Patent US9598411 (2017)
BindingDB Entry DOI: 10.7270/Q2ZG6V9S |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Rattus norvegicus) | BDBM50180197

((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...)Show SMILES CNC(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C18H18ClIN6O3S/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from rat adenosine A3 receptor expressed in CHO cells |
J Med Chem 51: 6609-13 (2008)
Article DOI: 10.1021/jm8008647
BindingDB Entry DOI: 10.7270/Q2XG9QZG |
More data for this
Ligand-Target Pair | |
Ras guanyl-releasing protein 3
(Homo sapiens (Human)) | BDBM50244449

((E)-(2-(hydroxymethyl)-4-((1-methyl-1H-indol-3-yl)...)Show SMILES CCCC(CCC)C(=O)OCC1(CO)C\C(=C/c2cn(C)c3ccccc23)C(=O)O1 Show InChI InChI=1S/C24H31NO5/c1-4-8-17(9-5-2)22(27)29-16-24(15-26)13-18(23(28)30-24)12-19-14-25(3)21-11-7-6-10-20(19)21/h6-7,10-12,14,17,26H,4-5,8-9,13,15-16H2,1-3H3/b18-12+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting |
J Med Chem 61: 6261-6276 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00661
BindingDB Entry DOI: 10.7270/Q28P632D |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50582798

(CHEMBL5082323)Show SMILES C[C@@H]1CCC[C@H](N2CCC(=CC2=O)c2cc(Cl)ccc2-n2ccnn2)c2cc(ccn2)-c2c(NC1=O)cnn2C(F)F |r,c:9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00613
BindingDB Entry DOI: 10.7270/Q20005Z7 |
More data for this
Ligand-Target Pair | |
Opioid growth factor receptor-like protein 1
(Homo sapiens (Human)) | BDBM220363
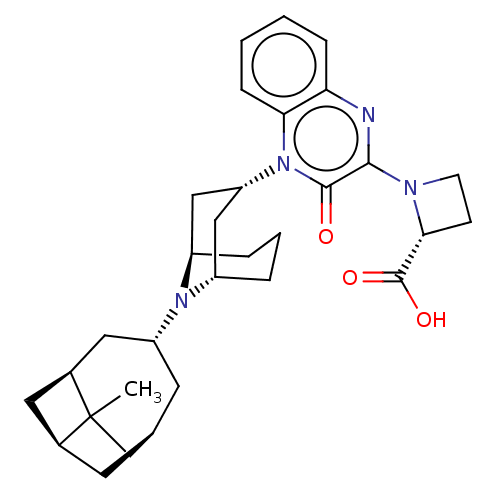
(US9290488, B20a(i))Show SMILES CC12C[C@@H]3C[C@H]1C[C@H]2C[C@@H](C3)N1[C@H]2CCC[C@@H]1C[C@@H](C2)n1c2ccccc2nc(N2CC[C@@H]2C(O)=O)c1=O |r,THB:9:11:18.19.17:13.15.14| Show InChI InChI=1S/C31H40N4O3/c1-31-17-18-11-19(31)13-20(31)14-23(12-18)34-21-5-4-6-22(34)16-24(15-21)35-26-8-3-2-7-25(26)32-28(29(35)36)33-10-9-27(33)30(37)38/h2-3,7-8,18-24,27H,4-6,9-17H2,1H3,(H,37,38)/t18-,19+,20+,21-,22+,23-,24+,27-,31?/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.890 | -51.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... |
