

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12941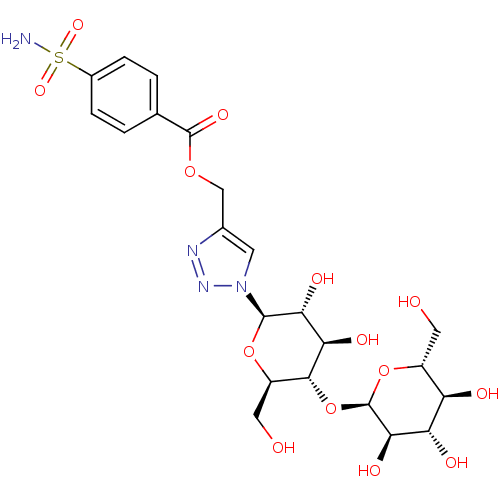 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12916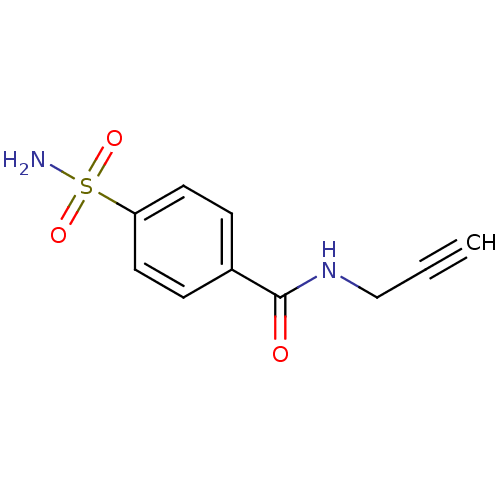 (CHEMBL386542 | N-(Prop-2-ynyl)-4-sulfamoylbenzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50200451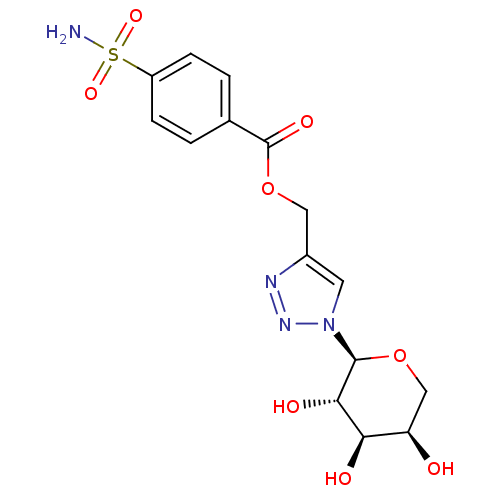 (4-sulfamoyl-benzoic acid 1-((2S,3S,4R,5R)-3,4,5-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12939 (4-({[4-(Aminosulfonyl)benzoyl]amino}methyl-1-(beta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10881 (CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12943 (4-sulfamoyl-N-[(1-{[(2R,3S,4S,5R,6S)-3,4,5-trihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12932 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2-acet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM15231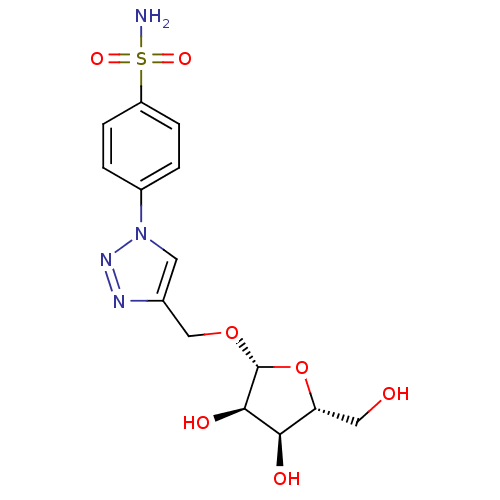 (4-[4-({[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 50: 1651-7 (2007) Article DOI: 10.1021/jm061320h BindingDB Entry DOI: 10.7270/Q2668BFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12918 (4-({[4-(Aminosulfonyl)benzoyl]amino}methyl-1-(2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12929 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12936 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM15224 (4-(4-{[(2,3,4,6-tetra-O-acetyl-beta-D-galactopyran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 50: 1651-7 (2007) Article DOI: 10.1021/jm061320h BindingDB Entry DOI: 10.7270/Q2668BFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12924 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2,3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM15230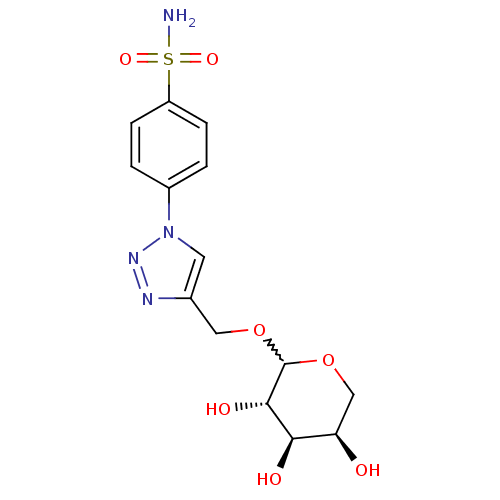 (4-[4-({[(3S,4R,5R)-3,4,5-trihydroxyoxan-2-yl]oxy}m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 50: 1651-7 (2007) Article DOI: 10.1021/jm061320h BindingDB Entry DOI: 10.7270/Q2668BFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM15224 (4-(4-{[(2,3,4,6-tetra-O-acetyl-beta-D-galactopyran...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 50: 1651-7 (2007) Article DOI: 10.1021/jm061320h BindingDB Entry DOI: 10.7270/Q2668BFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM15228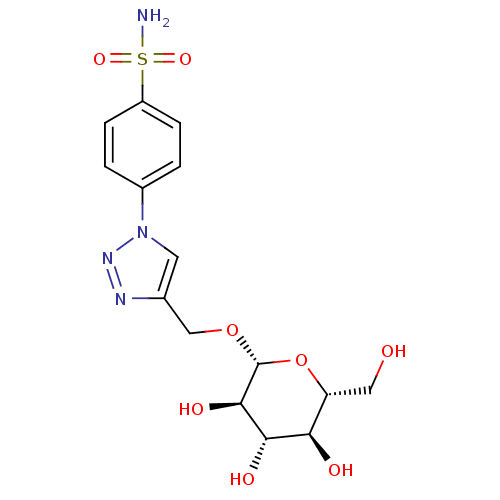 (4-[4-({[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 50: 1651-7 (2007) Article DOI: 10.1021/jm061320h BindingDB Entry DOI: 10.7270/Q2668BFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12921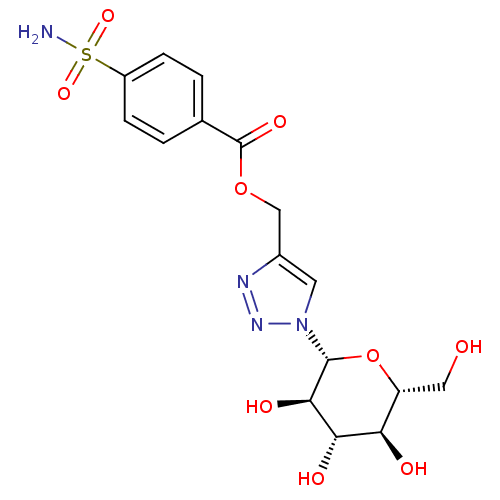 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12936 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12921 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12937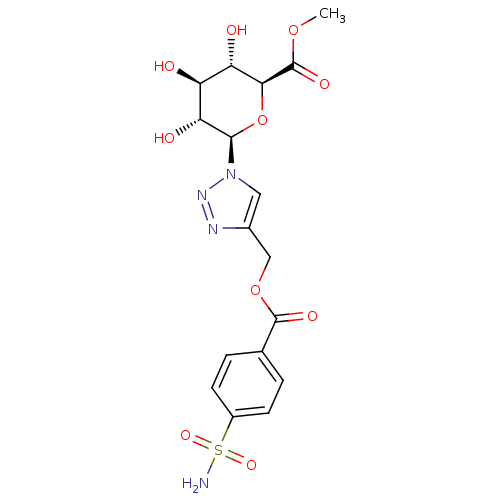 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12941 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12945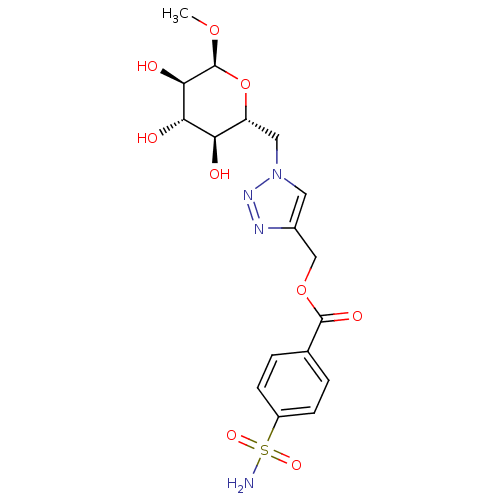 ((1-{[(2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-methoxyox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12933 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2-acet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM15232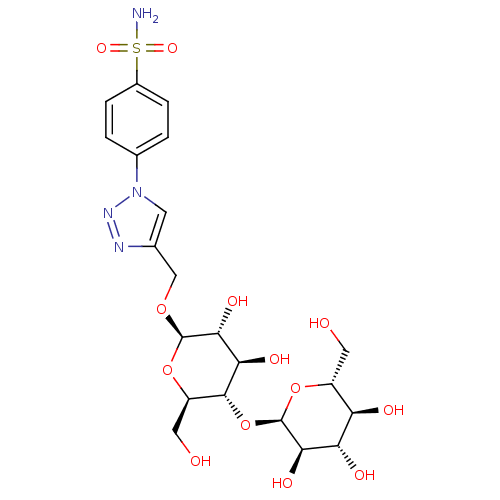 (4-(4-{[(4-O-alpha-D-glucopyranosyl-beta-D-glucopyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.30 | -46.