 Found 226 hits with Last Name = 'hrelia' and Initial = 'p'
Found 226 hits with Last Name = 'hrelia' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Irreversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot ... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM15579

(CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...)Show InChI InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Irreversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated ... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10512
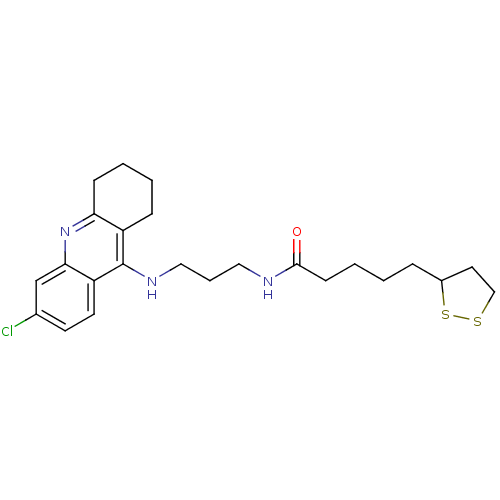
(CHEMBL194823 | N-{3-[(6-chloro-1,2,3,4-tetrahydroa...)Show SMILES Clc1ccc2c(NCCCNC(=O)CCCCC3CCSS3)c3CCCCc3nc2c1 Show InChI InChI=1S/C24H32ClN3OS2/c25-17-10-11-20-22(16-17)28-21-8-3-2-7-19(21)24(20)27-14-5-13-26-23(29)9-4-1-6-18-12-15-30-31-18/h10-11,16,18H,1-9,12-15H2,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 155 | -40.4 | 0.253 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... |
J Med Chem 48: 360-3 (2005)
Article DOI: 10.1021/jm049112h
BindingDB Entry DOI: 10.7270/Q2JQ0Z7J |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50261233
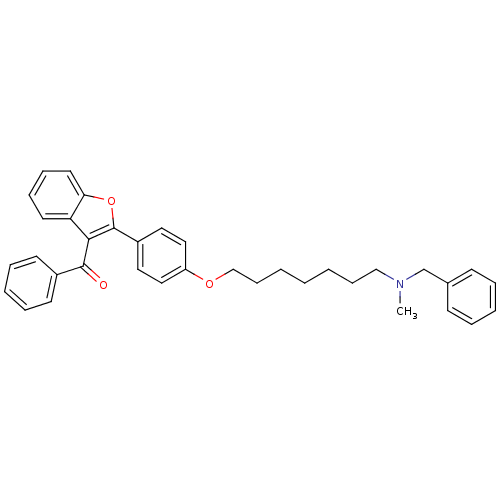
((2-{4-[7-(benzylmethylamino)heptyloxy]phenyl}benzo...)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C36H37NO3/c1-37(27-28-15-7-5-8-16-28)25-13-3-2-4-14-26-39-31-23-21-30(22-24-31)36-34(32-19-11-12-20-33(32)40-36)35(38)29-17-9-6-10-18-29/h5-12,15-24H,2-4,13-14,25-27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50394567
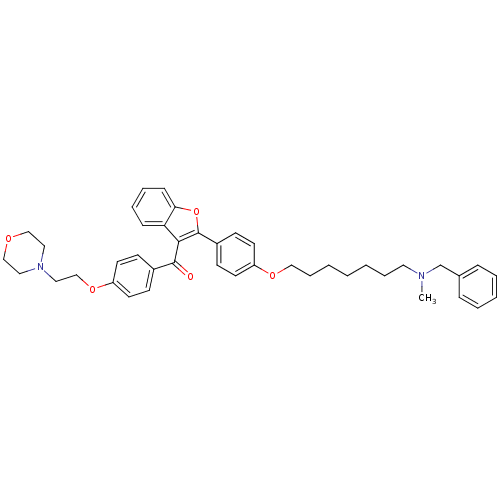
(CHEMBL2160225)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1ccc(OCCN2CCOCC2)cc1)Cc1ccccc1 Show InChI InChI=1S/C42H48N2O5/c1-43(32-33-12-6-5-7-13-33)24-10-3-2-4-11-28-47-36-22-18-35(19-23-36)42-40(38-14-8-9-15-39(38)49-42)41(45)34-16-20-37(21-17-34)48-31-27-44-25-29-46-30-26-44/h5-9,12-23H,2-4,10-11,24-32H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50394567