US Patent US9290488 (2016)
BindingDB Entry DOI: 10.7270/Q2GF0SCW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398013
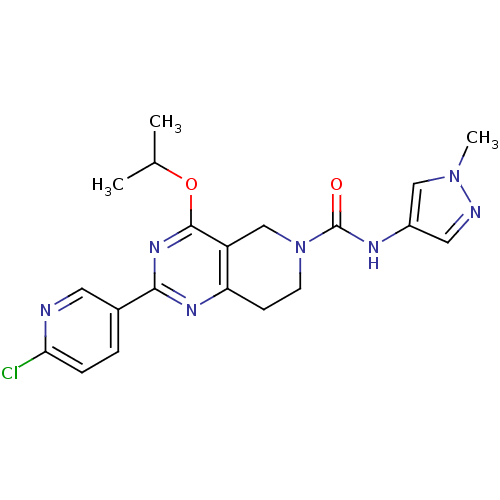
(CHEMBL2180421 | US8492392, K-2)Show SMILES CC(C)Oc1nc(nc2CCN(Cc12)C(=O)Nc1cnn(C)c1)-c1ccc(Cl)nc1 Show InChI InChI=1S/C20H22ClN7O2/c1-12(2)30-19-15-11-28(20(29)24-14-9-23-27(3)10-14)7-6-16(15)25-18(26-19)13-4-5-17(21)22-8-13/h4-5,8-10,12H,6-7,11H2,1-3H3,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
Opioid growth factor receptor-like protein 1
(Homo sapiens (Human)) | BDBM172419
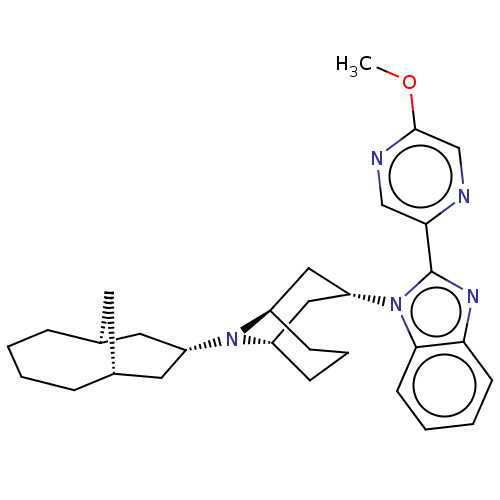
(US9090618, ZA42 | US9598411, Ref. No. ZA42)Show SMILES COc1cnc(cn1)-c1nc2ccccc2n1[C@@H]1C[C@@H]2CCC[C@H](C1)N2[C@H]1C[C@@H]2C[C@H](C1)CCCC2 |r,THB:26:25:17.18.24:20.22.21| Show InChI InChI=1S/C30H39N5O/c1-36-29-19-31-27(18-32-29)30-33-26-11-4-5-12-28(26)35(30)25-16-22-9-6-10-23(17-25)34(22)24-14-20-7-2-3-8-21(13-20)15-24/h4-5,11-12,18-25H,2-3,6-10,13-17H2,1H3/t20-,21+,22-,23+,24-,25+ | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.920 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 ug membrane protein in a fin... |
US Patent US9090618 (2015)
BindingDB Entry DOI: 10.7270/Q2G73CGB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM172419

(US9090618, ZA42 | US9598411, Ref. No. ZA42)Show SMILES COc1cnc(cn1)-c1nc2ccccc2n1[C@@H]1C[C@@H]2CCC[C@H](C1)N2[C@H]1C[C@@H]2C[C@H](C1)CCCC2 |r,THB:26:25:17.18.24:20.22.21| Show InChI InChI=1S/C30H39N5O/c1-36-29-19-31-27(18-32-29)30-33-26-11-4-5-12-28(26)35(30)25-16-22-9-6-10-23(17-25)34(22)24-14-20-7-2-3-8-21(13-20)15-24/h4-5,11-12,18-25H,2-3,6-10,13-17H2,1H3/t20-,21+,22-,23+,24-,25+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
US Patent
