 Found 5040 hits with Last Name = 'hruby' and Initial = 'vj'
Found 5040 hits with Last Name = 'hruby' and Initial = 'vj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50295070
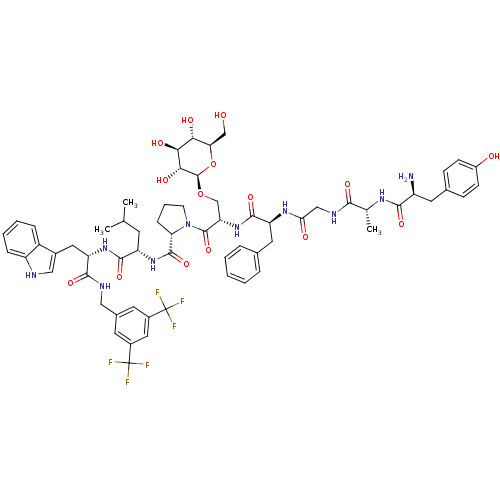
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C63H76F6N10O15/c1-32(2)20-44(57(89)76-46(25-37-28-71-43-13-8-7-12-41(37)43)56(88)72-27-36-21-38(62(64,65)66)26-39(22-36)63(67,68)69)77-59(91)48-14-9-19-79(48)60(92)47(31-93-61-53(85)52(84)51(83)49(30-80)94-61)78-58(90)45(24-34-10-5-4-6-11-34)75-50(82)29-73-54(86)33(3)74-55(87)42(70)23-35-15-17-40(81)18-16-35/h4-8,10-13,15-18,21-22,26,28,32-33,42,44-49,51-53,61,71,80-81,83-85H,9,14,19-20,23-25,27,29-31,70H2,1-3H3,(H,72,88)(H,73,86)(H,74,87)(H,75,82)(H,76,89)(H,77,91)(H,78,90)/t33-,42+,44+,45+,46+,47+,48+,49-,51-,52+,53-,61-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane |
J Med Chem 52: 5164-75 (2010)
Article DOI: 10.1021/jm900473p
BindingDB Entry DOI: 10.7270/Q2MC90ZZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50295070

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C63H76F6N10O15/c1-32(2)20-44(57(89)76-46(25-37-28-71-43-13-8-7-12-41(37)43)56(88)72-27-36-21-38(62(64,65)66)26-39(22-36)63(67,68)69)77-59(91)48-14-9-19-79(48)60(92)47(31-93-61-53(85)52(84)51(83)49(30-80)94-61)78-58(90)45(24-34-10-5-4-6-11-34)75-50(82)29-73-54(86)33(3)74-55(87)42(70)23-35-15-17-40(81)18-16-35/h4-8,10-13,15-18,21-22,26,28,32-33,42,44-49,51-53,61,71,80-81,83-85H,9,14,19-20,23-25,27,29-31,70H2,1-3H3,(H,72,88)(H,73,86)(H,74,87)(H,75,82)(H,76,89)(H,77,91)(H,78,90)/t33-,42+,44+,45+,46+,47+,48+,49-,51-,52+,53-,61-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane |
J Med Chem 52: 5164-75 (2010)
Article DOI: 10.1021/jm900473p
BindingDB Entry DOI: 10.7270/Q2MC90ZZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50295069
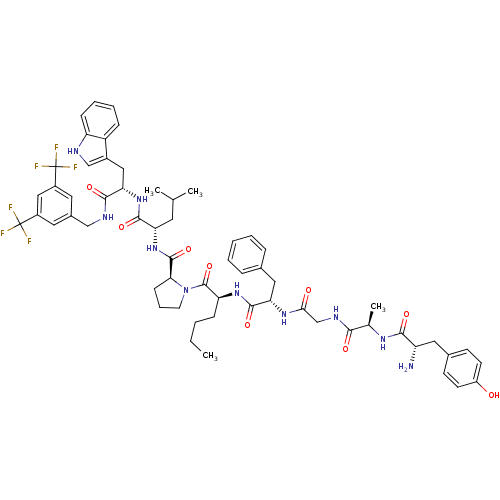
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C60H72F6N10O9/c1-5-6-16-46(73-56(83)48(28-36-13-8-7-9-14-36)72-51(78)33-70-52(79)35(4)71-53(80)44(67)27-37-19-21-42(77)22-20-37)58(85)76-23-12-18-50(76)57(84)75-47(24-34(2)3)55(82)74-49(29-39-32-68-45-17-11-10-15-43(39)45)54(81)69-31-38-25-40(59(61,62)63)30-41(26-38)60(64,65)66/h7-11,13-15,17,19-22,25-26,30,32,34-35,44,46-50,68,77H,5-6,12,16,18,23-24,27-29,31,33,67H2,1-4H3,(H,69,81)(H,70,79)(H,71,80)(H,72,78)(H,73,83)(H,74,82)(H,75,84)/t35-,44+,46+,47+,48+,49+,50+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane |
J Med Chem 52: 5164-75 (2010)
Article DOI: 10.1021/jm900473p
BindingDB Entry DOI: 10.7270/Q2MC90ZZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50295069

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C60H72F6N10O9/c1-5-6-16-46(73-56(83)48(28-36-13-8-7-9-14-36)72-51(78)33-70-52(79)35(4)71-53(80)44(67)27-37-19-21-42(77)22-20-37)58(85)76-23-12-18-50(76)57(84)75-47(24-34(2)3)55(82)74-49(29-39-32-68-45-17-11-10-15-43(39)45)54(81)69-31-38-25-40(59(61,62)63)30-41(26-38)60(64,65)66/h7-11,13-15,17,19-22,25-26,30,32,34-35,44,46-50,68,77H,5-6,12,16,18,23-24,27-29,31,33,67H2,1-4H3,(H,69,81)(H,70,79)(H,71,80)(H,72,78)(H,73,83)(H,74,82)(H,75,84)/t35-,44+,46+,47+,48+,49+,50+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane |
J Med Chem 52: 5164-75 (2010)
Article DOI: 10.1021/jm900473p
BindingDB Entry DOI: 10.7270/Q2MC90ZZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50498575
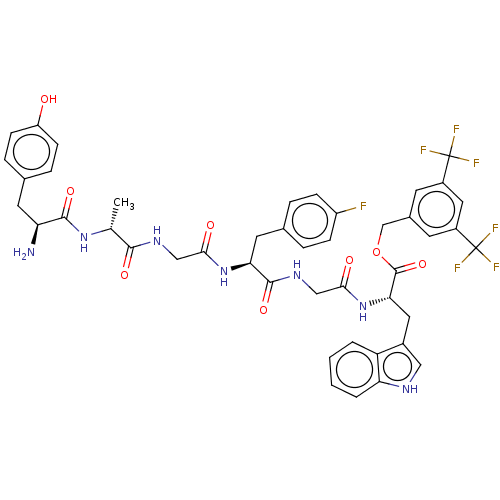
(CHEMBL3608939)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C45H44F7N7O8/c1-24(57-41(64)34(53)16-25-8-12-32(60)13-9-25)40(63)55-21-38(61)58-36(17-26-6-10-31(46)11-7-26)42(65)56-22-39(62)59-37(18-28-20-54-35-5-3-2-4-33(28)35)43(66)67-23-27-14-29(44(47,48)49)19-30(15-27)45(50,51)52/h2-15,19-20,24,34,36-37,54,60H,16-18,21-23,53H2,1H3,(H,55,63)(H,56,65)(H,57,64)(H,58,61)(H,59,62)/t24-,34+,36+,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor transfected in CHO cells |
Bioorg Med Chem Lett 25: 3716-20 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.030
BindingDB Entry DOI: 10.7270/Q2R78J7N |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50498577
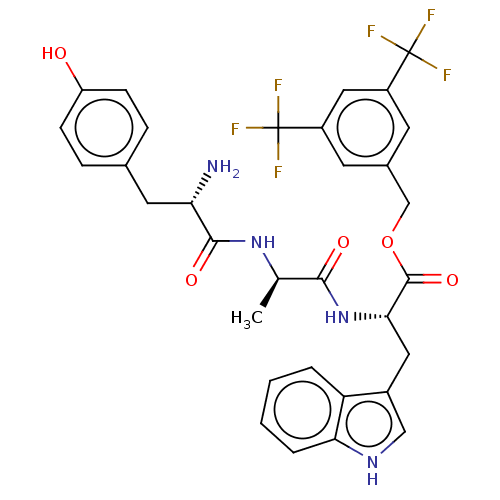
(CHEMBL3609619)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C32H30F6N4O5/c1-17(41-29(45)25(39)12-18-6-8-23(43)9-7-18)28(44)42-27(13-20-15-40-26-5-3-2-4-24(20)26)30(46)47-16-19-10-21(31(33,34)35)14-22(11-19)32(36,37)38/h2-11,14-15,17,25,27,40,43H,12-13,16,39H2,1H3,(H,41,45)(H,42,44)/t17-,25+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor transfected in CHO cells |
Bioorg Med Chem Lett 25: 3716-20 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.030
BindingDB Entry DOI: 10.7270/Q2R78J7N |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50323869
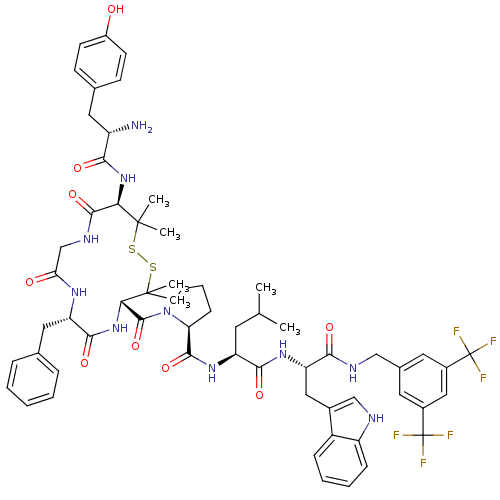
((S)-1-((4S,7S,13R)-13-((S)-2-amino-3-(4-hydroxyphe...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(C)(C)SSC1(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C61H72F6N10O9S2/c1-33(2)23-44(53(82)73-46(28-37-31-69-43-16-11-10-15-41(37)43)52(81)70-30-36-24-38(60(62,63)64)29-39(25-36)61(65,66)67)74-55(84)47-17-12-22-77(47)57(86)50-59(5,6)88-87-58(3,4)49(75-51(80)42(68)26-35-18-20-40(78)21-19-35)56(85)71-32-48(79)72-45(54(83)76-50)27-34-13-8-7-9-14-34/h7-11,13-16,18-21,24-25,29,31,33,42,44-47,49-50,69,78H,12,17,22-23,26-28,30,32,68H2,1-6H3,(H,70,81)(H,71,85)(H,72,79)(H,73,82)(H,74,84)(H,75,80)(H,76,83)/t42-,44-,45-,46-,47-,49+,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P frome human NK1 receptor |
J Med Chem 53: 5491-501 (2010)
Article DOI: 10.1021/jm100157m
BindingDB Entry DOI: 10.7270/Q21N81BD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50323869