(CHEMBL2160225)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1ccc(OCCN2CCOCC2)cc1)Cc1ccccc1 Show InChI InChI=1S/C42H48N2O5/c1-43(32-33-12-6-5-7-13-33)24-10-3-2-4-11-28-47-36-22-18-35(19-23-36)42-40(38-14-8-9-15-39(38)49-42)41(45)34-16-20-37(21-17-34)48-31-27-44-25-29-46-30-26-44/h5-9,12-23H,2-4,10-11,24-32H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50394568
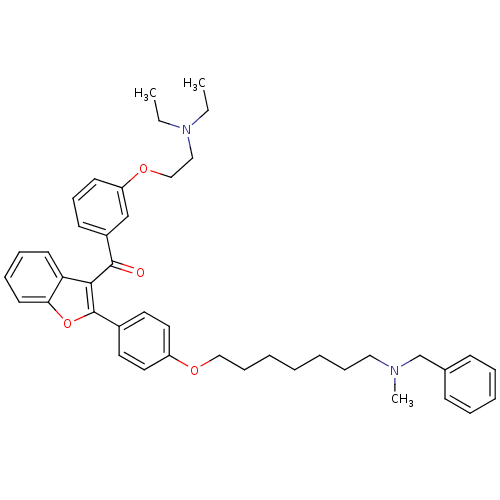
(CHEMBL2160223)Show SMILES CCN(CC)CCOc1cccc(c1)C(=O)c1c(oc2ccccc12)-c1ccc(OCCCCCCCN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C42H50N2O4/c1-4-44(5-2)28-30-47-37-20-16-19-35(31-37)41(45)40-38-21-12-13-22-39(38)48-42(40)34-23-25-36(26-24-34)46-29-15-8-6-7-14-27-43(3)32-33-17-10-9-11-18-33/h9-13,16-26,31H,4-8,14-15,27-30,32H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50394569
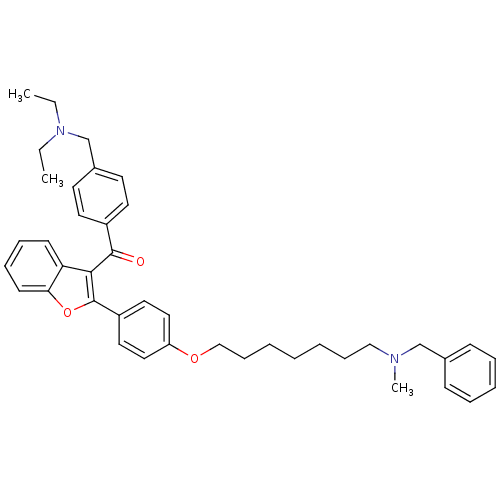
(CHEMBL2160222)Show SMILES CCN(CC)Cc1ccc(cc1)C(=O)c1c(oc2ccccc12)-c1ccc(OCCCCCCCN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C41H48N2O3/c1-4-43(5-2)31-33-20-22-34(23-21-33)40(44)39-37-18-12-13-19-38(37)46-41(39)35-24-26-36(27-25-35)45-29-15-8-6-7-14-28-42(3)30-32-16-10-9-11-17-32/h9-13,16-27H,4-8,14-15,28-31H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50394570
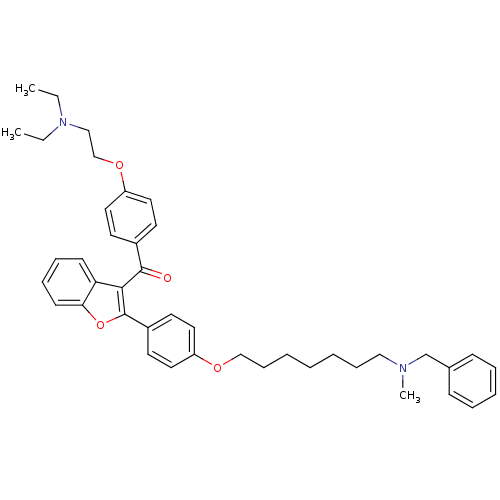
(CHEMBL2160224)Show SMILES CCN(CC)CCOc1ccc(cc1)C(=O)c1c(oc2ccccc12)-c1ccc(OCCCCCCCN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C42H50N2O4/c1-4-44(5-2)29-31-47-37-24-20-34(21-25-37)41(45)40-38-18-12-13-19-39(38)48-42(40)35-22-26-36(27-23-35)46-30-15-8-6-7-14-28-43(3)32-33-16-10-9-11-17-33/h9-13,16-27H,4-8,14-15,28-32H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50394572
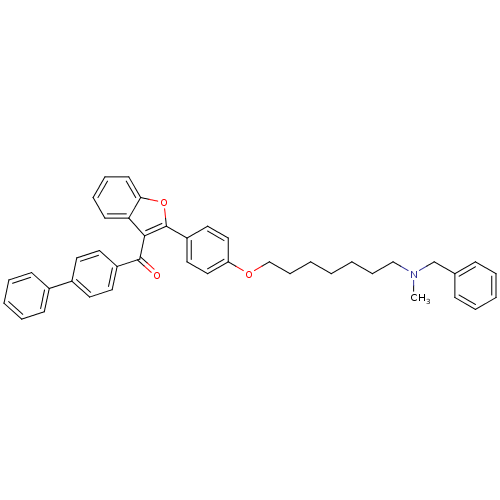
(CHEMBL2160219)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1ccc(cc1)-c1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C42H41NO3/c1-43(31-32-15-7-5-8-16-32)29-13-3-2-4-14-30-45-37-27-25-36(26-28-37)42-40(38-19-11-12-20-39(38)46-42)41(44)35-23-21-34(22-24-35)33-17-9-6-10-18-33/h5-12,15-28H,2-4,13-14,29-31H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM11022
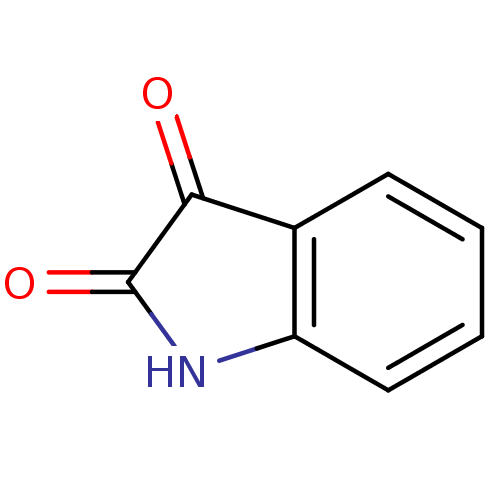
(2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...)Show InChI InChI=1S/C8H5NO2/c10-7-5-3-1-2-4-6(5)9-8(7)11/h1-4H,(H,9,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated f... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50394568

