

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM92520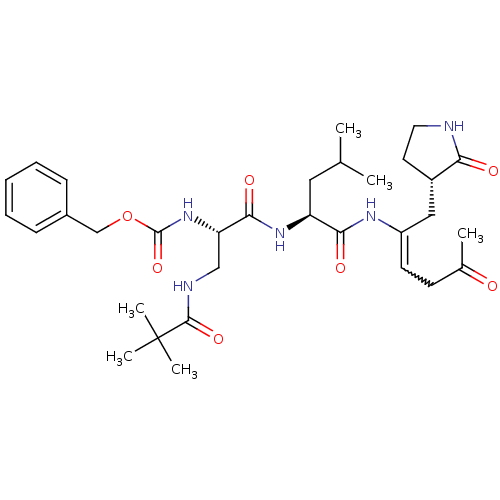 (TG-0204998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | -42.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | -41.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232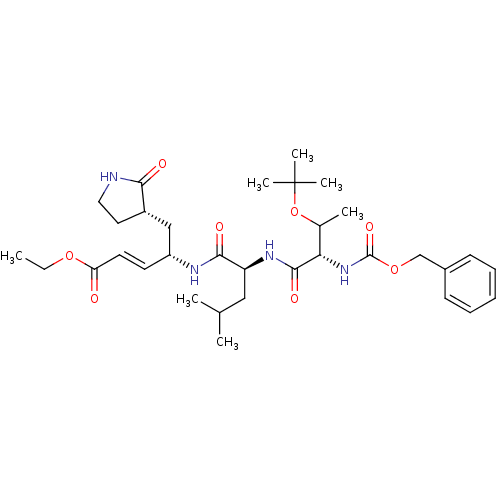 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM92521 (TG-0205486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 99 | -40.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92521 (TG-0205486) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -36.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11231 (N-[(benzyloxy)carbonyl]-L-valyl-N1-((1S,2E)-4-etho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 660 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92520 (TG-0204998) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11232 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230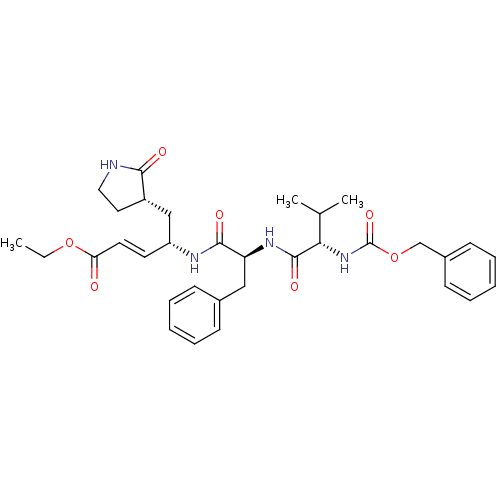 (AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.26E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11229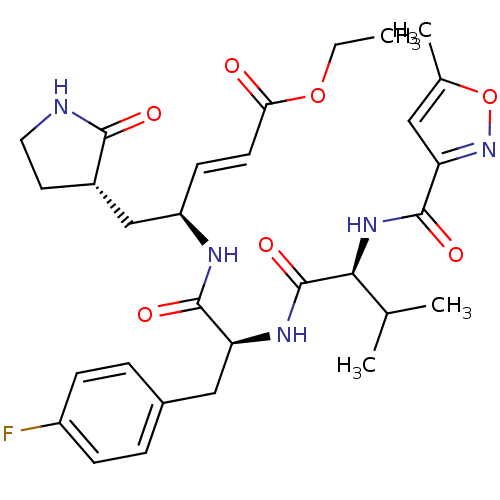 (AG7088 analogue 2a | CHEMBL20636 | N-[(5-methyliso...