((S)-1-((4S,7S,13R)-13-((S)-2-amino-3-(4-hydroxyphe...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(C)(C)SSC1(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C61H72F6N10O9S2/c1-33(2)23-44(53(82)73-46(28-37-31-69-43-16-11-10-15-41(37)43)52(81)70-30-36-24-38(60(62,63)64)29-39(25-36)61(65,66)67)74-55(84)47-17-12-22-77(47)57(86)50-59(5,6)88-87-58(3,4)49(75-51(80)42(68)26-35-18-20-40(78)21-19-35)56(85)71-32-48(79)72-45(54(83)76-50)27-34-13-8-7-9-14-34/h7-11,13-16,18-21,24-25,29,31,33,42,44-47,49-50,69,78H,12,17,22-23,26-28,30,32,68H2,1-6H3,(H,70,81)(H,71,85)(H,72,79)(H,73,82)(H,74,84)(H,75,80)(H,76,83)/t42-,44-,45-,46-,47-,49+,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P frome human NK1 receptor |
J Med Chem 53: 5491-501 (2010)
Article DOI: 10.1021/jm100157m
BindingDB Entry DOI: 10.7270/Q21N81BD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439327
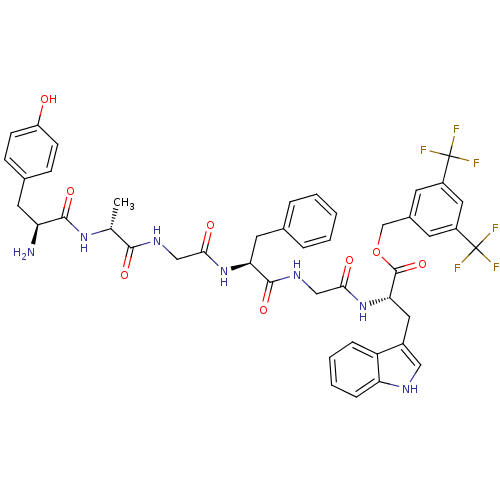
(CHEMBL2419544)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C45H45F6N7O8/c1-25(56-41(63)34(52)17-27-11-13-32(59)14-12-27)40(62)54-22-38(60)57-36(18-26-7-3-2-4-8-26)42(64)55-23-39(61)58-37(19-29-21-53-35-10-6-5-9-33(29)35)43(65)66-24-28-15-30(44(46,47)48)20-31(16-28)45(49,50)51/h2-16,20-21,25,34,36-37,53,59H,17-19,22-24,52H2,1H3,(H,54,62)(H,55,64)(H,56,63)(H,57,60)(H,58,61)/t25-,34+,36+,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50264406
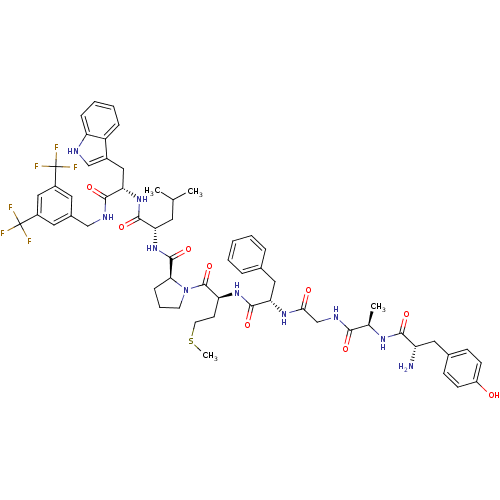
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C59H70F6N10O9S/c1-33(2)23-46(54(81)73-48(28-38-31-67-44-14-9-8-13-42(38)44)53(80)68-30-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)74-56(83)49-15-10-21-75(49)57(84)45(20-22-85-4)72-55(82)47(27-35-11-6-5-7-12-35)71-50(77)32-69-51(78)34(3)70-52(79)43(66)26-36-16-18-41(76)19-17-36/h5-9,11-14,16-19,24-25,29,31,33-34,43,45-49,67,76H,10,15,20-23,26-28,30,32,66H2,1-4H3,(H,68,80)(H,69,78)(H,70,79)(H,71,77)(H,72,82)(H,73,81)(H,74,83)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting |
J Med Chem 51: 6334-47 (2008)
Article DOI: 10.1021/jm800389v
BindingDB Entry DOI: 10.7270/Q26T0MF1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50264406

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C59H70F6N10O9S/c1-33(2)23-46(54(81)73-48(28-38-31-67-44-14-9-8-13-42(38)44)53(80)68-30-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)74-56(83)49-15-10-21-75(49)57(84)45(20-22-85-4)72-55(82)47(27-35-11-6-5-7-12-35)71-50(77)32-69-51(78)34(3)70-52(79)43(66)26-36-16-18-41(76)19-17-36/h5-9,11-14,16-19,24-25,29,31,33-34,43,45-49,67,76H,10,15,20-23,26-28,30,32,66H2,1-4H3,(H,68,80)(H,69,78)(H,70,79)(H,71,77)(H,72,82)(H,73,81)(H,74,83)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P frome human NK1 receptor |
J Med Chem 53: 5491-501 (2010)
Article DOI: 10.1021/jm100157m
BindingDB Entry DOI: 10.7270/Q21N81BD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50264406

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C59H70F6N10O9S/c1-33(2)23-46(54(81)73-48(28-38-31-67-44-14-9-8-13-42(38)44)53(80)68-30-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)74-56(83)49-15-10-21-75(49)57(84)45(20-22-85-4)72-55(82)47(27-35-11-6-5-7-12-35)71-50(77)32-69-51(78)34(3)70-52(79)43(66)26-36-16-18-41(76)19-17-36/h5-9,11-14,16-19,24-25,29,31,33-34,43,45-49,67,76H,10,15,20-23,26-28,30,32,66H2,1-4H3,(H,68,80)(H,69,78)(H,70,79)(H,71,77)(H,72,82)(H,73,81)(H,74,83)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membranes |
Bioorg Med Chem 17: 7337-43 (2009)
Article DOI: 10.1016/j.bmc.2009.08.035
BindingDB Entry DOI: 10.7270/Q2CJ8DK5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50264406