(CHEMBL2160223)Show SMILES CCN(CC)CCOc1cccc(c1)C(=O)c1c(oc2ccccc12)-c1ccc(OCCCCCCCN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C42H50N2O4/c1-4-44(5-2)28-30-47-37-20-16-19-35(31-37)41(45)40-38-21-12-13-22-39(38)48-42(40)34-23-25-36(26-24-34)46-29-15-8-6-7-14-27-43(3)32-33-17-10-9-11-18-33/h9-13,16-26,31H,4-8,14-15,27-30,32H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50261202
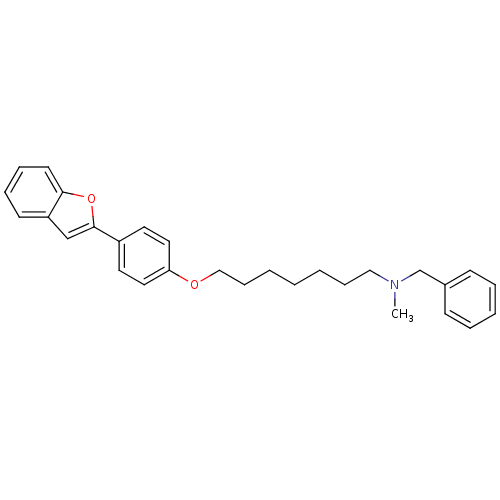
(CHEMBL497755 | [7-(4-Benzofuran-2-yl-phenoxy)hepht...)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1cc2ccccc2o1)Cc1ccccc1 Show InChI InChI=1S/C29H33NO2/c1-30(23-24-12-6-5-7-13-24)20-10-3-2-4-11-21-31-27-18-16-25(17-19-27)29-22-26-14-8-9-15-28(26)32-29/h5-9,12-19,22H,2-4,10-11,20-21,23H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50394572

(CHEMBL2160219)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1ccc(cc1)-c1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C42H41NO3/c1-43(31-32-15-7-5-8-16-32)29-13-3-2-4-14-30-45-37-27-25-36(26-28-37)42-40(38-19-11-12-20-39(38)46-42)41(44)35-23-21-34(22-24-35)33-17-9-6-10-18-33/h5-12,15-28H,2-4,13-14,29-31H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50494371

(CHEMBL3086339)Show InChI InChI=1S/C21H34N2S/c1-23(19-21-15-11-10-12-16-21)18-14-9-7-5-3-2-4-6-8-13-17-22-20-24/h10-12,15-16H,2-9,13-14,17-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Competitive inhibition of MAOA in human SH-SY5Y cells using p-tyramine as substrate preincubated for 5 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50494371

(CHEMBL3086339)Show InChI InChI=1S/C21H34N2S/c1-23(19-21-15-11-10-12-16-21)18-14-9-7-5-3-2-4-6-8-13-17-22-20-24/h10-12,15-16H,2-9,13-14,17-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Irreversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot ... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50494371

(CHEMBL3086339)Show InChI InChI=1S/C21H34N2S/c1-23(19-21-15-11-10-12-16-21)18-14-9-7-5-3-2-4-6-8-13-17-22-20-24/h10-12,15-16H,2-9,13-14,17-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated f... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50394570