| Assay Description
δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... |
US Patent US9598411 (2017)
BindingDB Entry DOI: 10.7270/Q2ZG6V9S |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398011
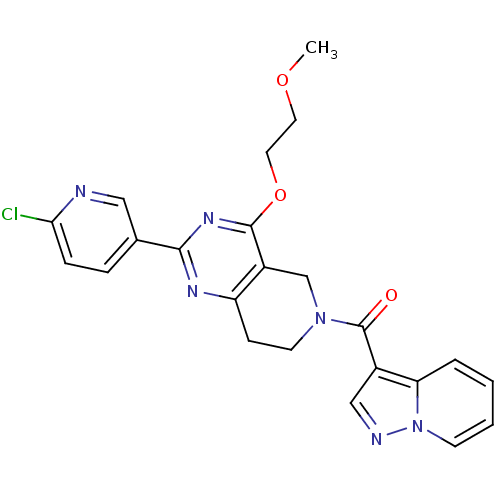
(CHEMBL2180423 | US8492392, O-2)Show SMILES COCCOc1nc(nc2CCN(Cc12)C(=O)c1cnn2ccccc12)-c1ccc(Cl)nc1 Show InChI InChI=1S/C23H21ClN6O3/c1-32-10-11-33-22-17-14-29(23(31)16-13-26-30-8-3-2-4-19(16)30)9-7-18(17)27-21(28-22)15-5-6-20(24)25-12-15/h2-6,8,12-13H,7,9-11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398004
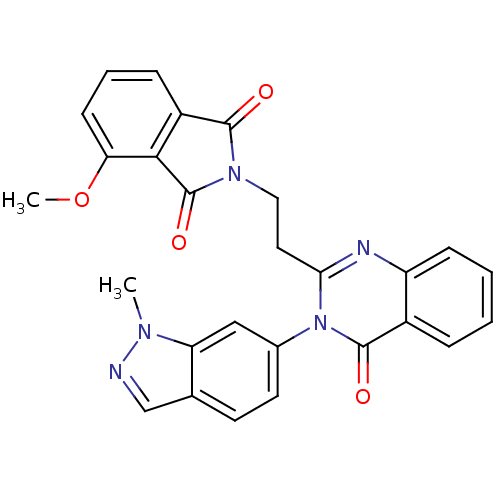
(CHEMBL2180401)Show SMILES COc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc4cnn(C)c4c3)C(=O)c12 Show InChI InChI=1S/C27H21N5O4/c1-30-21-14-17(11-10-16(21)15-28-30)32-23(29-20-8-4-3-6-18(20)26(32)34)12-13-31-25(33)19-7-5-9-22(36-2)24(19)27(31)35/h3-11,14-15H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50293031
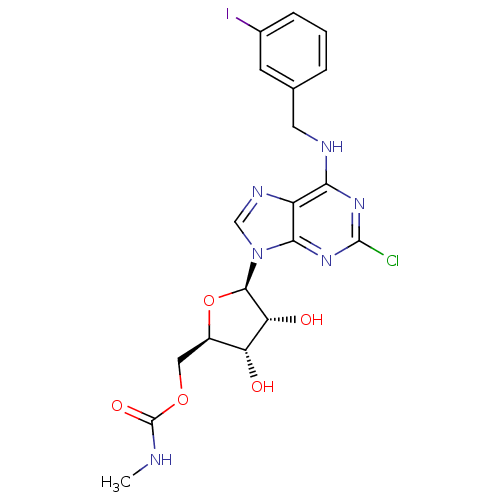
(2-chloro-N6-(3-iodobenzyl)-5'-N-methylcarbamoylade...)Show SMILES CNC(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C19H20ClIN6O5/c1-22-19(30)31-7-11-13(28)14(29)17(32-11)27-8-24-12-15(25-18(20)26-16(12)27)23-6-9-3-2-4-10(21)5-9/h2-5,8,11,13-14,17,28-29H,6-7H2,1H3,(H,22,30)(H,23,25,26)/t11-,13-,14-,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
J Med Chem 55: 342-56 (2012)
Article DOI: 10.1021/jm201229j
BindingDB Entry DOI: 10.7270/Q2VQ334S |
More data for this
Ligand-Target Pair | |
Ras guanyl-releasing protein 3
(Homo sapiens (Human)) | BDBM50280765
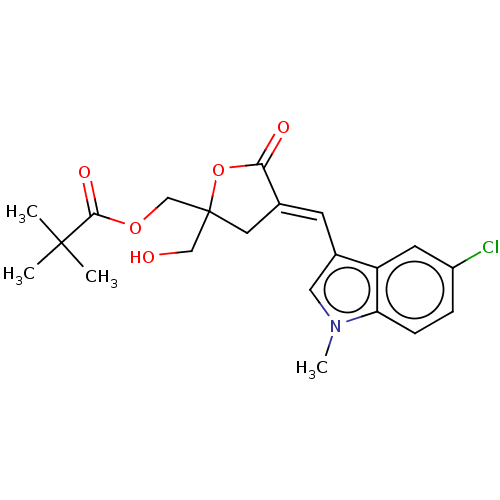
(CHEMBL4160647)Show SMILES Cn1cc(\C=C2/CC(CO)(COC(=O)C(C)(C)C)OC2=O)c2cc(Cl)ccc12 Show InChI InChI=1S/C21H24ClNO5/c1-20(2,3)19(26)27-12-21(11-24)9-13(18(25)28-21)7-14-10-23(4)17-6-5-15(22)8-16(14)17/h5-8,10,24H,9,11-12H2,1-4H3/b13-7+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting |
J Med Chem 61: 6261-6276 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00661
BindingDB Entry DOI: 10.7270/Q28P632D |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118812
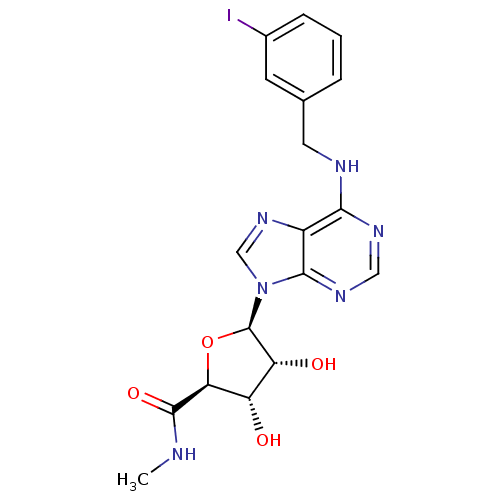
((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells |
J Med Chem 51: 6609-13 (2008)
Article DOI: 10.1021/jm8008647
BindingDB Entry DOI: 10.7270/Q2XG9QZG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50240887
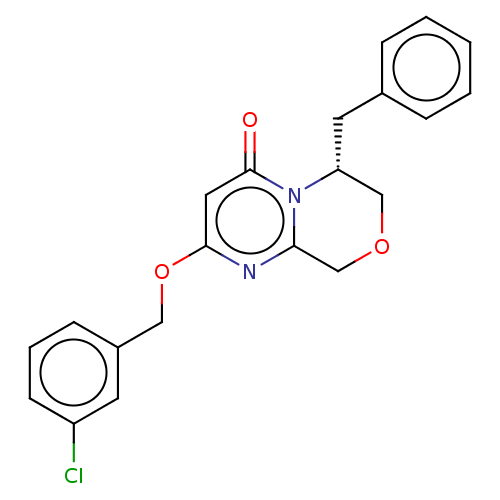
(CHEMBL4066731)Show SMILES Clc1cccc(COc2cc(=O)n3[C@H](Cc4ccccc4)COCc3n2)c1 |r| Show InChI InChI=1S/C21H19ClN2O3/c22-17-8-4-7-16(9-17)12-27-20-11-21(25)24-18(13-26-14-19(24)23-20)10-15-5-2-1-3-6-15/h1-9,11,18H,10,12-14H2/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting |
J Med Chem 60: 7764-7780 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00604
BindingDB Entry DOI: 10.7270/Q2DJ5HS8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM303064
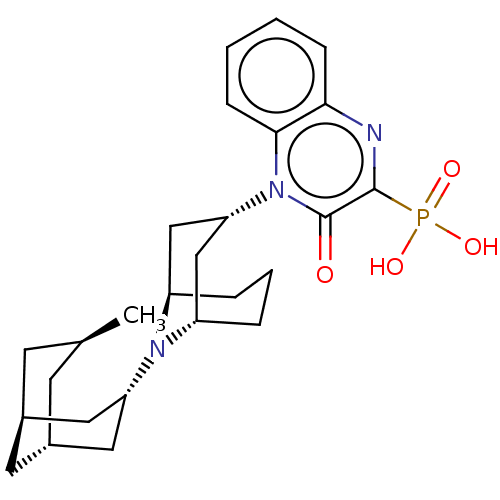
(US9598447, Ref No. FF)Show SMILES C[C@H]1C[C@H]2C[C@@H](C1)C[C@@H](C2)N1[C@H]2CCC[C@@H]1C[C@@H](C2)n1c2ccccc2nc(c1=O)P(O)(O)=O |r,THB:8:10:17.18.16:12.14.13| Show InChI InChI=1S/C26H36N3O4P/c1-16-9-17-11-18(10-16)13-21(12-17)28-19-5-4-6-20(28)15-22(14-19)29-24-8-3-2-7-23(24)27-25(26(29)30)34(31,32)33/h2-3,7-8,16-22H,4-6,9-15H2,1H3,(H2,31,32,33)/t16-,17-,18+,19-,20+,21+,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in ... |
US Patent US9598447 (2017)
BindingDB Entry DOI: 10.7270/Q2P27168 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50439674
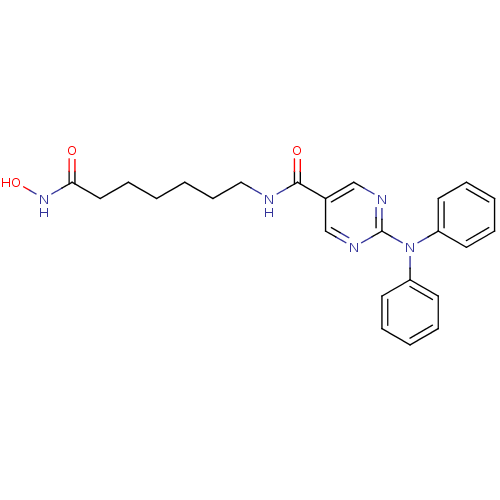
(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00853
BindingDB Entry DOI: 10.7270/Q2XD15N3 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide
(RAT) | BDBM82450