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C59H70F6N10O9S/c1-33(2)23-46(54(81)73-48(28-38-31-67-44-14-9-8-13-42(38)44)53(80)68-30-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)74-56(83)49-15-10-21-75(49)57(84)45(20-22-85-4)72-55(82)47(27-35-11-6-5-7-12-35)71-50(77)32-69-51(78)34(3)70-52(79)43(66)26-36-16-18-41(76)19-17-36/h5-9,11-14,16-19,24-25,29,31,33-34,43,45-49,67,76H,10,15,20-23,26-28,30,32,66H2,1-4H3,(H,68,80)(H,69,78)(H,70,79)(H,71,77)(H,72,82)(H,73,81)(H,74,83)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membranes |
Bioorg Med Chem 17: 7337-43 (2009)
Article DOI: 10.1016/j.bmc.2009.08.035
BindingDB Entry DOI: 10.7270/Q2CJ8DK5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341318
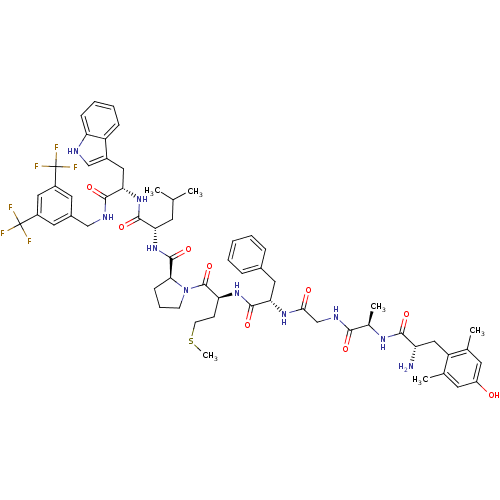
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C61H74F6N10O9S/c1-33(2)21-48(56(83)75-50(27-39-31-69-46-16-11-10-15-43(39)46)55(82)70-30-38-24-40(60(62,63)64)28-41(25-38)61(65,66)67)76-58(85)51-17-12-19-77(51)59(86)47(18-20-87-6)74-57(84)49(26-37-13-8-7-9-14-37)73-52(79)32-71-53(80)36(5)72-54(81)45(68)29-44-34(3)22-42(78)23-35(44)4/h7-11,13-16,22-25,28,31,33,36,45,47-51,69,78H,12,17-21,26-27,29-30,32,68H2,1-6H3,(H,70,82)(H,71,80)(H,72,81)(H,73,79)(H,74,84)(H,75,83)(H,76,85)/t36-,45+,47+,48+,49+,50+,51+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341318

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C61H74F6N10O9S/c1-33(2)21-48(56(83)75-50(27-39-31-69-46-16-11-10-15-43(39)46)55(82)70-30-38-24-40(60(62,63)64)28-41(25-38)61(65,66)67)76-58(85)51-17-12-19-77(51)59(86)47(18-20-87-6)74-57(84)49(26-37-13-8-7-9-14-37)73-52(79)32-71-53(80)36(5)72-54(81)45(68)29-44-34(3)22-42(78)23-35(44)4/h7-11,13-16,22-25,28,31,33,36,45,47-51,69,78H,12,17-21,26-27,29-30,32,68H2,1-6H3,(H,70,82)(H,71,80)(H,72,81)(H,73,79)(H,74,84)(H,75,83)(H,76,85)/t36-,45+,47+,48+,49+,50+,51+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439329
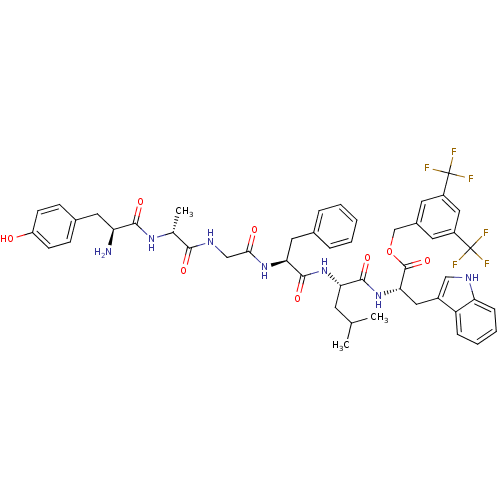
(CHEMBL2419542)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C49H53F6N7O8/c1-27(2)17-39(45(67)62-41(22-32-24-57-38-12-8-7-11-36(32)38)47(69)70-26-31-18-33(48(50,51)52)23-34(19-31)49(53,54)55)61-46(68)40(21-29-9-5-4-6-10-29)60-42(64)25-58-43(65)28(3)59-44(66)37(56)20-30-13-15-35(63)16-14-30/h4-16,18-19,23-24,27-28,37,39-41,57,63H,17,20-22,25-26,56H2,1-3H3,(H,58,65)(H,59,66)(H,60,64)(H,61,68)(H,62,67)/t28-,37+,39+,40+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50498573
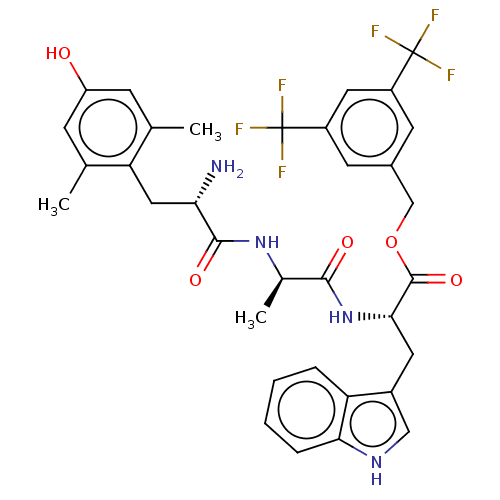
(CHEMBL3609620)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C34H34F6N4O5/c1-17-8-24(45)9-18(2)26(17)14-27(41)31(47)43-19(3)30(46)44-29(12-21-15-42-28-7-5-4-6-25(21)28)32(48)49-16-20-10-22(33(35,36)37)13-23(11-20)34(38,39)40/h4-11,13,15,19,27,29,42,45H,12,14,16,41H2,1-3H3,(H,43,47)(H,44,46)/t19-,27+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor transfected in CHO cells |
Bioorg Med Chem Lett 25: 3716-20 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.030
BindingDB Entry DOI: 10.7270/Q2R78J7N |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50498569
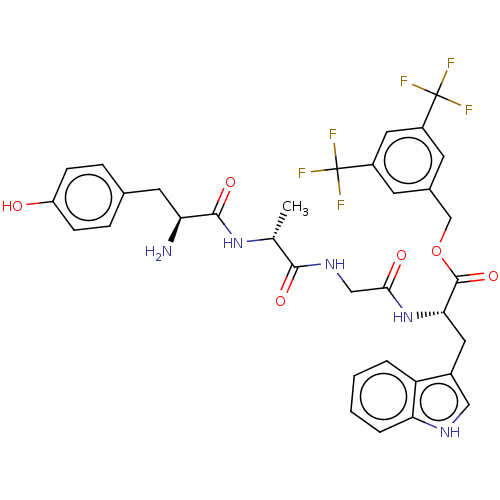
(CHEMBL3609617)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C34H33F6N5O6/c1-18(44-31(49)26(41)12-19-6-8-24(46)9-7-19)30(48)43-16-29(47)45-28(13-21-15-42-27-5-3-2-4-25(21)27)32(50)51-17-20-10-22(33(35,36)37)14-23(11-20)34(38,39)40/h2-11,14-15,18,26,28,42,46H,12-13,16-17,41H2,1H3,(H,43,48)(H,44,49)(H,45,47)/t18-,26+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor transfected in CHO cells |
Bioorg Med Chem Lett 25: 3716-20 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.030
BindingDB Entry DOI: 10.7270/Q2R78J7N |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439326
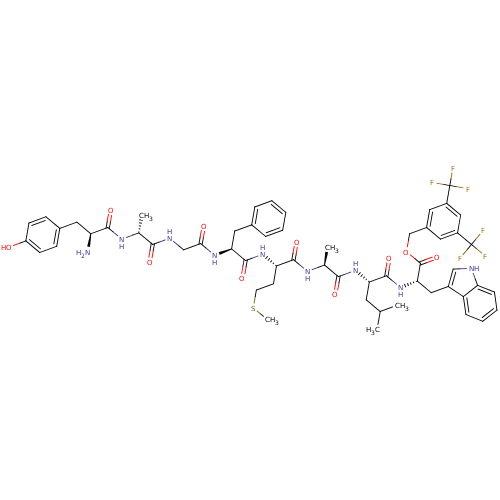
(CHEMBL2419537)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C57H67F6N9O10S/c1-31(2)21-45(53(79)72-47(26-37-28-65-43-14-10-9-13-41(37)43)55(81)82-30-36-22-38(56(58,59)60)27-39(23-36)57(61,62)63)71-50(76)33(4)68-52(78)44(19-20-83-5)70-54(80)46(25-34-11-7-6-8-12-34)69-48(74)29-66-49(75)32(3)67-51(77)42(64)24-35-15-17-40(73)18-16-35/h6-18,22-23,27-28,31-33,42,44-47,65,73H,19-21,24-26,29-30,64H2,1-5H3,(H,66,75)(H,67,77)(H,68,78)(H,69,74)(H,70,80)(H,71,76)(H,72,79)/t32-,33+,42+,44+,45+,46+,47+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50498575