(CHEMBL2160224)Show SMILES CCN(CC)CCOc1ccc(cc1)C(=O)c1c(oc2ccccc12)-c1ccc(OCCCCCCCN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C42H50N2O4/c1-4-44(5-2)29-31-47-37-24-20-34(21-25-37)41(45)40-38-18-12-13-19-39(38)48-42(40)35-22-26-36(27-23-35)46-30-15-8-6-7-14-28-43(3)32-33-16-10-9-11-17-33/h9-13,16-27H,4-8,14-15,28-32H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50394580
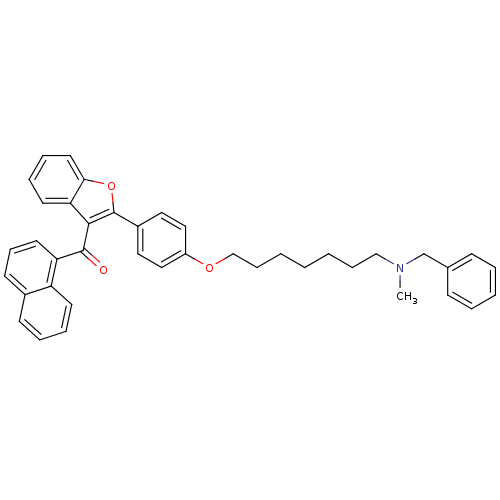
(CHEMBL2160217)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1cccc2ccccc12)Cc1ccccc1 Show InChI InChI=1S/C40H39NO3/c1-41(29-30-15-6-5-7-16-30)27-12-3-2-4-13-28-43-33-25-23-32(24-26-33)40-38(36-20-10-11-22-37(36)44-40)39(42)35-21-14-18-31-17-8-9-19-34(31)35/h5-11,14-26H,2-4,12-13,27-29H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50394581
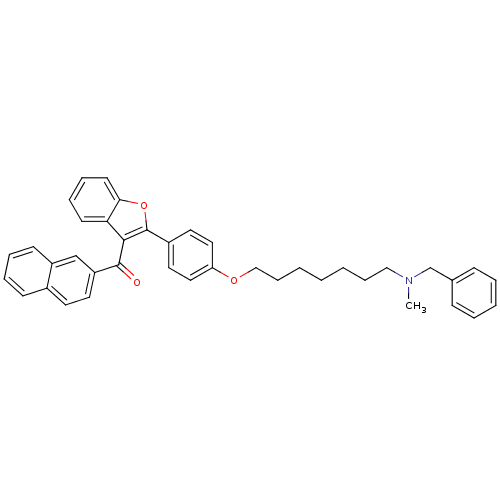
(CHEMBL2160218)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1ccc2ccccc2c1)Cc1ccccc1 Show InChI InChI=1S/C40H39NO3/c1-41(29-30-14-6-5-7-15-30)26-12-3-2-4-13-27-43-35-24-22-32(23-25-35)40-38(36-18-10-11-19-37(36)44-40)39(42)34-21-20-31-16-8-9-17-33(31)28-34/h5-11,14-25,28H,2-4,12-13,26-27,29H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50394582
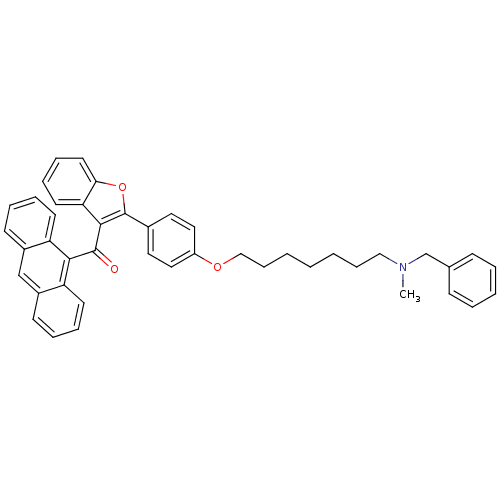
(CHEMBL2160220)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1c2ccccc2cc2ccccc12)Cc1ccccc1 Show InChI InChI=1S/C44H41NO3/c1-45(31-32-16-6-5-7-17-32)28-14-3-2-4-15-29-47-36-26-24-33(25-27-36)44-42(39-22-12-13-23-40(39)48-44)43(46)41-37-20-10-8-18-34(37)30-35-19-9-11-21-38(35)41/h5-13,16-27,30H,2-4,14-15,28-29,31H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50394577
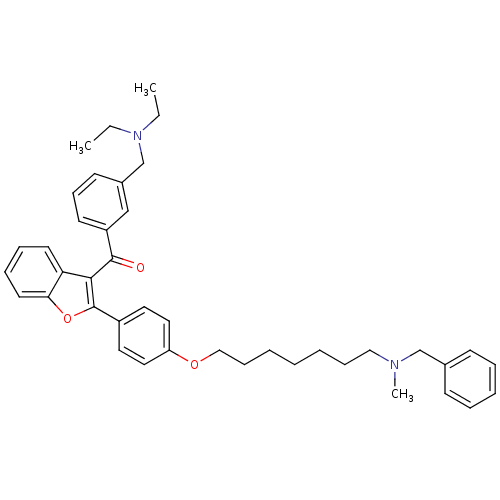
(CHEMBL2160221)Show SMILES CCN(CC)Cc1cccc(c1)C(=O)c1c(oc2ccccc12)-c1ccc(OCCCCCCCN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C41H48N2O3/c1-4-43(5-2)31-33-19-16-20-35(29-33)40(44)39-37-21-12-13-22-38(37)46-41(39)34-23-25-36(26-24-34)45-28-15-8-6-7-14-27-42(3)30-32-17-10-9-11-18-32/h9-13,16-26,29H,4-8,14-15,27-28,30-31H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50394569