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 50: 1651-7 (2007) Article DOI: 10.1021/jm061320h BindingDB Entry DOI: 10.7270/Q2668BFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12928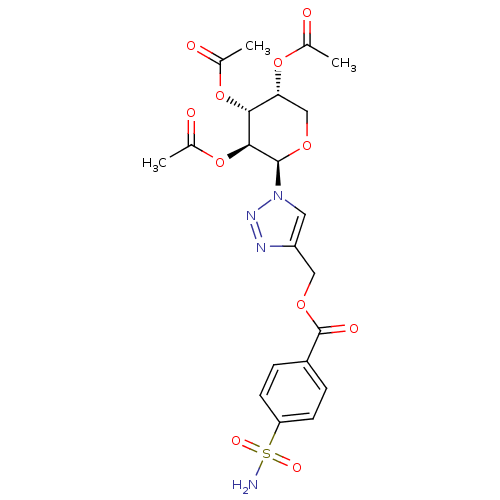 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12923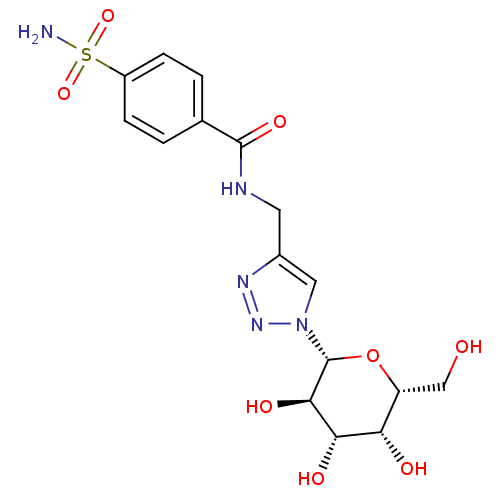 (4-(4-Sulfamoylbenzamido)methyl-1-(beta-D-galactopy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12937 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12933 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2-acet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12938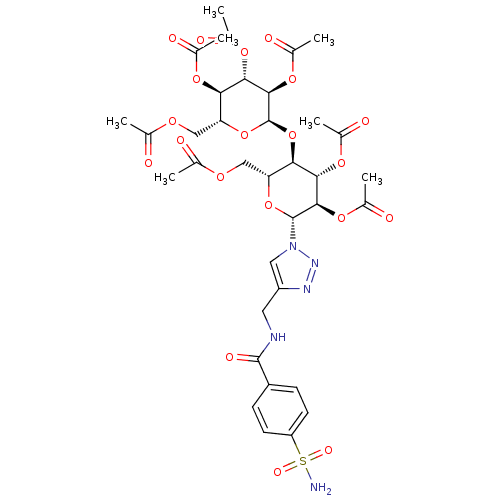 (4-({[4-(Aminosulfonyl)benzoyl]amino}methyl-1-(hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12924 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2,3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12925 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12934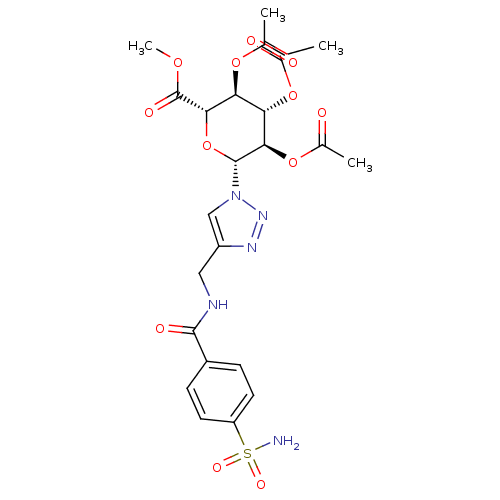 (4-({[4-(Aminosulfonyl)benzoyl]amino}methyl-1-(2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50200449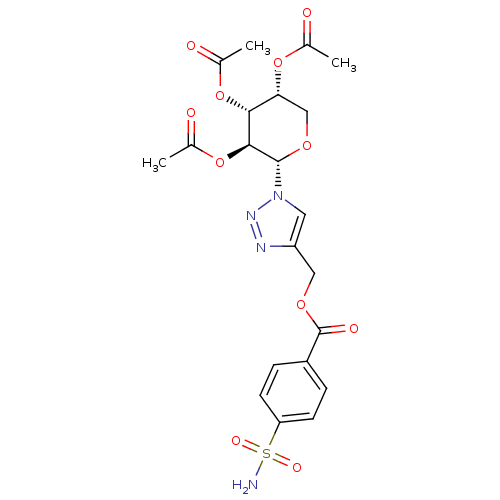 (4-sulfamoyl-benzoic acid 1-((2S,3S,4R,5R)-3,4,5-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM15226 (4-(4-{[(2,3,5-tri-O-benzoyl-beta-D-ribofuranosyl)o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.60 | -46.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 50: 1651-7 (2007) Article DOI: 10.1021/jm061320h BindingDB Entry DOI: 10.7270/Q2668BFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12940 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(hepta-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12920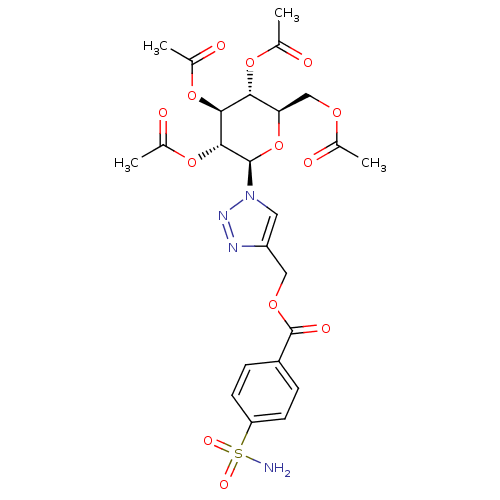 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2,3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM12929 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.70 | -46.