(CHEMBL3608939)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C45H44F7N7O8/c1-24(57-41(64)34(53)16-25-8-12-32(60)13-9-25)40(63)55-21-38(61)58-36(17-26-6-10-31(46)11-7-26)42(65)56-22-39(62)59-37(18-28-20-54-35-5-3-2-4-33(28)35)43(66)67-23-27-14-29(44(47,48)49)19-30(15-27)45(50,51)52/h2-15,19-20,24,34,36-37,54,60H,16-18,21-23,53H2,1H3,(H,55,63)(H,56,65)(H,57,64)(H,58,61)(H,59,62)/t24-,34+,36+,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from rat NK1 receptor transfected in CHO cells |
Bioorg Med Chem Lett 25: 3716-20 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.030
BindingDB Entry DOI: 10.7270/Q2R78J7N |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM21007
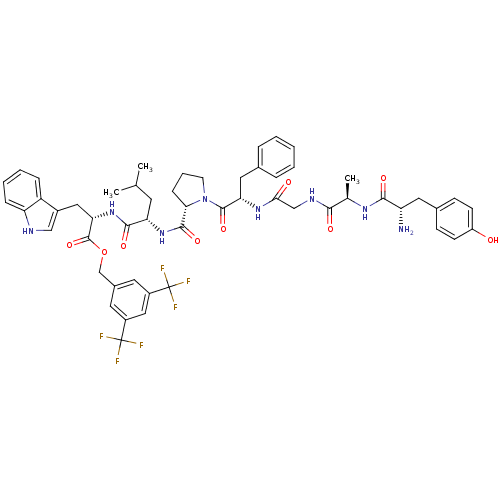
(C-terminal modified bifunctional peptide, 1 | H-Ty...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C54H60F6N8O9/c1-30(2)20-42(49(73)67-44(25-35-27-62-41-13-8-7-12-39(35)41)52(76)77-29-34-21-36(53(55,56)57)26-37(22-34)54(58,59)60)66-50(74)45-14-9-19-68(45)51(75)43(24-32-10-5-4-6-11-32)65-46(70)28-63-47(71)31(3)64-48(72)40(61)23-33-15-17-38(69)18-16-33/h4-8,10-13,15-18,21-22,26-27,30-31,40,42-45,62,69H,9,14,19-20,23-25,28-29,61H2,1-3H3,(H,63,71)(H,64,72)(H,65,70)(H,66,74)(H,67,73)/t31-,40+,42+,43+,44+,45+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | -59.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 51: 1369-76 (2008)
Article DOI: 10.1021/jm070332f
BindingDB Entry DOI: 10.7270/Q2X63K7C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50498575

(CHEMBL3608939)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C45H44F7N7O8/c1-24(57-41(64)34(53)16-25-8-12-32(60)13-9-25)40(63)55-21-38(61)58-36(17-26-6-10-31(46)11-7-26)42(65)56-22-39(62)59-37(18-28-20-54-35-5-3-2-4-33(28)35)43(66)67-23-27-14-29(44(47,48)49)19-30(15-27)45(50,51)52/h2-15,19-20,24,34,36-37,54,60H,16-18,21-23,53H2,1H3,(H,55,63)(H,56,65)(H,57,64)(H,58,61)(H,59,62)/t24-,34+,36+,37+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from rat mu opioid receptor transfected in HN9.10 cells |
Bioorg Med Chem Lett 25: 3716-20 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.030
BindingDB Entry DOI: 10.7270/Q2R78J7N |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50498576
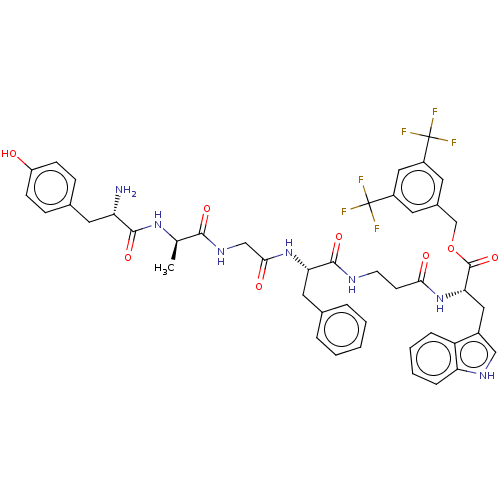
(CHEMBL3608937)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C46H47F6N7O8/c1-26(57-42(64)35(53)19-28-11-13-33(60)14-12-28)41(63)56-24-40(62)58-37(20-27-7-3-2-4-8-27)43(65)54-16-15-39(61)59-38(21-30-23-55-36-10-6-5-9-34(30)36)44(66)67-25-29-17-31(45(47,48)49)22-32(18-29)46(50,51)52/h2-14,17-18,22-23,26,35,37-38,55,60H,15-16,19-21,24-25,53H2,1H3,(H,54,65)(H,56,63)(H,57,64)(H,58,62)(H,59,61)/t26-,35+,37+,38+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor transfected in CHO cells |
Bioorg Med Chem Lett 25: 3716-20 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.030
BindingDB Entry DOI: 10.7270/Q2R78J7N |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50295067
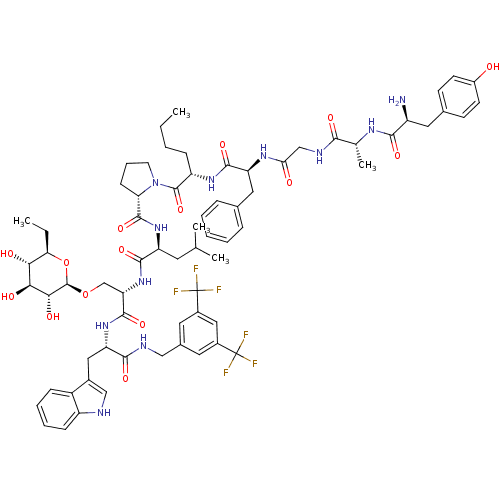
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO[C@@H]1O[C@H](CC)[C@@H](O)[C@H](O)[C@H]1O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C70H89F6N11O15/c1-6-8-18-49(83-64(97)51(30-39-15-10-9-11-16-39)82-56(89)35-80-60(93)38(5)81-61(94)47(77)29-40-21-23-45(88)24-22-40)67(100)87-25-14-20-54(87)66(99)85-50(26-37(3)4)63(96)86-53(36-101-68-59(92)58(91)57(90)55(7-2)102-68)65(98)84-52(31-42-34-78-48-19-13-12-17-46(42)48)62(95)79-33-41-27-43(69(71,72)73)32-44(28-41)70(74,75)76/h9-13,15-17,19,21-24,27-28,32,34,37-38,47,49-55,57-59,68,78,88,90-92H,6-8,14,18,20,25-26,29-31,33,35-36,77H2,1-5H3,(H,79,95)(H,80,93)(H,81,94)(H,82,89)(H,83,97)(H,84,98)(H,85,99)(H,86,96)/t38-,47+,49+,50+,51+,52+,53+,54+,55-,57-,58+,59-,68-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane |
J Med Chem 52: 5164-75 (2010)
Article DOI: 10.1021/jm900473p
BindingDB Entry DOI: 10.7270/Q2MC90ZZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50295067

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO[C@@H]1O[C@H](CC)[C@@H](O)[C@H](O)[C@H]1O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C70H89F6N11O15/c1-6-8-18-49(83-64(97)51(30-39-15-10-9-11-16-39)82-56(89)35-80-60(93)38(5)81-61(94)47(77)29-40-21-23-45(88)24-22-40)67(100)87-25-14-20-54(87)66(99)85-50(26-37(3)4)63(96)86-53(36-101-68-59(92)58(91)57(90)55(7-2)102-68)65(98)84-52(31-42-34-78-48-19-13-12-17-46(42)48)62(95)79-33-41-27-43(69(71,72)73)32-44(28-41)70(74,75)76/h9-13,15-17,19,21-24,27-28,32,34,37-38,47,49-55,57-59,68,78,88,90-92H,6-8,14,18,20,25-26,29-31,33,35-36,77H2,1-5H3,(H,79,95)(H,80,93)(H,81,94)(H,82,89)(H,83,97)(H,84,98)(H,85,99)(H,86,96)/t38-,47+,49+,50+,51+,52+,53+,54+,55-,57-,58+,59-,68-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane |
J Med Chem 52: 5164-75 (2010)
Article DOI: 10.1021/jm900473p
BindingDB Entry DOI: 10.7270/Q2MC90ZZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439325
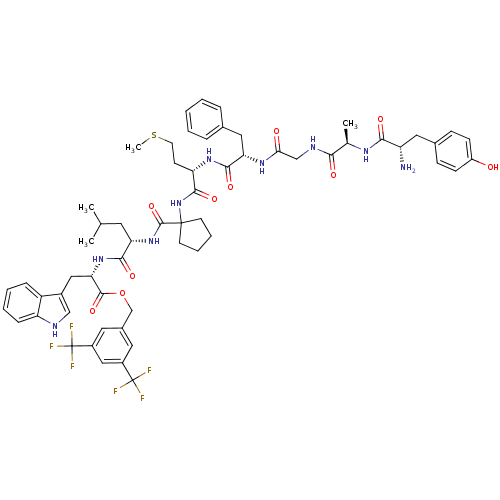
(CHEMBL2419538)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NC1(CCCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C60H71F6N9O10S/c1-34(2)24-47(53(80)73-49(29-39-31-68-45-15-9-8-14-43(39)45)56(83)85-33-38-25-40(59(61,62)63)30-41(26-38)60(64,65)66)74-57(84)58(21-10-11-22-58)75-55(82)46(20-23-86-4)72-54(81)48(28-36-12-6-5-7-13-36)71-50(77)32-69-51(78)35(3)70-52(79)44(67)27-37-16-18-42(76)19-17-37/h5-9,12-19,25-26,30-31,34-35,44,46-49,68,76H,10-11,20-24,27-29,32-33,67H2,1-4H3,(H,69,78)(H,70,79)(H,71,77)(H,72,81)(H,73,80)(H,74,84)(H,75,82)/t35-,44+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558721