([AcHis1]PACAP(1-27))Show SMILES CCC(C)[C@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O |r,wU:52.53,67.68,85.87,102.104,125.128,143.146,156.159,172.175,193.197,206.211,218.222,53.56,4.4,12.17,27.34,wD:41.41,59.60,73.74,91.93,114.117,151.155,181.184,201.206,211.215,134.137,163.166,21.23,(-14.46,-50.6,;-14.44,-52.33,;-15.67,-53.24,;-17,-52.47,;-15.67,-54.78,;-17,-55.55,;-18.34,-54.78,;-18.34,-53.24,;-19.43,-55.87,;-20.76,-55.1,;-22.14,-55.94,;-22.14,-57.48,;-23.47,-55.17,;-23.47,-53.63,;-24.56,-52.54,;-24.56,-51,;-25.89,-50.23,;-23.23,-50.23,;-24.56,-56.25,;-25.89,-55.48,;-25.96,-54.17,;-27.23,-56.25,;-27.23,-57.79,;-25.89,-58.56,;-28.56,-55.48,;-29.89,-56.25,;-29.89,-57.79,;-31.23,-55.48,;-31.23,-53.94,;-32,-52.61,;-32,-51.07,;-34.67,-51.07,;-34.67,-52.61,;-33.33,-53.38,;-32.54,-56.66,;-34.03,-56.26,;-34.8,-54.92,;-35.12,-57.35,;-14.34,-55.55,;-14.41,-57.12,;-13,-54.78,;-11.67,-55.55,;-11.67,-57.09,;-10.34,-57.86,;-9,-57.09,;-7.66,-57.86,;-7.66,-59.41,;-9,-60.18,;-10.34,-59.41,;-10.34,-54.78,;-10.34,-53.24,;-9,-55.55,;-7.67,-54.78,;-7.67,-53.24,;-9,-52.47,;-6.33,-52.47,;-6.33,-55.55,;-6.33,-57.09,;-5,-54.78,;-3.67,-55.55,;-3.67,-57.09,;-2.33,-57.86,;-1,-57.09,;-2.33,-59.4,;-2.33,-54.78,;-2.33,-53.24,;-1,-55.55,;.33,-54.78,;.33,-53.24,;1.67,-52.47,;1.67,-55.55,;1.67,-57.09,;3,-54.78,;4.34,-55.55,;4.34,-57.09,;5.67,-57.86,;7.01,-57.09,;8.35,-57.86,;8.35,-59.41,;9.68,-60.18,;7.01,-60.18,;5.67,-59.41,;5.67,-54.78,;5.67,-53.24,;7,-55.55,;8.34,-54.78,;8.34,-53.24,;9.67,-52.47,;9.67,-55.55,;9.67,-57.09,;11,-54.78,;12.34,-55.55,;12.34,-57.09,;13.67,-57.86,;13.67,-59.4,;15,-60.17,;15,-61.71,;13.67,-62.48,;16.34,-62.48,;13.67,-54.78,;13.67,-53.24,;15,-55.55,;16.34,-54.78,;16.34,-53.24,;17.67,-52.47,;19.01,-53.25,;20.35,-52.47,;20.35,-50.93,;21.68,-50.16,;19.01,-50.16,;17.67,-50.93,;17.67,-55.55,;17.67,-57.09,;19.01,-54.78,;20.34,-55.55,;20.34,-57.09,;21.67,-57.86,;21.67,-59.4,;23.01,-60.17,;23.01,-61.71,;21.67,-62.48,;24.34,-62.48,;21.67,-54.78,;21.67,-53.24,;23.01,-55.55,;24.34,-54.78,;24.