(CHEMBL2160222)Show SMILES CCN(CC)Cc1ccc(cc1)C(=O)c1c(oc2ccccc12)-c1ccc(OCCCCCCCN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C41H48N2O3/c1-4-43(5-2)31-33-20-22-34(23-21-33)40(44)39-37-18-12-13-19-38(37)46-41(39)35-24-26-36(27-25-35)45-29-15-8-6-7-14-28-42(3)30-32-16-10-9-11-17-32/h9-13,16-27H,4-8,14-15,28-31H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50394581

(CHEMBL2160218)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1ccc2ccccc2c1)Cc1ccccc1 Show InChI InChI=1S/C40H39NO3/c1-41(29-30-14-6-5-7-15-30)26-12-3-2-4-13-27-43-35-24-22-32(23-25-35)40-38(36-18-10-11-19-37(36)44-40)39(42)34-21-20-31-16-8-9-17-33(31)28-34/h5-11,14-25,28H,2-4,12-13,26-27,29H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50394582

(CHEMBL2160220)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1c2ccccc2cc2ccccc12)Cc1ccccc1 Show InChI InChI=1S/C44H41NO3/c1-45(31-32-16-6-5-7-17-32)28-14-3-2-4-15-29-47-36-26-24-33(25-27-36)44-42(39-22-12-13-23-40(39)48-44)43(46)41-37-20-10-8-18-34(37)30-35-19-9-11-21-38(35)41/h5-13,16-27,30H,2-4,14-15,28-29,31H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50394577

(CHEMBL2160221)Show SMILES CCN(CC)Cc1cccc(c1)C(=O)c1c(oc2ccccc12)-c1ccc(OCCCCCCCN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C41H48N2O3/c1-4-43(5-2)31-33-19-16-20-35(29-33)40(44)39-37-21-12-13-22-38(37)46-41(39)34-23-25-36(26-24-34)45-28-15-8-6-7-14-27-42(3)30-32-17-10-9-11-18-32/h9-13,16-26,29H,4-8,14-15,27-28,30-31H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
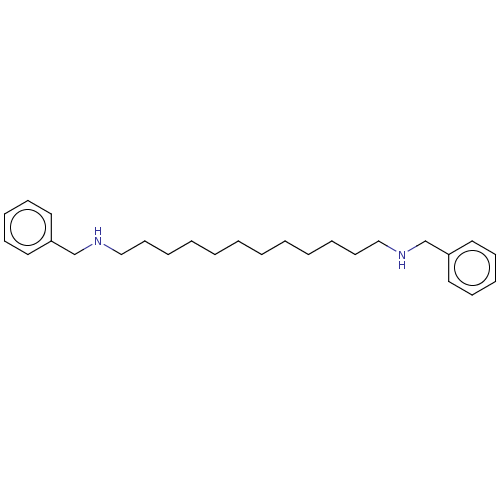
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261233

((2-{4-[7-(benzylmethylamino)heptyloxy]phenyl}benzo...)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C36H37NO3/c1-37(27-28-15-7-5-8-16-28)25-13-3-2-4-14-26-39-31-23-21-30(22-24-31)36-34(32-19-11-12-20-33(32)40-36)35(38)29-17-9-6-10-18-29/h5-12,15-24H,2-4,13-14,25-27H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261202

(CHEMBL497755 | [7-(4-Benzofuran-2-yl-phenoxy)hepht...)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1cc2ccccc2o1)Cc1ccccc1 Show InChI InChI=1S/C29H33NO2/c1-30(23-24-12-6-5-7-13-24)20-10-3-2-4-11-21-31-27-18-16-25(17-19-27)29-22-26-14-8-9-15-28(26)32-29/h5-9,12-19,22H,2-4,10-11,20-21,23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50394580