(CHEMBL4784791)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341314
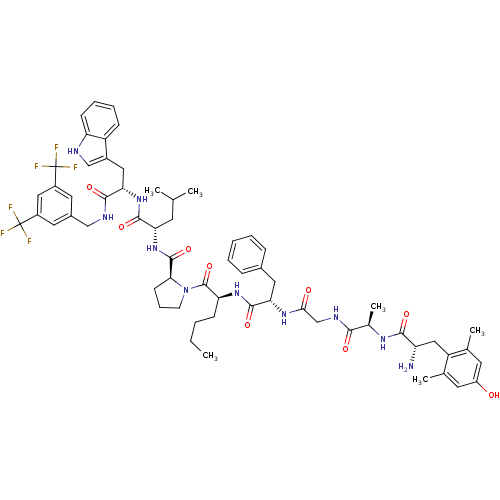
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C62H76F6N10O9/c1-7-8-18-48(75-58(85)50(27-38-15-10-9-11-16-38)74-53(80)33-72-54(81)37(6)73-55(82)46(69)30-45-35(4)23-43(79)24-36(45)5)60(87)78-21-14-20-52(78)59(86)77-49(22-34(2)3)57(84)76-51(28-40-32-70-47-19-13-12-17-44(40)47)56(83)71-31-39-25-41(61(63,64)65)29-42(26-39)62(66,67)68/h9-13,15-17,19,23-26,29,32,34,37,46,48-52,70,79H,7-8,14,18,20-22,27-28,30-31,33,69H2,1-6H3,(H,71,83)(H,72,81)(H,73,82)(H,74,80)(H,75,85)(H,76,84)(H,77,86)/t37-,46+,48+,49+,50+,51+,52+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341314

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C62H76F6N10O9/c1-7-8-18-48(75-58(85)50(27-38-15-10-9-11-16-38)74-53(80)33-72-54(81)37(6)73-55(82)46(69)30-45-35(4)23-43(79)24-36(45)5)60(87)78-21-14-20-52(78)59(86)77-49(22-34(2)3)57(84)76-51(28-40-32-70-47-19-13-12-17-44(40)47)56(83)71-31-39-25-41(61(63,64)65)29-42(26-39)62(66,67)68/h9-13,15-17,19,23-26,29,32,34,37,46,48-52,70,79H,7-8,14,18,20-22,27-28,30-31,33,69H2,1-6H3,(H,71,83)(H,72,81)(H,73,82)(H,74,80)(H,75,85)(H,76,84)(H,77,86)/t37-,46+,48+,49+,50+,51+,52+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50295072
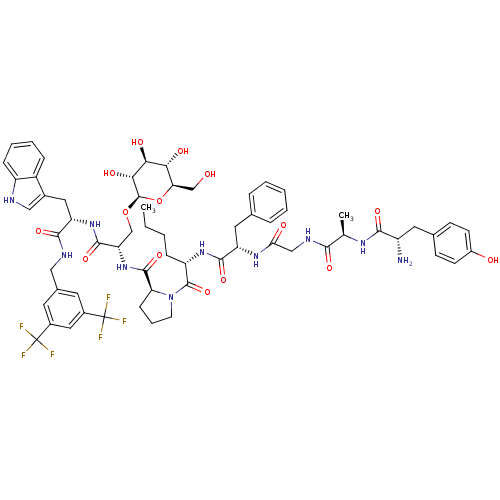
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C63H76F6N10O15/c1-3-4-14-44(76-57(89)45(25-34-11-6-5-7-12-34)75-50(82)30-73-54(86)33(2)74-55(87)42(70)24-35-17-19-40(81)20-18-35)60(92)79-21-10-16-48(79)59(91)78-47(32-93-61-53(85)52(84)51(83)49(31-80)94-61)58(90)77-46(26-37-29-71-43-15-9-8-13-41(37)43)56(88)72-28-36-22-38(62(64,65)66)27-39(23-36)63(67,68)69/h5-9,11-13,15,17-20,22-23,27,29,33,42,44-49,51-53,61,71,80-81,83-85H,3-4,10,14,16,21,24-26,28,30-32,70H2,1-2H3,(H,72,88)(H,73,86)(H,74,87)(H,75,82)(H,76,89)(H,77,90)(H,78,91)/t33-,42+,44+,45+,46+,47+,48+,49-,51-,52+,53-,61-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane |
J Med Chem 52: 5164-75 (2010)
Article DOI: 10.1021/jm900473p
BindingDB Entry DOI: 10.7270/Q2MC90ZZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM21021
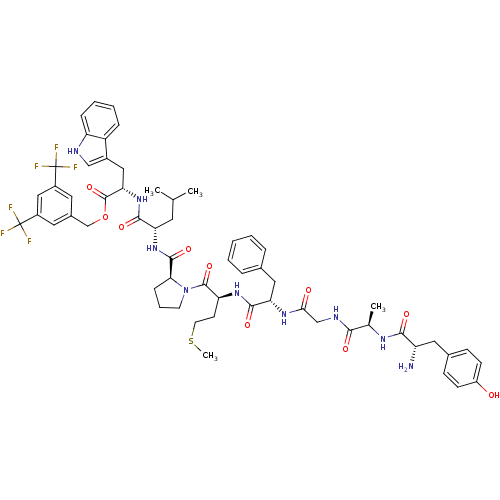
(Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C59H69F6N9O10S/c1-33(2)23-46(53(79)73-48(28-38-30-67-44-14-9-8-13-42(38)44)57(83)84-32-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)72-55(81)49-15-10-21-74(49)56(82)45(20-22-85-4)71-54(80)47(27-35-11-6-5-7-12-35)70-50(76)31-68-51(77)34(3)69-52(78)43(66)26-36-16-18-41(75)19-17-36/h5-9,11-14,16-19,24-25,29-30,33-34,43,45-49,67,75H,10,15,20-23,26-28,31-32,66H2,1-4H3,(H,68,77)(H,69,78)(H,70,76)(H,71,80)(H,72,81)(H,73,79)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM21021

(Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C59H69F6N9O10S/c1-33(2)23-46(53(79)73-48(28-38-30-67-44-14-9-8-13-42(38)44)57(83)84-32-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)72-55(81)49-15-10-21-74(49)56(82)45(20-22-85-4)71-54(80)47(27-35-11-6-5-7-12-35)70-50(76)31-68-51(77)34(3)69-52(78)43(66)26-36-16-18-41(75)19-17-36/h5-9,11-14,16-19,24-25,29-30,33-34,43,45-49,67,75H,10,15,20-23,26-28,31-32,66H2,1-4H3,(H,68,77)(H,69,78)(H,70,76)(H,71,80)(H,72,81)(H,73,79)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting |
J Med Chem 51: 6334-47 (2008)
Article DOI: 10.1021/jm800389v
BindingDB Entry DOI: 10.7270/Q26T0MF1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50439329