34,-53.24,;25.67,-52.47,;25.67,-50.93,;27.01,-50.16,;27.01,-48.62,;25.67,-55.55,;25.67,-57.09,;27.01,-54.78,;28.34,-55.55,;28.34,-57.09,;29.67,-57.86,;29.67,-59.4,;28.34,-60.17,;31.01,-60.17,;29.67,-54.78,;29.67,-53.24,;31.01,-55.55,;32.34,-54.78,;32.34,-53.24,;33.68,-52.47,;33.68,-50.93,;35.01,-50.16,;33.68,-55.55,;33.68,-57.09,;35.01,-54.78,;36.34,-55.55,;36.34,-57.09,;37.68,-54.78,;37.68,-53.24,;39.01,-55.55,;40.34,-54.78,;40.34,-53.24,;41.68,-52.47,;39.01,-52.47,;41.68,-55.55,;41.68,-57.09,;43.01,-54.78,;44.34,-55.55,;44.34,-57.09,;45.68,-57.86,;45.68,-59.4,;47.01,-60.17,;47.01,-61.71,;45.68,-54.78,;45.68,-53.24,;47.01,-55.55,;48.35,-54.78,;48.35,-53.24,;49.68,-52.47,;49.68,-50.93,;51.01,-50.16,;51.01,-48.62,;49.68,-55.55,;49.68,-57.09,;51.01,-54.78,;52.35,-55.55,;52.35,-57.09,;53.68,-57.86,;55.02,-57.09,;56.36,-57.86,;56.36,-59.41,;57.69,-60.18,;55.02,-60.18,;53.68,-59.41,;53.68,-54.78,;53.68,-53.24,;55.01,-55.55,;56.35,-54.78,;56.35,-53.24,;57.68,-52.47,;57.68,-50.93,;59.01,-53.24,;57.68,-55.55,;57.68,-57.09,;59.01,-54.78,;60.35,-55.55,;60.35,-57.09,;61.68,-54.78,;61.68,-53.24,;63.02,-55.55,;64.35,-54.78,;64.35,-53.24,;65.68,-55.55,;65.68,-57.09,;67.02,-54.78,;68.35,-55.55,;68.35,-57.09,;69.68,-57.86,;67.02,-57.86,;69.68,-54.78,;69.68,-53.24,;71.02,-55.55,;72.35,-54.78,;72.35,-53.24,;73.68,-52.47,;73.68,-50.93,;75.02,-53.24,;73.68,-55.55,;75.02,-54.78,;73.68,-57.09,)| Show InChI InChI=1S/C145H228N40O40S/c1-17-77(10)116(182-111(195)68-158-122(204)96(49-51-112(196)197)168-138(220)108(70-187)180-134(216)105(163-82(15)190)65-87-67-155-72-159-87)142(224)177-104(61-83-29-19-18-20-30-83)136(218)185-117(81(14)189)143(225)178-106(66-113(198)199)135(217)181-109(71-188)139(221)176-103(64-86-40-46-90(193)47-41-86)133(215)179-107(69-186)137(219)167-95(35-28-57-157-145(153)154)128(210)174-101(62-84-36-42-88(191)43-37-84)131(213)166-94(34-27-56-156-144(151)152)126(