

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50369460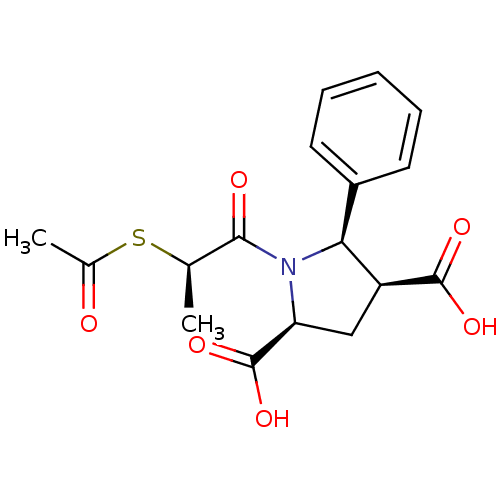 (CHEMBL1788109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Enzyme inhibitory activity towards Angiotensin I converting enzyme | J Med Chem 42: 3743-78 (1999) BindingDB Entry DOI: 10.7270/Q22Z167W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50399749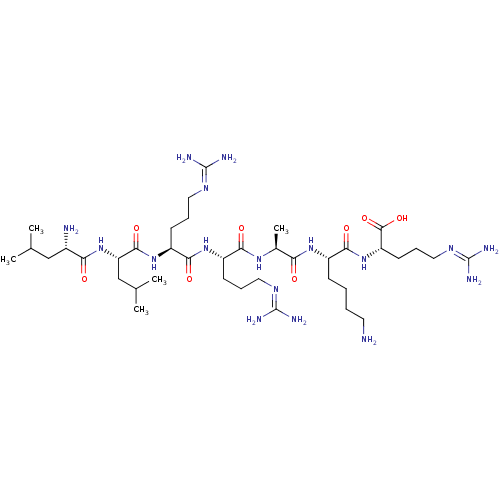 (CHEMBL2179430) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PACE4 expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50399753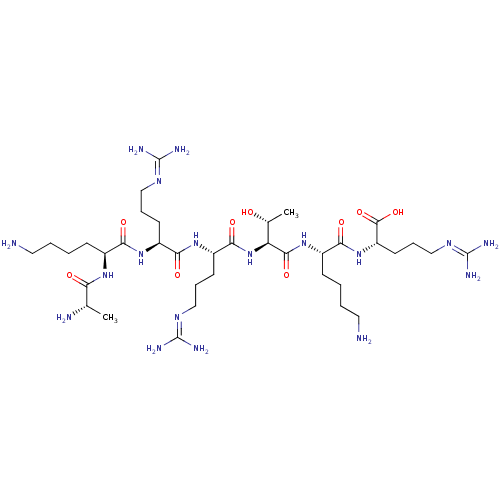 (CHEMBL2179835) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PACE4 expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM21842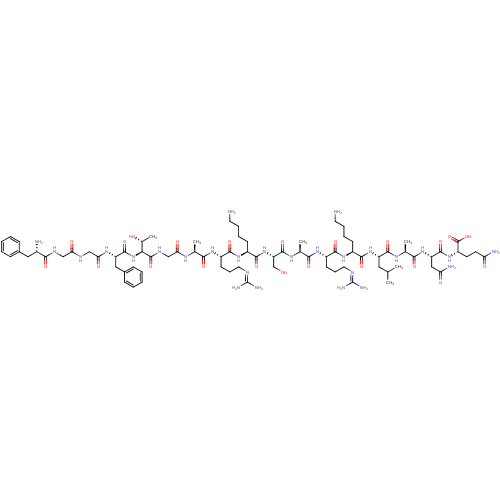 ((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071073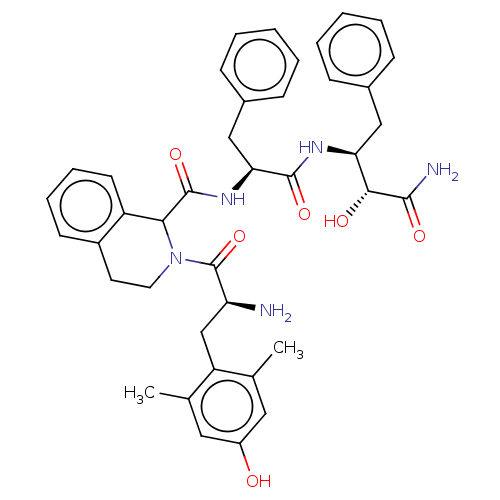 (CHEMBL3409763) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85194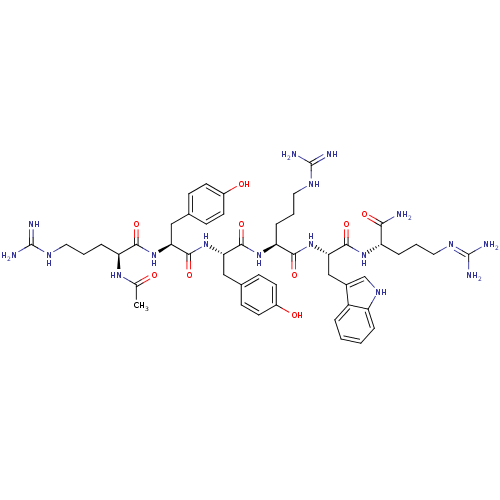 (Ac-RYYRWR-NH2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155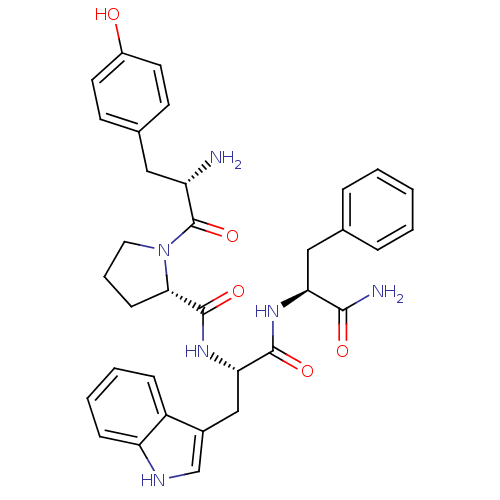 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071072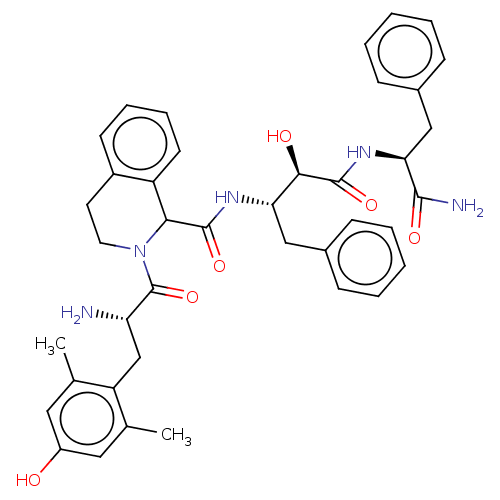 (CHEMBL3409762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85191 (Ac-RYYRWK-NH2 | CAS_200959-47-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50266734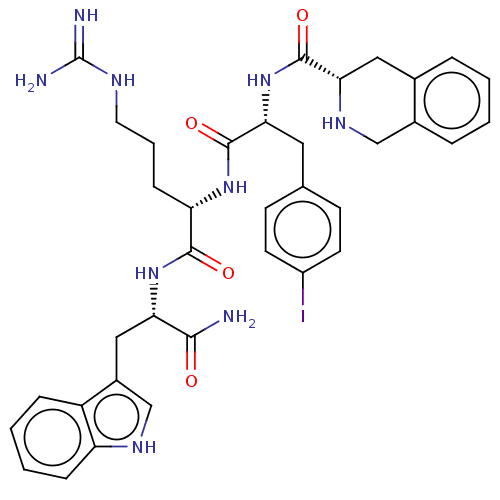 (CHEMBL4096081) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Antagonist activity at human MC4R expressed in CHO cells assessed as inhibition of aplha MSH-induced cAMP activation after 45 mins | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071071 (CHEMBL3409761) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140041 (CHEMBL3765059) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071070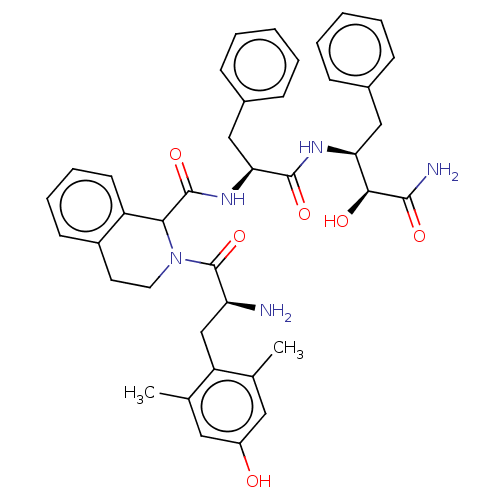 (CHEMBL3409760) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071093 (CHEMBL3409764) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071094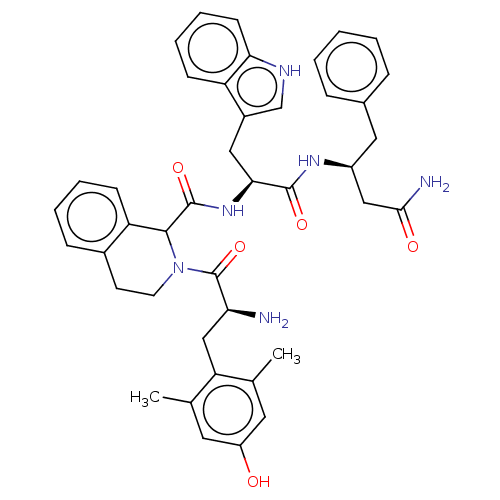 (CHEMBL3409765) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071095 (CHEMBL3409766) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574259 (CHEMBL4865888) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50399754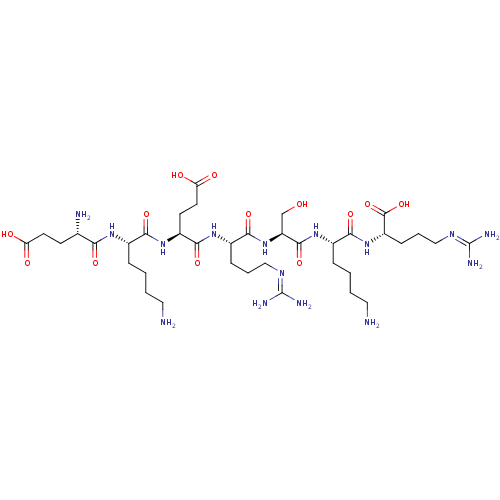 (CHEMBL2179836) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PACE4 expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50399752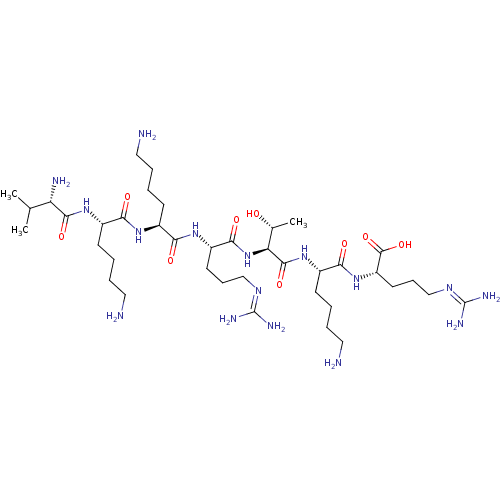 (CHEMBL2179429) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PACE4 expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50399754 (CHEMBL2179836) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant furin expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85193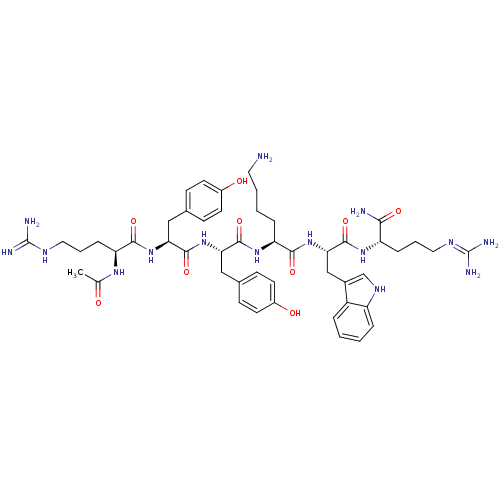 (Ac-RYYKWR-NH2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85192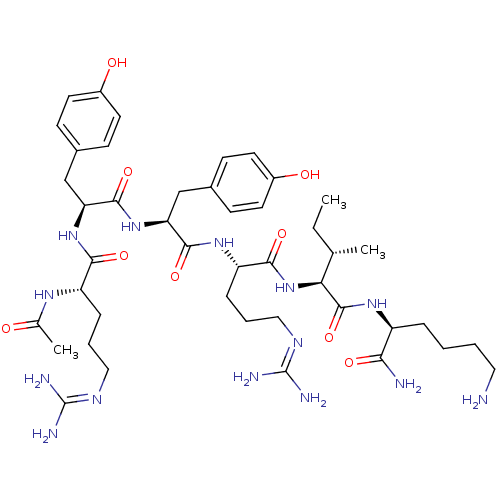 (Ac-RYYRIK-NH2 | CAS_200959-48-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574231 (CHEMBL4850510) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50399753 (CHEMBL2179835) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant furin expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140039 (CHEMBL3764556) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071069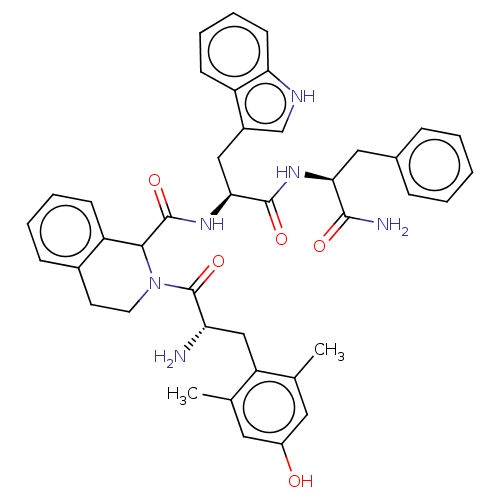 (CHEMBL3409759) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574246 (CHEMBL4852297) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071068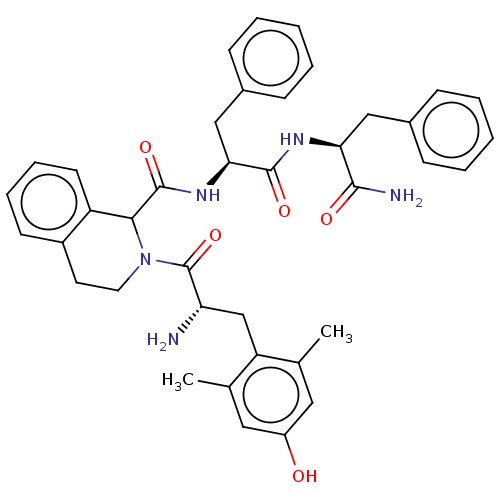 (CHEMBL3409758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50399751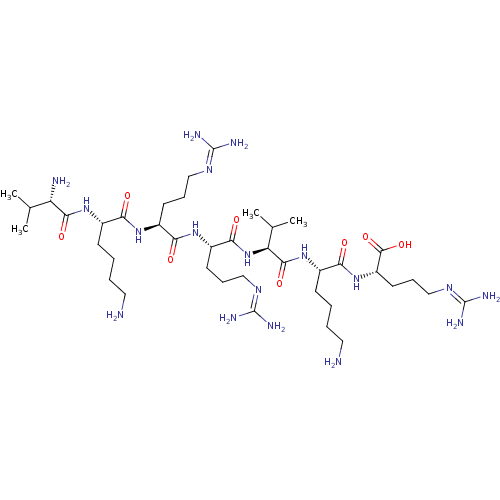 (CHEMBL2179431) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PACE4 expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139929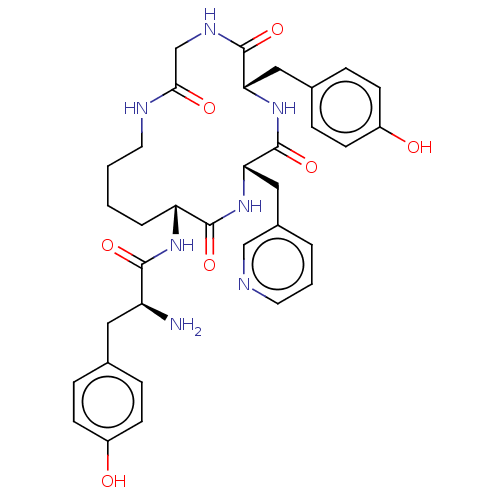 (CHEMBL3763920) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574230 (CHEMBL4851302) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574244 (CHEMBL4852476) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50399752 (CHEMBL2179429) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant furin expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013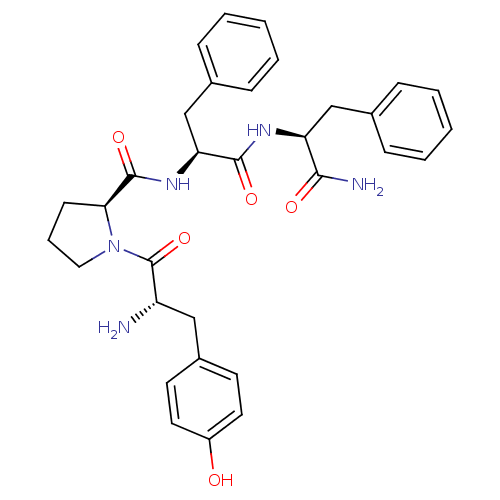 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071071 (CHEMBL3409761) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574238 (CHEMBL4848870) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574229 (CHEMBL4848905) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574236 (CHEMBL4848948) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50120216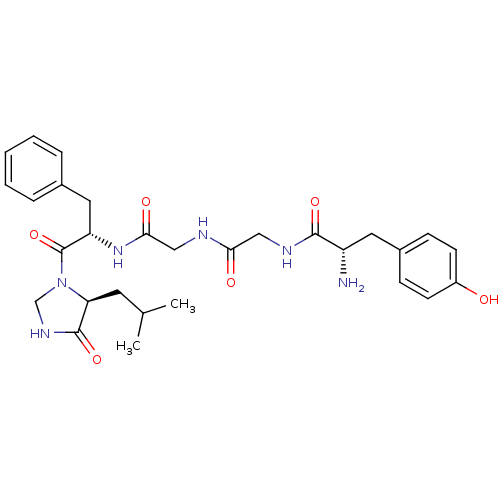 (2-Amino-N-[({[1-benzyl-2-(5-isobutyl-4-oxo-imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Molecular Studies Curated by ChEMBL | Assay Description Binding affinity of the compound towards Opioid receptor mu 1 was determined using ([d-Ala2,MePhe4, Gly5-ol]-enkephalin (DAMGO) as the radioligand. | Bioorg Med Chem Lett 12: 3175-8 (2002) BindingDB Entry DOI: 10.7270/Q2862FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071069 (CHEMBL3409759) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574242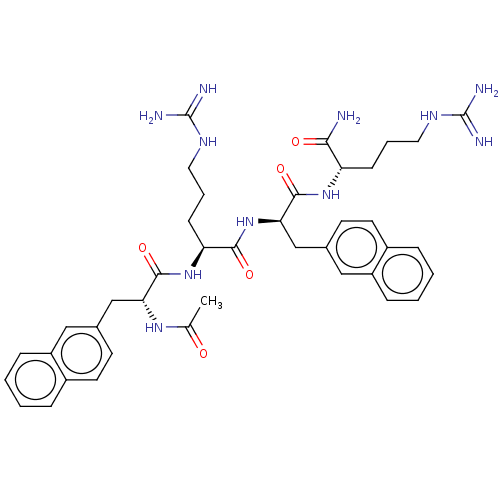 (CHEMBL4859395) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071068 (CHEMBL3409758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574245 (CHEMBL4861645) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574253 (CHEMBL4863587) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50399751 (CHEMBL2179431) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant furin expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071095 (CHEMBL3409766) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50574240 (CHEMBL4872732) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at mouse melanocortin receptor 4 expressed in HEK293 cells assessed as inhibition of NDP-MSH-induced cAMP accumulation incubated ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01417 BindingDB Entry DOI: 10.7270/Q2RR231G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50399750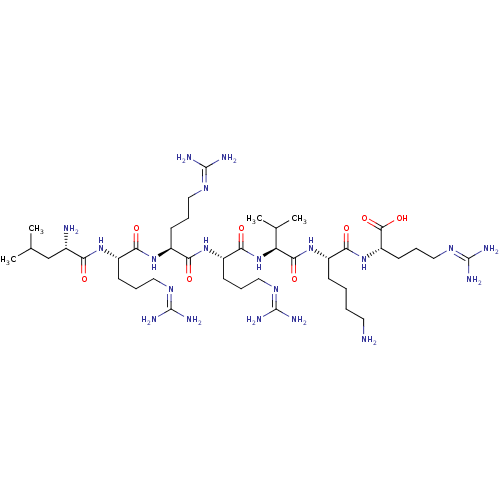 (CHEMBL2179428) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PACE4 expressed in Drosophila S2 cells using pyroGlu-Arg-Val-Lys-Arg-methyl-coumaryl-7-amide as substrate... | J Med Chem 55: 10501-11 (2012) Article DOI: 10.1021/jm3011178 BindingDB Entry DOI: 10.7270/Q2NV9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071072 (CHEMBL3409762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1575 total ) | Next | Last >> |