(CHEMBL2419542)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C49H53F6N7O8/c1-27(2)17-39(45(67)62-41(22-32-24-57-38-12-8-7-11-36(32)38)47(69)70-26-31-18-33(48(50,51)52)23-34(19-31)49(53,54)55)61-46(68)40(21-29-9-5-4-6-10-29)60-42(64)25-58-43(65)28(3)59-44(66)37(56)20-30-13-15-35(63)16-14-30/h4-16,18-19,23-24,27-28,37,39-41,57,63H,17,20-22,25-26,56H2,1-3H3,(H,58,65)(H,59,66)(H,60,64)(H,61,68)(H,62,67)/t28-,37+,39+,40+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439330
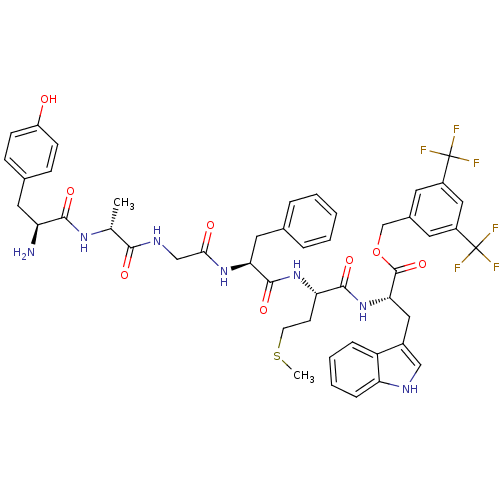
(CHEMBL2419541)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C48H51F6N7O8S/c1-27(58-43(65)36(55)20-29-12-14-34(62)15-13-29)42(64)57-25-41(63)59-39(21-28-8-4-3-5-9-28)45(67)60-38(16-17-70-2)44(66)61-40(22-31-24-56-37-11-7-6-10-35(31)37)46(68)69-26-30-18-32(47(49,50)51)23-33(19-30)48(52,53)54/h3-15,18-19,23-24,27,36,38-40,56,62H,16-17,20-22,25-26,55H2,1-2H3,(H,57,64)(H,58,65)(H,59,63)(H,60,67)(H,61,66)/t27-,36+,38+,39+,40+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50491858
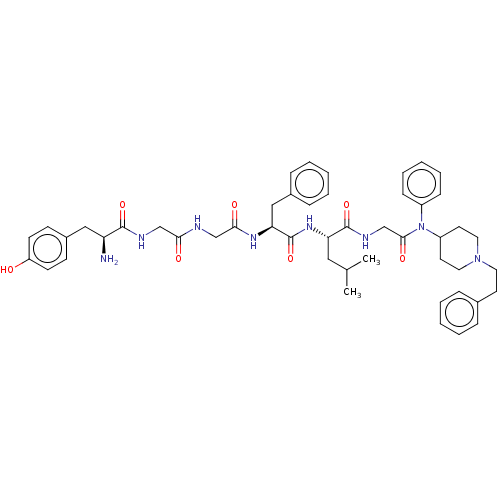
(CHEMBL2387338)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 |r| Show InChI InChI=1S/C49H62N8O7/c1-34(2)28-42(48(63)53-33-46(61)57(38-16-10-5-11-17-38)39-23-26-56(27-24-39)25-22-35-12-6-3-7-13-35)55-49(64)43(30-36-14-8-4-9-15-36)54-45(60)32-51-44(59)31-52-47(62)41(50)29-37-18-20-40(58)21-19-37/h3-21,34,39,41-43,58H,22-33,50H2,1-2H3,(H,51,59)(H,52,62)(H,53,63)(H,54,60)(H,55,64)/t41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cell membranes |
Bioorg Med Chem Lett 23: 3434-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.065
BindingDB Entry DOI: 10.7270/Q2T72MCM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM214798
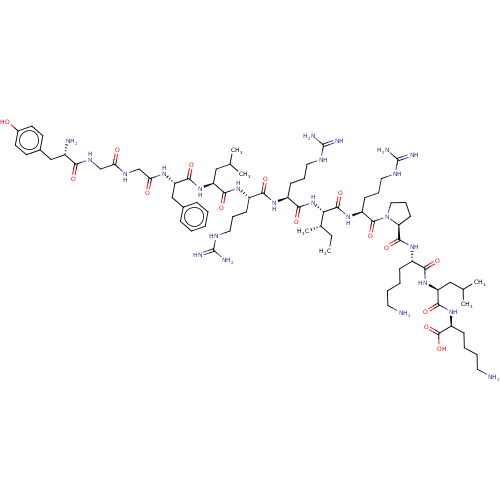
(Dynorphin A (1-17) | YGGFLRRIRPKLK)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439328
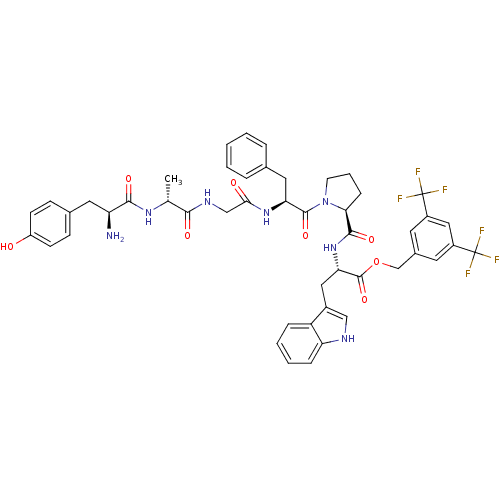
(CHEMBL2419543)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C48H49F6N7O8/c1-27(58-43(65)36(55)20-29-13-15-34(62)16-14-29)42(64)57-25-41(63)59-38(21-28-8-3-2-4-9-28)45(67)61-17-7-12-40(61)44(66)60-39(22-31-24-56-37-11-6-5-10-35(31)37)46(68)69-26-30-18-32(47(49,50)51)23-33(19-30)48(52,53)54/h2-6,8-11,13-16,18-19,23-24,27,36,38-40,56,62H,7,12,17,20-22,25-26,55H2,1H3,(H,57,64)(H,58,65)(H,59,63)(H,60,66)/t27-,36+,38+,39+,40+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50304490
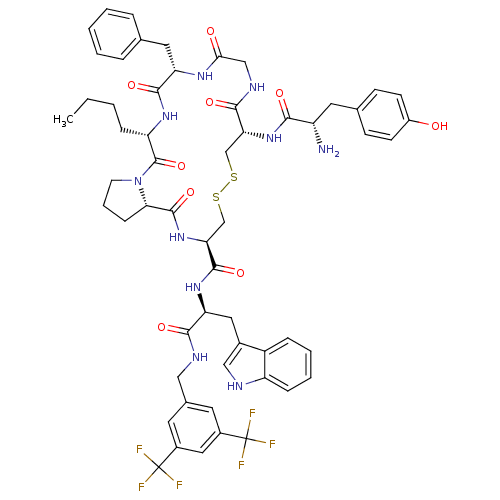
(CHEMBL611926 | H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-Cys]-...)Show SMILES CCCC[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](CSSC[C@H](NC(=O)[C@@H]2CCCN2C1=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F)NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C57H64F6N10O9S2/c1-2-3-13-42-55(82)73-20-9-15-47(73)54(81)72-46(53(80)70-44(25-35-28-65-41-14-8-7-12-39(35)41)50(77)66-27-34-21-36(56(58,59)60)26-37(22-34)57(61,62)63)31-84-83-30-45(71-49(76)40(64)23-33-16-18-38(74)19-17-33)51(78)67-29-48(75)68-43(52(79)69-42)24-32-10-5-4-6-11-32/h4-8,10-12,14,16-19,21-22,26,28,40,42-47,65,74H,2-3,9,13,15,20,23-25,27,29-31,64H2,1H3,(H,66,77)(H,67,78)(H,68,75)(H,69,79)(H,70,80)(H,71,76)(H,72,81)/t40-,42-,43-,44-,45+,46-,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membranes |
Bioorg Med Chem 17: 7337-43 (2009)
Article DOI: 10.1016/j.bmc.2009.08.035
BindingDB Entry DOI: 10.7270/Q2CJ8DK5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010704
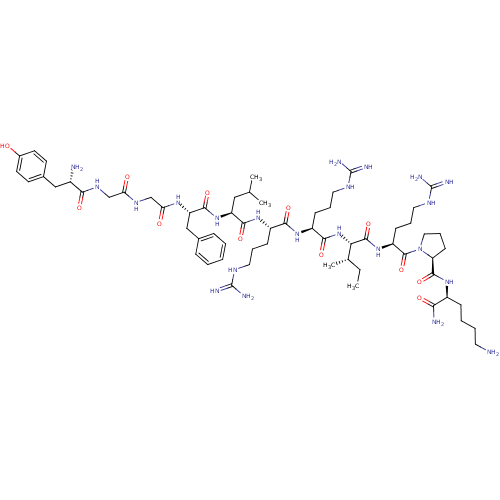
(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50122099
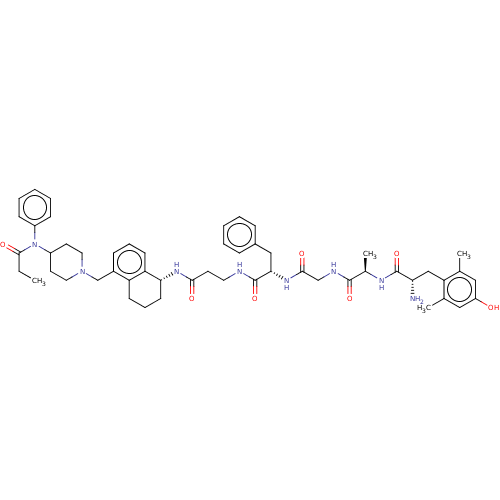
(CHEMBL3617471)Show SMILES CCC(=O)N(C1CCN(Cc2cccc3[C@@H](CCCc23)NC(=O)CCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)CC1)c1ccccc1 |r| Show InChI InChI=1S/C53H68N8O7/c1-5-50(65)61(39-17-10-7-11-18-39)40-23-26-60(27-24-40)33-38-16-12-20-43-42(38)19-13-21-46(43)58-48(63)22-25-55-53(68)47(30-37-14-8-6-9-15-37)59-49(64)32-56-51(66)36(4)57-52(67)45(54)31-44-34(2)28-41(62)29-35(44)3/h6-12,14-18,20,28-29,36,40,45-47,62H,5,13,19,21-27,30-33,54H2,1-4H3,(H,55,68)(H,56,66)(H,57,67)(H,58,63)(H,59,64)/t36-,45+,46-,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H] DAMGO from rat mu-opioid receptor |
Bioorg Med Chem Lett 25: 4683-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.064
BindingDB Entry DOI: 10.7270/Q2N29ZR2 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084
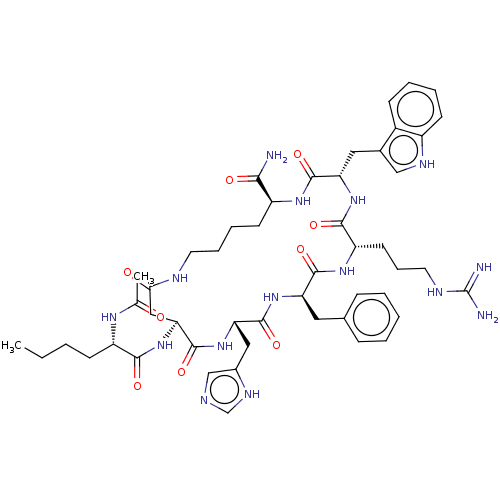
(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R LBD expressed in HEK293 cell membranes incubated for 16 to 23 hrs in dark by scintillation proxi... |
J Med Chem 61: 3674-3684 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00170
BindingDB Entry DOI: 10.7270/Q2736TCZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439324
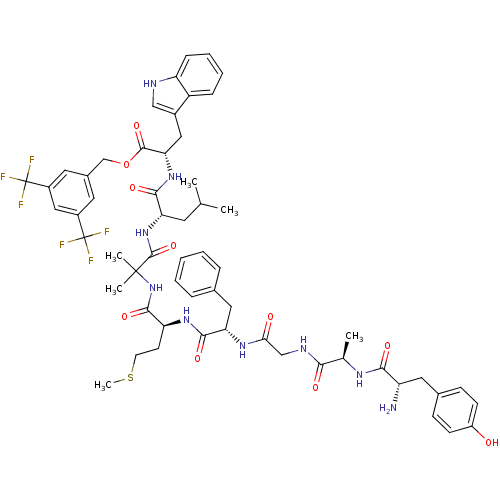
(CHEMBL2419539)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NC(C)(C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C58H69F6N9O10S/c1-32(2)22-45(51(78)71-47(27-37-29-66-43-15-11-10-14-41(37)43)54(81)83-31-36-23-38(57(59,60)61)28-39(24-36)58(62,63)64)72-55(82)56(4,5)73-53(80)44(20-21-84-6)70-52(79)46(26-34-12-8-7-9-13-34)69-48(75)30-67-49(76)33(3)68-50(77)42(65)25-35-16-18-40(74)19-17-35/h7-19,23-24,28-29,32-33,42,44-47,66,74H,20-22,25-27,30-31,65H2,1-6H3,(H,67,76)(H,68,77)(H,69,75)(H,70,79)(H,71,78)(H,72,82)(H,73,80)/t33-,42+,44+,45+,46+,47+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50001157
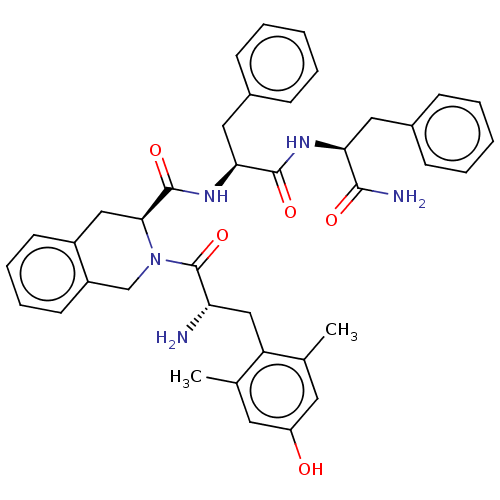
(CHEMBL538700)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C39H43N5O5/c1-24-17-30(45)18-25(2)31(24)22-32(40)39(49)44-23-29-16-10-9-15-28(29)21-35(44)38(48)43-34(20-27-13-7-4-8-14-27)37(47)42-33(36(41)46)19-26-11-5-3-6-12-26/h3-18,32-35,45H,19-23,40H2,1-2H3,(H2,41,46)(H,42,47)(H,43,48)/t32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in human/mouse HN9.10 cell membranes after 3 hrs by liquid scintillation countin... |
ACS Med Chem Lett 4: 656-659 (2013)
Article DOI: 10.1021/ml400115n
BindingDB Entry DOI: 10.7270/Q2CN76VX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50498572