(CHEMBL2160217)Show SMILES CN(CCCCCCCOc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1cccc2ccccc12)Cc1ccccc1 Show InChI InChI=1S/C40H39NO3/c1-41(29-30-15-6-5-7-16-30)27-12-3-2-4-13-28-43-33-25-23-32(24-26-33)40-38(36-20-10-11-22-37(36)44-40)39(42)35-21-14-18-31-17-8-9-19-34(31)35/h5-11,14-26H,2-4,12-13,27-29H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
Eur J Med Chem 58: 519-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.045
BindingDB Entry DOI: 10.7270/Q2XW4KW5 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM11022

(2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...)Show InChI InChI=1S/C8H5NO2/c10-7-5-3-1-2-4-6(5)9-8(7)11/h1-4H,(H,9,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot a... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50494369

(CHEMBL3086344)Show InChI InChI=1S/C15H30N4S/c16-7-4-10-17-8-1-2-9-18-11-5-12-19-14-15-6-3-13-20-15/h3,6,13,17-19H,1-2,4-5,7-12,14,16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Mixed type inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50494370
(CHEMBL3086341)Show InChI InChI=1S/C26H40N2/c1(3-5-7-15-21-27-23-25-17-11-9-12-18-25)2-4-6-8-16-22-28-24-26-19-13-10-14-20-26/h9-14,17-20,27-28H,1-8,15-16,21-24H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Mixed type inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50494370

(CHEMBL3086341)Show InChI InChI=1S/C26H40N2/c1(3-5-7-15-21-27-23-25-17-11-9-12-18-25)2-4-6-8-16-22-28-24-26-19-13-10-14-20-26/h9-14,17-20,27-28H,1-8,15-16,21-24H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Mixed type inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50494368

(CHEMBL1185605)Show InChI InChI=1S/C17H32N4/c18-10-6-13-19-11-4-5-12-20-14-7-15-21-16-17-8-2-1-3-9-17/h1-3,8-9,19-21H,4-7,10-16,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Mixed type inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50494370

(CHEMBL3086341)Show InChI InChI=1S/C26H40N2/c1(3-5-7-15-21-27-23-25-17-11-9-12-18-25)2-4-6-8-16-22-28-24-26-19-13-10-14-20-26/h9-14,17-20,27-28H,1-8,15-16,21-24H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50494368

(CHEMBL1185605)Show InChI InChI=1S/C17H32N4/c18-10-6-13-19-11-4-5-12-20-14-7-15-21-16-17-8-2-1-3-9-17/h1-3,8-9,19-21H,4-7,10-16,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50494369

(CHEMBL3086344)Show InChI InChI=1S/C15H30N4S/c16-7-4-10-17-8-1-2-9-18-11-5-12-19-14-15-6-3-13-20-15/h3,6,13,17-19H,1-2,4-5,7-12,14,16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Mixed type inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50494368

(CHEMBL1185605)Show InChI InChI=1S/C17H32N4/c18-10-6-13-19-11-4-5-12-20-14-7-15-21-16-17-8-2-1-3-9-17/h1-3,8-9,19-21H,4-7,10-16,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Mixed type inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50494369

(CHEMBL3086344)Show InChI InChI=1S/C15H30N4S/c16-7-4-10-17-8-1-2-9-18-11-5-12-19-14-15-6-3-13-20-15/h3,6,13,17-19H,1-2,4-5,7-12,14,16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50494368

(CHEMBL1185605)Show InChI InChI=1S/C17H32N4/c18-10-6-13-19-11-4-5-12-20-14-7-15-21-16-17-8-2-1-3-9-17/h1-3,8-9,19-21H,4-7,10-16,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50494369

(CHEMBL3086344)Show InChI InChI=1S/C15H30N4S/c16-7-4-10-17-8-1-2-9-18-11-5-12-19-14-15-6-3-13-20-15/h3,6,13,17-19H,1-2,4-5,7-12,14,16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50494370

(CHEMBL3086341)Show InChI InChI=1S/C26H40N2/c1(3-5-7-15-21-27-23-25-17-11-9-12-18-25)2-4-6-8-16-22-28-24-26-19-13-10-14-20-26/h9-14,17-20,27-28H,1-8,15-16,21-24H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.05E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... |
Eur J Med Chem 70: 88-101 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.005
BindingDB Entry DOI: 10.7270/Q2T156MV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50355819
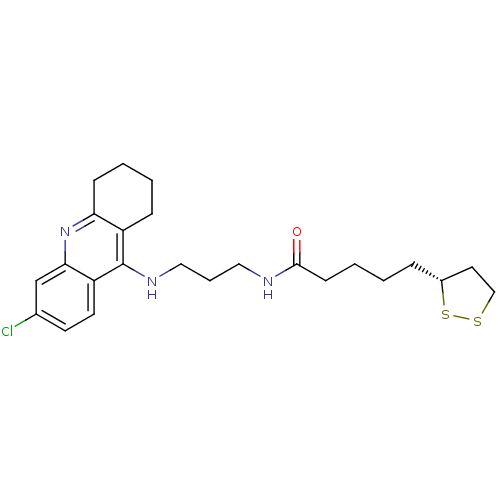
(CHEMBL1912059)Show SMILES Clc1ccc2c(NCCCNC(=O)CCCC[C@@H]3CCSS3)c3CCCCc3nc2c1 |r| Show InChI InChI=1S/C24H32ClN3OS2/c25-17-10-11-20-22(16-17)28-21-8-3-2-7-19(21)24(20)27-14-5-13-26-23(29)9-4-1-6-18-12-15-30-31-18/h10-11,16,18H,1-9,12-15H2,(H,26,29)(H,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum recombinant AChE after 20 mins using acetylthiocholine iodide as a substrate by Ellman's assay |
Eur J Med Chem 46: 5435-42 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.001
BindingDB Entry DOI: 10.7270/Q2ZS2WXV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10512

