

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50118810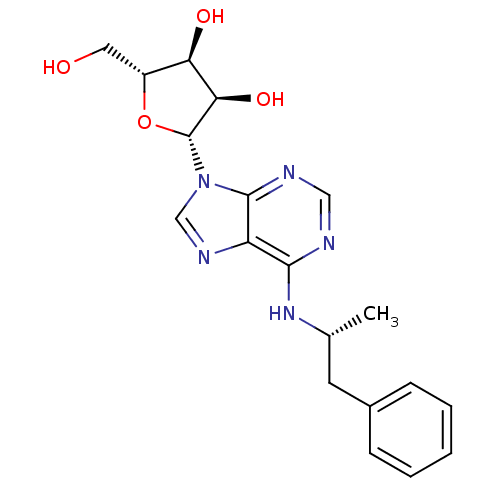 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor in cerebral cortices of Sprague-Dawley male rats using [3H]-CHA | J Med Chem 33: 2240-54 (1990) BindingDB Entry DOI: 10.7270/Q2PR7WKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50009552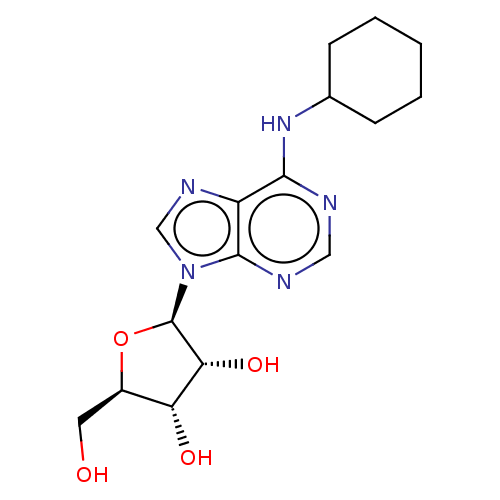 (2-[6-Amino-2-(2-morpholin-4-yl-ethylamino)-purin-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor in corpora striata of rats using [3H]NECA | J Med Chem 33: 2240-54 (1990) BindingDB Entry DOI: 10.7270/Q2PR7WKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007692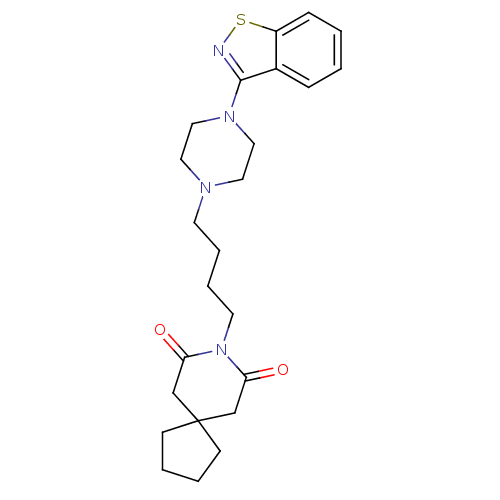 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50118810 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards the Adenosine A1 receptor in corpora striata of rats using [3H]CHA as radioligand. | J Med Chem 33: 2240-54 (1990) BindingDB Entry DOI: 10.7270/Q2PR7WKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50009552 (2-[6-Amino-2-(2-morpholin-4-yl-ethylamino)-purin-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor in cerebral cortices of Sprague-Dawley male rats using [3H]CHA | J Med Chem 33: 2240-54 (1990) BindingDB Entry DOI: 10.7270/Q2PR7WKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048806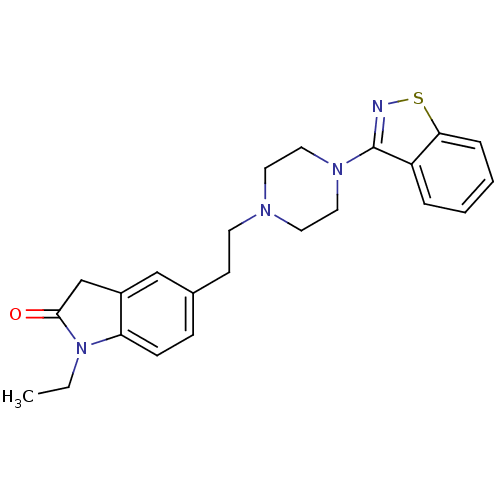 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM42467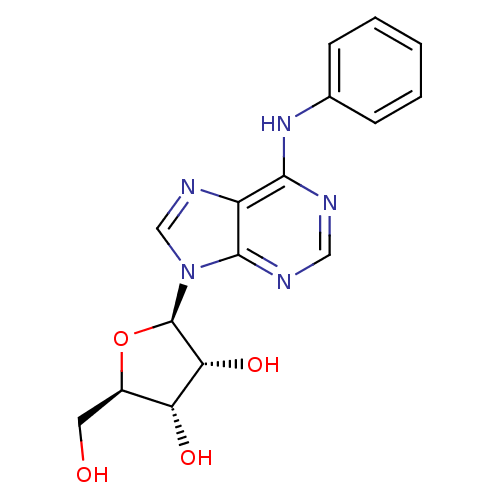 ((2R,3R,4S,5R)-2-(6-anilino-9-purinyl)-5-(hydroxyme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards the Adenosine A1 receptor in corpora striata of rats using [3H]CHA as radioligand. | J Med Chem 33: 