(CHEMBL3609616)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C43H42F6N6O7/c1-24(53-39(59)33(50)17-26-11-13-31(56)14-12-26)38(58)52-22-37(57)54-35(18-25-7-3-2-4-8-25)40(60)55-36(19-28-21-51-34-10-6-5-9-32(28)34)41(61)62-23-27-15-29(42(44,45)46)20-30(16-27)43(47,48)49/h2-16,20-21,24,33,35-36,51,56H,17-19,22-23,50H2,1H3,(H,52,58)(H,53,59)(H,54,57)(H,55,60)/t24-,33+,35+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor transfected in CHO cells |
Bioorg Med Chem Lett 25: 3716-20 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.030
BindingDB Entry DOI: 10.7270/Q2R78J7N |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50040123
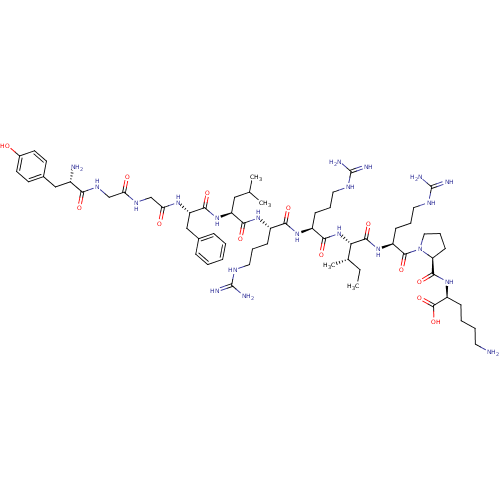
(CHEMBL438223 | HTry-Gly-Gly-Phe-Leu-Arg-Arg-lle-Ar...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C63H103N21O13/c1-5-37(4)51(58(94)80-44(20-13-29-74-63(70)71)59(95)84-30-14-21-48(84)57(93)81-45(60(96)97)17-9-10-26-64)83-54(90)43(19-12-28-73-62(68)69)78-53(89)42(18-11-27-72-61(66)67)79-55(91)46(31-36(2)3)82-56(92)47(33-38-15-7-6-8-16-38)77-50(87)35-75-49(86)34-76-52(88)41(65)32-39-22-24-40(85)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,85H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H,75,86)(H,76,88)(H,77,87)(H,78,89)(H,79,91)(H,80,94)(H,81,93)(H,82,92)(H,83,90)(H,96,97)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321606
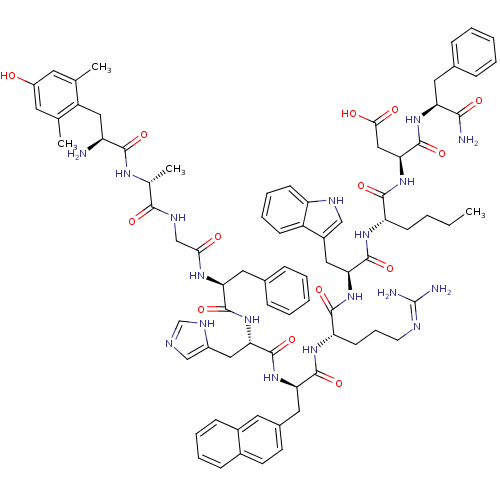
((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:8.20,58.71,93.99,78.84,wD:73.79,48.59,22.31,33.48,4.4,101.107,(24.59,-5.48,;25.93,-4.72,;25.93,-3.18,;24.59,-2.4,;24.59,-.86,;23.26,-.1,;21.93,-.87,;21.93,-2.41,;20.59,-.11,;20.59,1.44,;22,2.07,;23.25,1.19,;24.48,2.12,;23.98,3.58,;24.73,4.92,;23.94,6.24,;22.4,6.22,;21.65,4.87,;22.44,3.55,;19.25,-.87,;17.91,-.12,;17.91,1.43,;16.57,-.89,;16.57,-2.43,;17.91,-3.2,;17.91,-4.73,;19.24,-5.51,;19.24,-7.05,;17.93,-7.8,;20.61,-7.81,;15.25,-.1,;13.92,-.87,;13.92,-2.41,;12.59,-.1,;12.6,1.44,;13.92,2.21,;15.26,1.44,;16.59,2.21,;16.6,3.75,;17.93,4.53,;17.92,6.07,;16.58,6.84,;15.25,6.06,;15.26,4.52,;13.92,3.75,;11.26,-.86,;9.92,-.1,;9.92,1.45,;8.58,-.87,;8.58,-2.41,;9.92,-3.18,;11.32,-2.55,;12.36,-3.69,;11.59,-5.03,;10.08,-4.71,;7.25,-.09,;5.91,-.85,;5.91,-2.39,;4.58,-.08,;4.58,1.46,;5.91,2.23,;7.25,1.47,;8.58,2.24,;8.58,3.78,;7.24,4.55,;5.91,3.78,;3.25,-.85,;1.92,-.07,;1.92,1.47,;.58,-.85,;-.75,-.07,;-2.08,-.85,;-2.08,-2.38,;-3.41,-.07,;-3.41,1.47,;-4.75,-.84,;-6.09,-.08,;-6.09,1.47,;-7.42,-.86,;-7.4,-2.4,;-8.75,-.1,;-10.08,-.88,;-10.06,-2.42,;-8.72,-3.17,;-11.4,-3.2,;-12.73,-2.44,;-14.07,-3.22,;-12.76,-.9,;-11.41,-.13,;-11.43,1.41,;25.93,-.09,;25.93,1.45,;27.26,-.87,;28.59,-.09,;28.59,1.45,;29.93,2.21,;31.25,1.45,;29.93,3.76,;29.93,-.87,;29.93,-2.4,;31.26,-.1,;32.6,-.87,;32.6,-2.4,;33.93,-3.17,;35.28,-2.4,;36.61,-3.17,;36.61,-4.71,;35.27,-5.48,;33.94,-4.71,;33.93,-.1,;35.27,-.86,;33.93,1.45,)| Show InChI InChI=1S/C80H98N18O14/c1-5-6-25-60(73(106)98-67(40-69(101)102)79(112)94-62(70(82)103)34-48-18-9-7-10-19-48)92-77(110)65(37-53-41-87-59-26-16-15-24-56(53)59)96-74(107)61(27-17-30-86-80(83)84)93-76(109)64(36-50-28-29-51-22-13-14-23-52(51)33-50)95-78(111)66(38-54-42-85-44-89-54)97-75(108)63(35-49-20-11-8-12-21-49)91-68(100)43-88-71(104)47(4)90-72(105)58(81)39-57-45(2)31-55(99)32-46(57)3/h7-16,18-24,26,28-29,31-33,41-42,44,47,58,60-67,87,99H,5-6,17,25,27,30,34-40,43,81H2,1-4H3,(H2,82,103)(H,85,89)(H,88,104)(H,90,105)(H,91,100)(H,92,110)(H,93,109)(H,94,112)(H,95,111)(H,96,107)(H,97,108)(H,98,106)(H,101,102)(H4,83,84,86)/t47-,58+,60+,61+,62+,63+,64-,65+,66+,67+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50586821
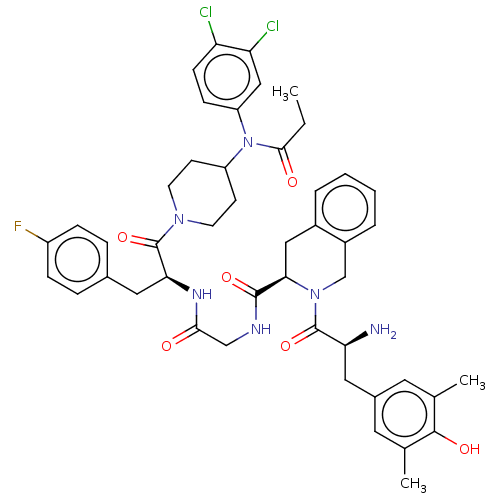
(CHEMBL5089012)Show SMILES CCC(=O)N(C1CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1cc(C)c(O)c(C)c1)c1ccc(Cl)c(Cl)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in mouse HN9.10 cells assessed as inhibition constant by radioligand binding a... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116509
BindingDB Entry DOI: 10.7270/Q2FX7FCX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321606