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM15227 (4-[4-({[2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.80 | -46.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 50: 1651-7 (2007) Article DOI: 10.1021/jm061320h BindingDB Entry DOI: 10.7270/Q2668BFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12938 (4-({[4-(Aminosulfonyl)benzoyl]amino}methyl-1-(hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12942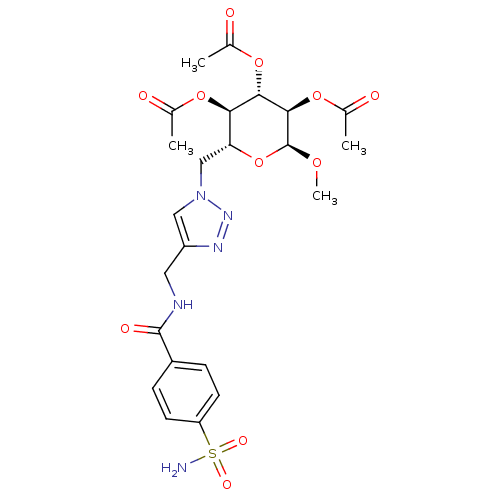 ((2R,3R,4S,5R,6S)-4,5-bis(acetyloxy)-6-methoxy-2-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 50: 1651-7 (2007) Article DOI: 10.1021/jm061320h BindingDB Entry DOI: 10.7270/Q2668BFJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12925 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM15227 (4-[4-({[2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.10 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 50: 1651-7 (2007) Article DOI: 10.1021/jm061320h BindingDB Entry DOI: 10.7270/Q2668BFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12944 ((1-{[(2R,3R,4S,5R,6S)-3,4,5-tris(acetyloxy)-6-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12919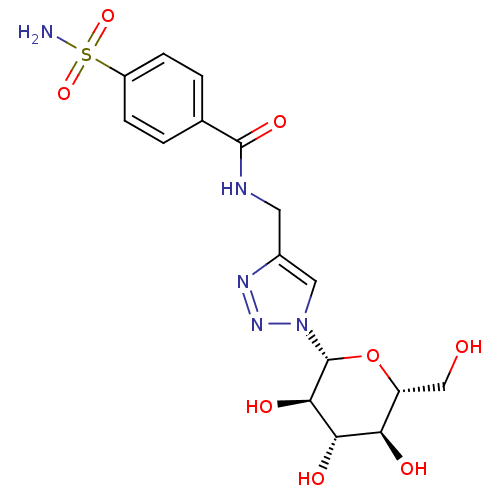 (4-({[4-(Aminosulfonyl)benzoyl]amino}methyl-1-(beta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12917 (CHEMBL217569 | O-propynyl ester derivative 3 | Pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM15230 (4-[4-({[(3S,4R,5R)-3,4,5-trihydroxyoxan-2-yl]oxy}m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.5 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 50: 1651-7 (2007) Article DOI: 10.1021/jm061320h BindingDB Entry DOI: 10.7270/Q2668BFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 242 total ) | Next | Last >> |