2240-54 (1990) BindingDB Entry DOI: 10.7270/Q2PR7WKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048807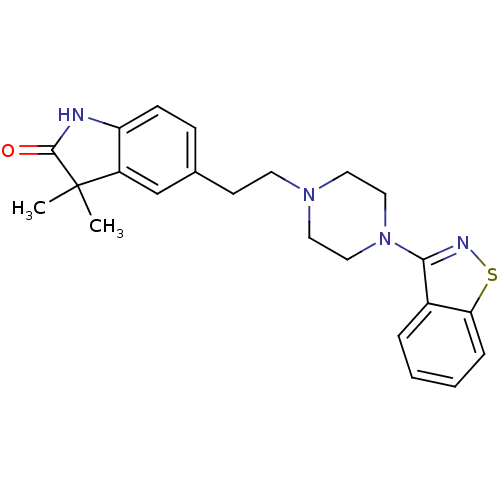 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048804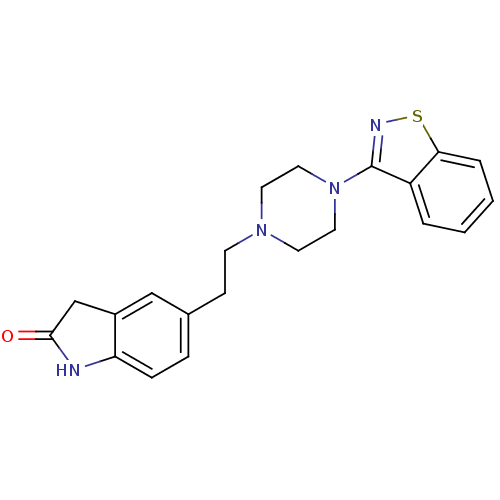 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048802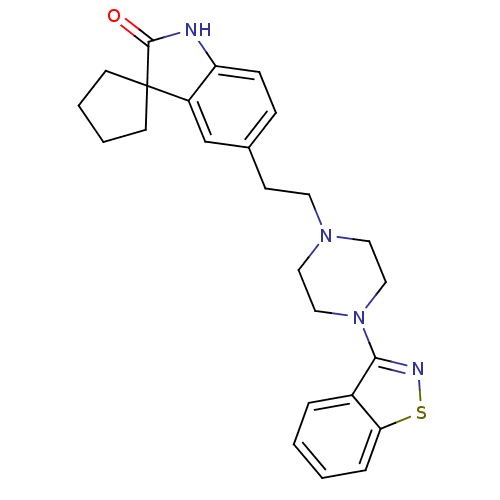 (5'-[2-(4-benzo[d]isothiazol-3-ylhexahydro-1-pyrazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048805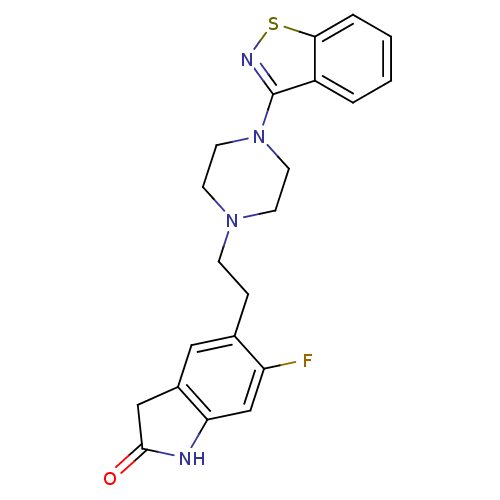 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM42467 ((2R,3R,4S,5R)-2-(6-anilino-9-purinyl)-5-(hydroxyme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor in corpora striata of rats using [3H]NECA | J Med Chem 33: 2240-54 (1990) BindingDB Entry DOI: 10.7270/Q2PR7WKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Binding affinity for Adenosine A2 receptor in corpora striata of rats using [3H]NECA | J Med Chem 33: 2240-54 (1990) BindingDB Entry DOI: 10.7270/Q2PR7WKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048800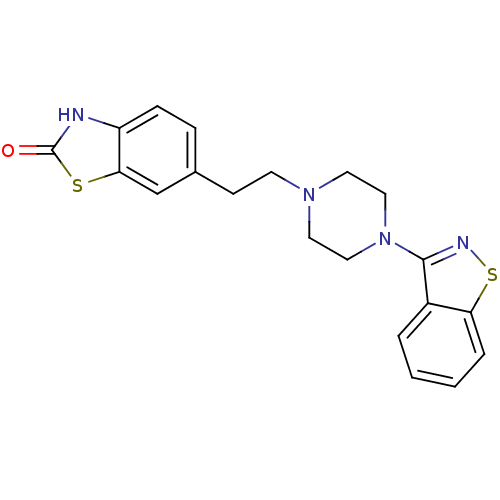 (6-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048801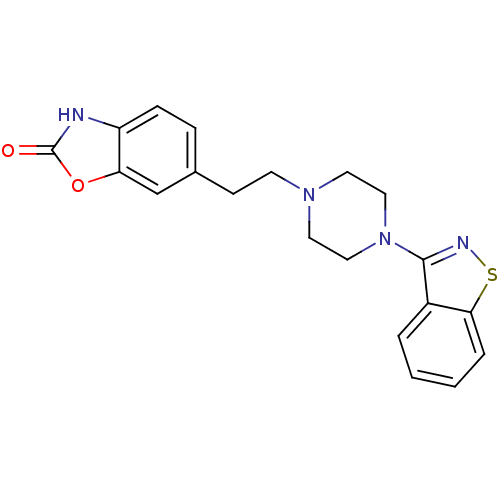 (6-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards the Adenosine A1 receptor in corpora striata of rats using [3H]CHA as radioligand. | J Med Chem 33: 2240-54 (1990) BindingDB Entry DOI: 10.7270/Q2PR7WKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM497388 (US11007200, Example 4a | US11426411, Example 4a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM497388 (US11007200, Example 4a | US11426411, Example 4a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497384 (US11007200, Example 