(CHEMBL194823 | N-{3-[(6-chloro-1,2,3,4-tetrahydroa...)Show SMILES Clc1ccc2c(NCCCNC(=O)CCCCC3CCSS3)c3CCCCc3nc2c1 Show InChI InChI=1S/C24H32ClN3OS2/c25-17-10-11-20-22(16-17)28-21-8-3-2-7-19(21)24(20)27-14-5-13-26-23(29)9-4-1-6-18-12-15-30-31-18/h10-11,16,18H,1-9,12-15H2,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.253 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum recombinant AChE after 20 mins using acetylthiocholine iodide as a substrate by Ellman's assay |
Eur J Med Chem 46: 5435-42 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.001
BindingDB Entry DOI: 10.7270/Q2ZS2WXV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50355820
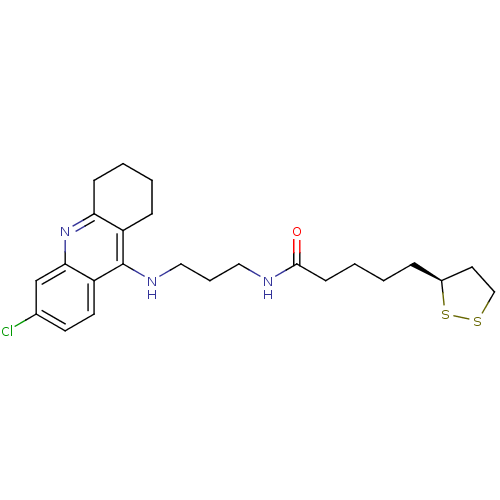
(CHEMBL1912058)Show SMILES Clc1ccc2c(NCCCNC(=O)CCCC[C@H]3CCSS3)c3CCCCc3nc2c1 |r| Show InChI InChI=1S/C24H32ClN3OS2/c25-17-10-11-20-22(16-17)28-21-8-3-2-7-19(21)24(20)27-14-5-13-26-23(29)9-4-1-6-18-12-15-30-31-18/h10-11,16,18H,1-9,12-15H2,(H,26,29)(H,27,28)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.471 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum recombinant AChE after 20 mins using acetylthiocholine iodide as a substrate by Ellman's assay |
Eur J Med Chem 46: 5435-42 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.001
BindingDB Entry DOI: 10.7270/Q2ZS2WXV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10509
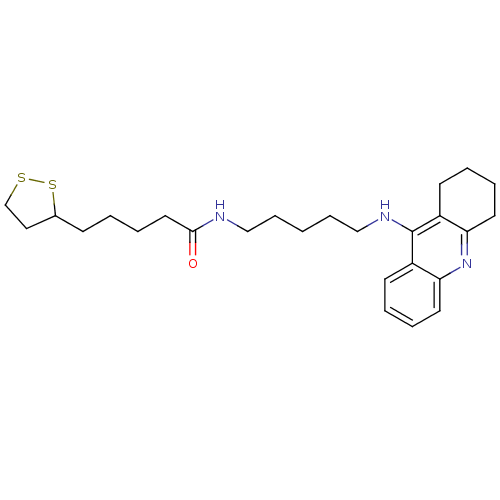
(5-(1,2-dithiolan-3-yl)-N-[5-(1,2,3,4-tetrahydroacr...)Show SMILES O=C(CCCCC1CCSS1)NCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C26H37N3OS2/c30-25(15-7-2-10-20-16-19-31-32-20)27-17-8-1-9-18-28-26-21-11-3-5-13-23(21)29-24-14-6-4-12-22(24)26/h3,5,11,13,20H,1-2,4,6-10,12,14-19H2,(H,27,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.48 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... |
J Med Chem 48: 360-3 (2005)
Article DOI: 10.1021/jm049112h
BindingDB Entry DOI: 10.7270/Q2JQ0Z7J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50231951
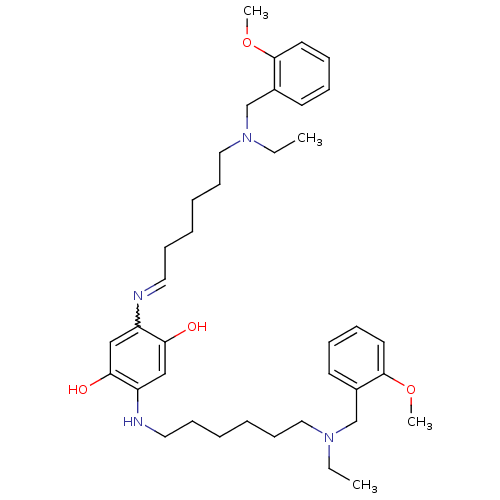
(2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:18.18| Show InChI InChI=1S/C38H56N4O4/c1-5-41(29-31-19-11-13-21-37(31)45-3)25-17-9-7-15-23-39-33-27-36(44)34(28-35(33)43)40-24-16-8-10-18-26-42(6-2)30-32-20-12-14-22-38(32)46-4/h11-14,19-23,27-28,40,43-44H,5-10,15-18,24-26,29-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum recombinant AChE after 20 mins using acetylthiocholine iodide as a substrate by Ellman's assay |
Eur J Med Chem 46: 5435-42 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.001
BindingDB Entry DOI: 10.7270/Q2ZS2WXV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221914
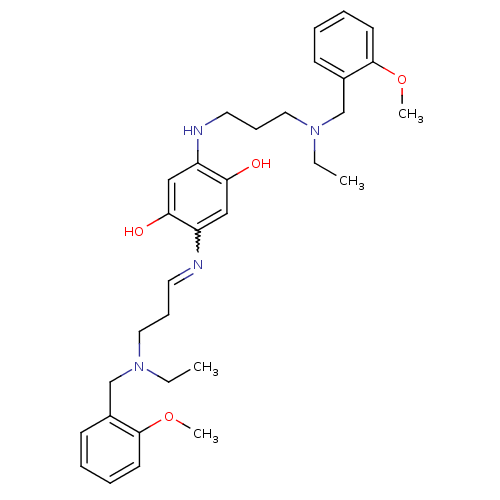
(2,5-bis{3-[ethyl(2-methoxybenzyl)amino]propylamino...)Show SMILES CCN(CCCNc1cc(O)c(cc1O)N=CCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:15.15| Show InChI InChI=1S/C32H44N4O4/c1-5-35(23-25-13-7-9-15-31(25)39-3)19-11-17-33-27-21-30(38)28(22-29(27)37)34-18-12-20-36(6-2)24-26-14-8-10-16-32(26)40-4/h7-10,13-17,21-22,34,37-38H,5-6,11-12,18-20,23-24H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221909