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:8.20,58.71,93.99,78.84,wD:73.79,48.59,22.31,33.48,4.4,101.107,(24.59,-5.48,;25.93,-4.72,;25.93,-3.18,;24.59,-2.4,;24.59,-.86,;23.26,-.1,;21.93,-.87,;21.93,-2.41,;20.59,-.11,;20.59,1.44,;22,2.07,;23.25,1.19,;24.48,2.12,;23.98,3.58,;24.73,4.92,;23.94,6.24,;22.4,6.22,;21.65,4.87,;22.44,3.55,;19.25,-.87,;17.91,-.12,;17.91,1.43,;16.57,-.89,;16.57,-2.43,;17.91,-3.2,;17.91,-4.73,;19.24,-5.51,;19.24,-7.05,;17.93,-7.8,;20.61,-7.81,;15.25,-.1,;13.92,-.87,;13.92,-2.41,;12.59,-.1,;12.6,1.44,;13.92,2.21,;15.26,1.44,;16.59,2.21,;16.6,3.75,;17.93,4.53,;17.92,6.07,;16.58,6.84,;15.25,6.06,;15.26,4.52,;13.92,3.75,;11.26,-.86,;9.92,-.1,;9.92,1.45,;8.58,-.87,;8.58,-2.41,;9.92,-3.18,;11.32,-2.55,;12.36,-3.69,;11.59,-5.03,;10.08,-4.71,;7.25,-.09,;5.91,-.85,;5.91,-2.39,;4.58,-.08,;4.58,1.46,;5.91,2.23,;7.25,1.47,;8.58,2.24,;8.58,3.78,;7.24,4.55,;5.91,3.78,;3.25,-.85,;1.92,-.07,;1.92,1.47,;.58,-.85,;-.75,-.07,;-2.08,-.85,;-2.08,-2.38,;-3.41,-.07,;-3.41,1.47,;-4.75,-.84,;-6.09,-.08,;-6.09,1.47,;-7.42,-.86,;-7.4,-2.4,;-8.75,-.1,;-10.08,-.88,;-10.06,-2.42,;-8.72,-3.17,;-11.4,-3.2,;-12.73,-2.44,;-14.07,-3.22,;-12.76,-.9,;-11.41,-.13,;-11.43,1.41,;25.93,-.09,;25.93,1.45,;27.26,-.87,;28.59,-.09,;28.59,1.45,;29.93,2.21,;31.25,1.45,;29.93,3.76,;29.93,-.87,;29.93,-2.4,;31.26,-.1,;32.6,-.87,;32.6,-2.4,;33.93,-3.17,;35.28,-2.4,;36.61,-3.17,;36.61,-4.71,;35.27,-5.48,;33.94,-4.71,;33.93,-.1,;35.27,-.86,;33.93,1.45,)| Show InChI InChI=1S/C80H98N18O14/c1-5-6-25-60(73(106)98-67(40-69(101)102)79(112)94-62(70(82)103)34-48-18-9-7-10-19-48)92-77(110)65(37-53-41-87-59-26-16-15-24-56(53)59)96-74(107)61(27-17-30-86-80(83)84)93-76(109)64(36-50-28-29-51-22-13-14-23-52(51)33-50)95-78(111)66(38-54-42-85-44-89-54)97-75(108)63(35-49-20-11-8-12-21-49)91-68(100)43-88-71(104)47(4)90-72(105)58(81)39-57-45(2)31-55(99)32-46(57)3/h7-16,18-24,26,28-29,31-33,41-42,44,47,58,60-67,87,99H,5-6,17,25,27,30,34-40,43,81H2,1-4H3,(H2,82,103)(H,85,89)(H,88,104)(H,90,105)(H,91,100)(H,92,110)(H,93,109)(H,94,112)(H,95,111)(H,96,107)(H,97,108)(H,98,106)(H,101,102)(H4,83,84,86)/t47-,58+,60+,61+,62+,63+,64-,65+,66+,67+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50492970

(CHEMBL2415082)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)CCCCCN=[N+]=[N-])C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C82H118N24O19/c1-4-5-21-55(97-79(123)64(45-108)103-76(120)60(38-49-27-29-52(109)30-28-49)100-78(122)63(44-107)95-66(110)26-10-7-15-35-93-105-87)72(116)98-57(31-32-68(112)113)74(118)102-62(40-51-42-88-46-92-51)77(121)99-59(37-48-18-8-6-9-19-48)75(119)96-56(24-16-34-89-82(85)86)73(117)101-61(39-50-41-90-54-22-12-11-20-53(50)54)71(115)91-43-67(111)94-58(23-13-14-33-83)81(125)106-36-17-25-65(106)80(124)104-69(47(2)3)70(84)114/h6,8-9,11-12,18-20,22,27-30,41-42,46-47,55-65,69,90,107-109H,4-5,7,10,13-17,21,23-26,31-40,43-45,83H2,1-3H3,(H2,84,114)(H,88,92)(H,91,115)(H,94,111)(H,95,110)(H,96,119)(H,97,123)(H,98,116)(H,99,121)(H,100,122)(H,101,117)(H,102,118)(H,103,120)(H,104,124)(H,112,113)(H4,85,86,89)/t55-,56-,57-,58-,59+,60-,61-,62-,63-,64-,65-,69-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of Eu-DTPA-PEGO-NDP-alpha-MSH-NH2 from human MC4R expressed in HEL293 cells after 1 hr by time-resolved fluorescence assay |
Bioorg Med Chem 21: 5029-38 (2013)
Article DOI: 10.1016/j.bmc.2013.06.052
BindingDB Entry DOI: 10.7270/Q26T0QK2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50491860

(CHEMBL2387215)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCNC(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C86H122ClN21O15/c1-52(2)45-69(105-81(119)70(49-58-28-30-59(87)31-29-58)100-73(111)51-98-75(113)55(5)99-76(114)64(88)50-63-53(3)46-62(109)47-54(63)4)80(118)102-66(27-18-40-95-85(92)93)77(115)101-65(26-17-39-94-84(90)91)78(116)103-67(32-33-72(89)110)79(117)106-71(48-57-21-11-7-12-22-57)82(120)104-68(83(121)122)25-15-16-38-96-86(123)97-41-34-74(112)108(60-23-13-8-14-24-60)61-36-43-107(44-37-61)42-35-56-19-9-6-10-20-56/h6-14,19-24,28-31,46-47,52,55,61,64-71,109H,15-18,25-27,32-45,48-51,88H2,1-5H3,(H2,89,110)(H,98,113)(H,99,114)(H,100,111)(H,101,115)(H,102,118)(H,103,116)(H,104,120)(H,105,119)(H,106,117)(H,121,122)(H4,90,91,94)(H4,92,93,95)(H2,96,97,123)/t55-,64+,65-,66+,67+,68+,69+,70+,71+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cell membranes |
Bioorg Med Chem Lett 23: 3434-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.065
BindingDB Entry DOI: 10.7270/Q2T72MCM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data