2a | US11426411, Example 2a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497384 (US11007200, Example 2a | US11426411, Example 2a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | Assay Description Procedures employed by the PDSP as described in the NIMH-PDSP Assay Protocol Book, Version II. The standard drug used in both Sigma subtype assays is... | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM497386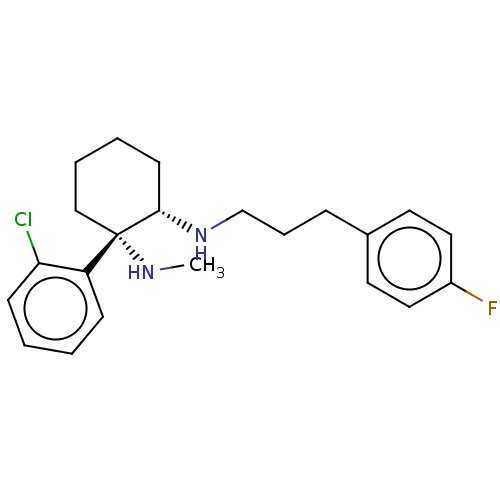 (US11007200, Example 3a | US11426411, Example 3a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM497386 (US11007200, Example 3a | US11426411, Example 3a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM247007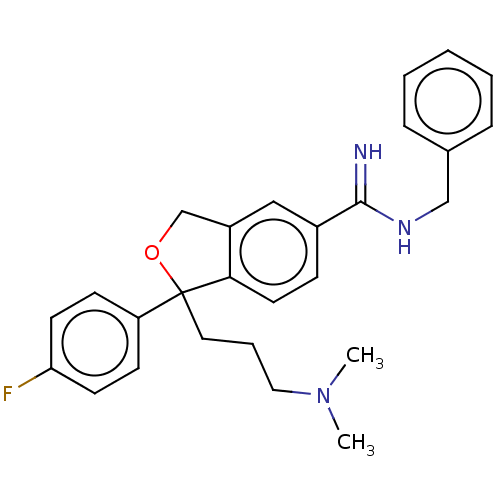 (US9700563, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergies, LLC US Patent | Assay Description The compounds were tested for activity vs. opioid receptor subtypes Kappa (KOR), Delta (DOR) and Mu (MOR) at an initial concentration of 10 μM e... | US Patent US9700563 (2017) BindingDB Entry DOI: 10.7270/Q22J6DV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497386 (US11007200, Example 3a | US11426411, Example 3a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 35.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497386 (US11007200, Example 3a | US11426411, Example 3a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 35.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | Assay Description Procedures employed by the PDSP as described in the NIMH-PDSP Assay Protocol Book, Version II. The standard drug used in both Sigma subtype assays is... | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM568457 (US11426411, Example 