(2,5-bis{5-[ethyl(2-methoxybenzyl)amino]pentylamino...)Show SMILES CCN(CCCCCNc1cc(O)c(cc1O)N=CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:17.17| Show InChI InChI=1S/C36H52N4O4/c1-5-39(27-29-17-9-11-19-35(29)43-3)23-15-7-13-21-37-31-25-34(42)32(26-33(31)41)38-22-14-8-16-24-40(6-2)28-30-18-10-12-20-36(30)44-4/h9-12,17-21,25-26,38,41-42H,5-8,13-16,22-24,27-28H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50355818
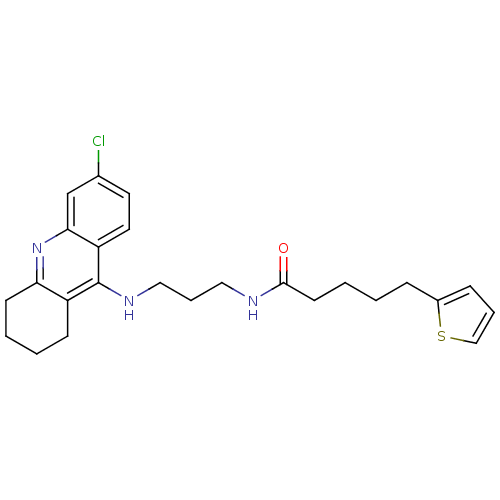
(CHEMBL1912060)Show SMILES Clc1ccc2c(NCCCNC(=O)CCCCc3cccs3)c3CCCCc3nc2c1 Show InChI InChI=1S/C25H30ClN3OS/c26-18-12-13-21-23(17-18)29-22-10-3-2-9-20(22)25(21)28-15-6-14-27-24(30)11-4-1-7-19-8-5-16-31-19/h5,8,12-13,16-17H,1-4,6-7,9-11,14-15H2,(H,27,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.66 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum recombinant AChE after 20 mins using acetylthiocholine iodide as a substrate by Ellman's assay |
Eur J Med Chem 46: 5435-42 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.001
BindingDB Entry DOI: 10.7270/Q2ZS2WXV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data