208)164-91(31-21-24-53-146)124(206)169-97(48-50-110(149)194)129(211)170-98(52-58-226-16)123(205)161-80(13)121(203)183-114(75(6)7)140(222)171-93(33-23-26-55-148)125(207)165-92(32-22-25-54-147)127(209)175-102(63-85-38-44-89(192)45-39-85)132(214)173-100(60-74(4)5)130(212)162-78(11)119(201)160-79(12)120(202)184-115(76(8)9)141(223)172-99(118(150)200)59-73(2)3/h18-20,29-30,36-47,67,72-81,91-109,114-117,186-189,191-193H,17,21-28,31-35,48-66,68-71,146-148H2,1-16H3,(H2,149,194)(H2,150,200)(H,155,159)(H,158,204)(H,160,201)(H,161,205)(H,162,212)(H,163,190)(H,164,208)(H,165,207)(H,166,213)(H,167,219)(H,168,220)(H,169,206)(H,170,211)(H,171,222)(H,172,223)(H,173,214)(H,174,210)(H,175,209)(H,176,221)(H,177,224)(H,178,225)(H,179,215)(H,180,216)(H,181,217)(H,182,195)(H,183,203)(H,184,202)(H,185,218)(H,196,197)(H,198,199)(H4,151,152,156)(H4,153,154,157)/t77?,78-,79-,80-,81+,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,114-,115-,116-,117-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 1189-95 (1994)
Article DOI: 10.1016/s0028-3908(05)80009-7
BindingDB Entry DOI: 10.7270/Q2251GP3 |
More data for this
Ligand-Target Pair | |
Opioid growth factor receptor-like protein 1
(Homo sapiens (Human)) | BDBM220366
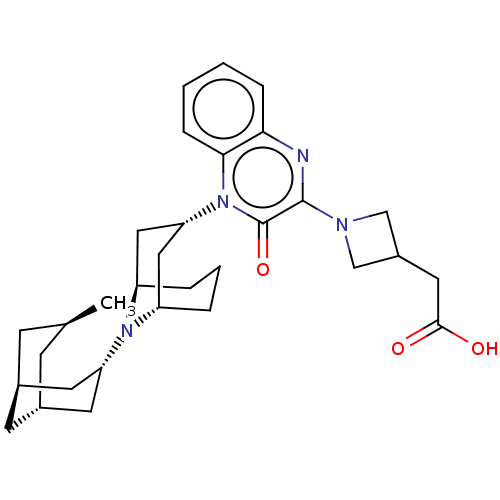
(US9290488, B34a)Show SMILES C[C@H]1C[C@H]2C[C@@H](C1)C[C@@H](C2)N1[C@H]2CCC[C@@H]1C[C@@H](C2)n1c2ccccc2nc(N2CC(CC(O)=O)C2)c1=O |r,THB:8:10:17.18.16:12.14.13| Show InChI InChI=1S/C31H42N4O3/c1-19-9-20-11-21(10-19)13-25(12-20)34-23-5-4-6-24(34)16-26(15-23)35-28-8-3-2-7-27(28)32-30(31(35)38)33-17-22(18-33)14-29(36)37/h2-3,7-8,19-26H,4-6,9-18H2,1H3,(H,36,37)/t19-,20-,21+,23-,24+,25+,26+ | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.03 | -51.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... |
US Patent US9290488 (2016)
BindingDB Entry DOI: 10.7270/Q2GF0SCW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data