2b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497391 (US11007200, Example 2b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | Assay Description Procedures employed by the PDSP as described in the NIMH-PDSP Assay Protocol Book, Version II. The standard drug used in both Sigma subtype assays is... | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to displace [3H]-ketanserin from 5-hydroxytryptamine 2A receptor in rat brain. | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM247009 (US9700563, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergies, LLC US Patent | Assay Description The compounds were tested for activity vs. opioid receptor subtypes Kappa (KOR), Delta (DOR) and Mu (MOR) at an initial concentration of 10 μM e... | US Patent US9700563 (2017) BindingDB Entry DOI: 10.7270/Q22J6DV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50080398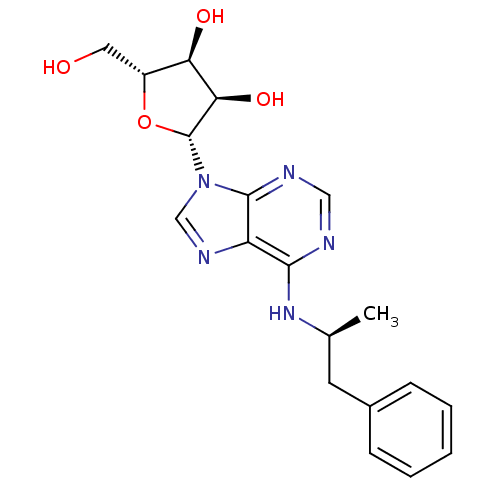 ((2R,3S,4R,5R)-2-Hydroxymethyl-5-[6-((S)-1-methyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor in cerebral cortices of Sprague-Dawley male rats using [3H]-CHA | J Med Chem 33: 2240-54 (1990) BindingDB Entry DOI: 10.7270/Q2PR7WKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM497384 (US11007200, Example 2a | US11426411, Example 2a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 59.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM497384 (US11007200, Example 2a | US11426411, Example 2a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 59.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497390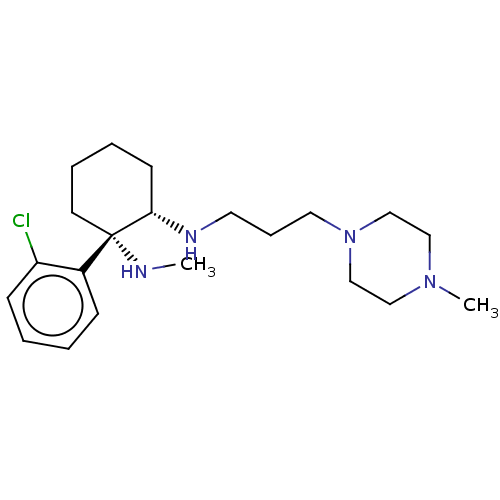 (US11007200, Example 13a | US11426411, Example 13a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497390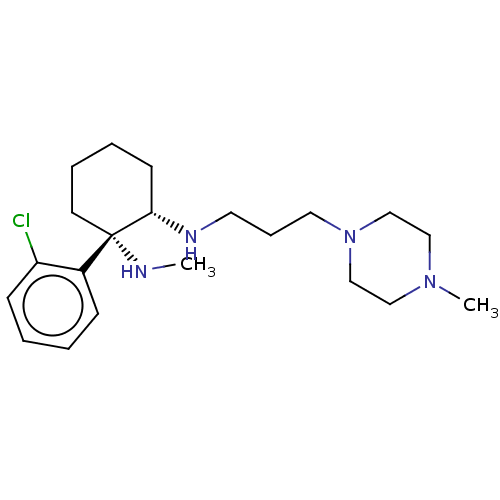 (US11007200, Example 13a | US11426411, Example 13a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | Assay Description Procedures employed by the PDSP as described in the NIMH-PDSP Assay Protocol Book, Version II. The standard drug used in both Sigma subtype assays is... | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497388 (US11007200, Example 4a | US11426411, Example 4a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 97.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497388 (US11007200, Example 4a | US11426411, Example 4a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 97.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | Assay Description Procedures employed by the PDSP as described in the NIMH-PDSP Assay Protocol Book, Version II. The standard drug used in both Sigma subtype assays is... | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM497390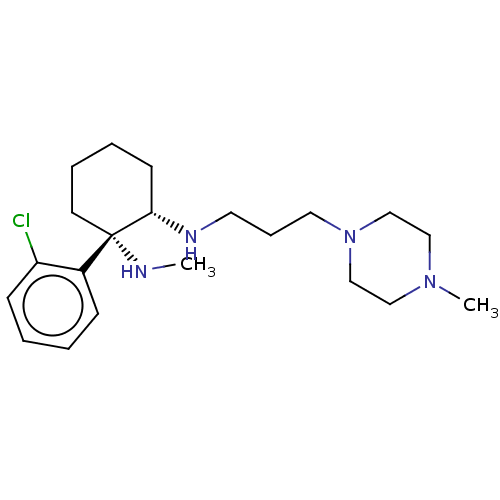 (US11007200, Example 13a | US11426411, Example 13a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM497390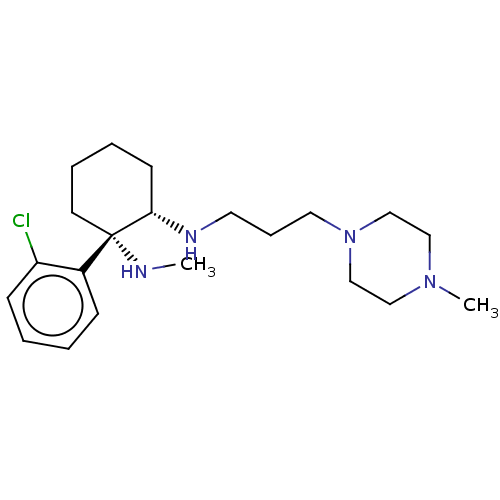 (US11007200, Example 13a | US11426411, Example 13a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497392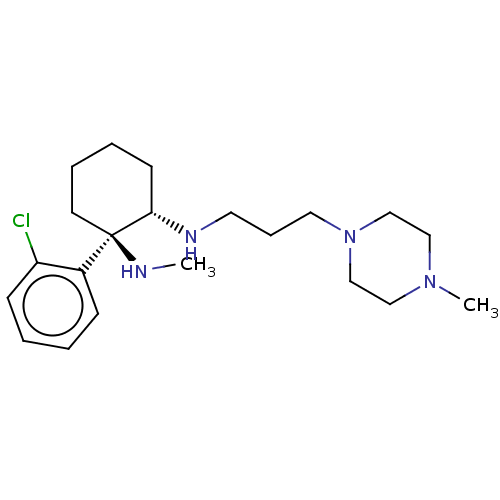 (US11007200, Example 13b | US11426411, Example 13b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497392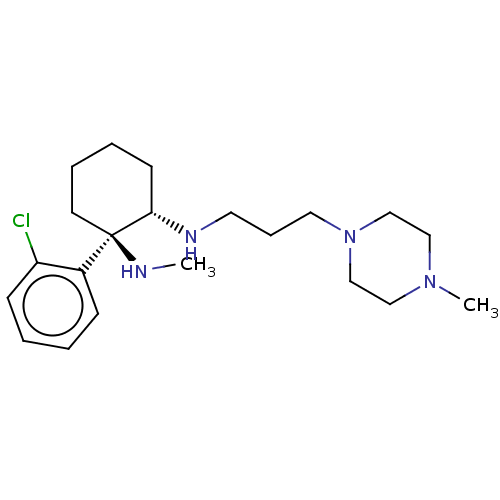 (US11007200, Example 13b | US11426411, Example 13b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | Assay Description Procedures employed by the PDSP as described in the NIMH-PDSP Assay Protocol Book, Version II. The standard drug used in both Sigma subtype assays is... | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50080398 ((2R,3S,4R,5R)-2-Hydroxymethyl-5-[6-((S)-1-methyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards the Adenosine A1 receptor in corpora striata of rats using [3H]CHA as radioligand. | J Med Chem 33: 2240-54 (1990) BindingDB Entry DOI: 10.7270/Q2PR7WKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497387 (US11007200, Example 3b | US11426411, Example 3b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | Assay Description Procedures employed by the PDSP as described in the NIMH-PDSP Assay Protocol Book, Version II. The standard drug used in both Sigma subtype assays is... | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM497387 (US11007200, Example 3b | US11426411, Example 3b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM247008 (US9700563, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergies, LLC US Patent | Assay Description The compounds were tested for activity vs. opioid receptor subtypes Kappa (KOR), Delta (DOR) and Mu (MOR) at an initial concentration of 10 μM e... | US Patent US9700563 (2017) BindingDB Entry DOI: 10.7270/Q22J6DV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM568457 (US11426411, Example 2b) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP). | Citation and Details BindingDB Entry DOI: 10.7270/Q29S1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM497391 (US11007200, Example 2b) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MediSynergics, LLC US Patent | US Patent US11007200 (2021) BindingDB Entry DOI: 10.7270/Q2NP27J7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 296 total ) | Next | Last >> |