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321857 (8-{4-(4-Fluorophenyl)-5-[4-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321856 (4-{2-[7-Hydroxy-5,6,7,8-tetrahydronaphthalen-1-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321853 (8-{4-Ethyl-5-[4-(trifluoromethyl)phenyl]oxazol-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321850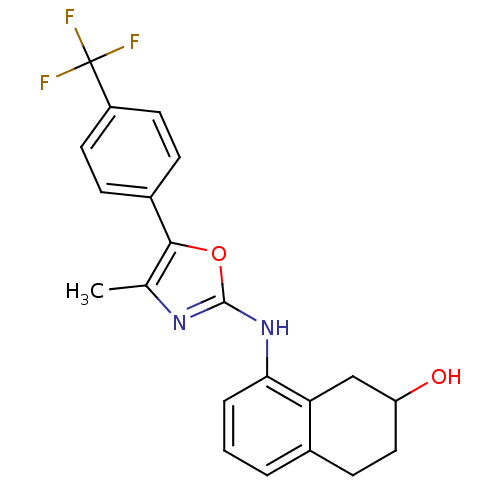 (8-{5-[3-Methyl-4-(trifluoromethyl)phenyl]oxazol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321852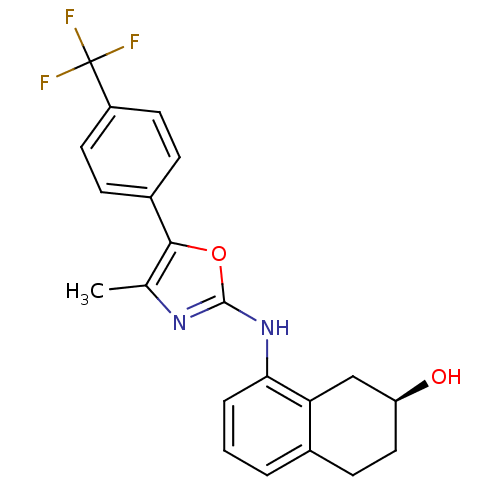 ((S)-8-{5-[3-Methyl-4-(trifluoromethyl)phenyl]oxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321836 (8-{5-[4-(Trifluoromethyl)phenyl]oxazol-2-ylamino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321855 (8-{4-Bromo-5-[4-(trifluoromethyl)phenyl]oxazol-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321839 (8-{5-[4-(Trifluoromethyl)phenyl]oxazol-2-ylamino}n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321845 (8-[5-(4-Chlorophenyl)oxazol-2-ylamino]-1,2,3,4-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321851 ((R)-8-{5-[3-Methyl-4-(trifluoromethyl)phenyl]oxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321844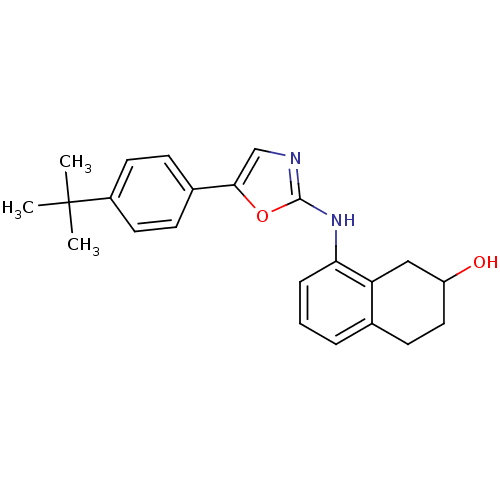 (8-[5-(4-tert-Butylphenyl)oxazol-2-ylamino]-1,2,3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50181911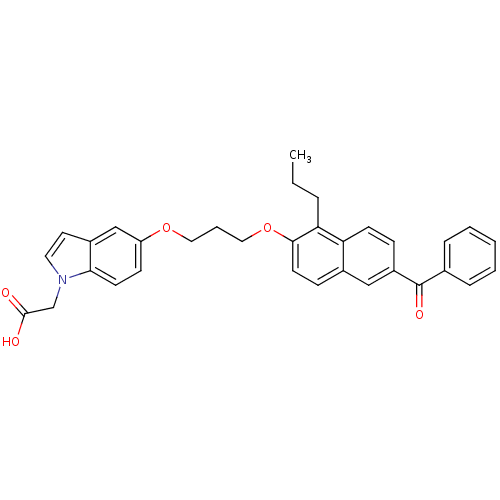 ((5-{3-[(6-BENZOYL-1-PROPYL-2-NAPHTHYL)OXY]PROPOXY}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from human PPAR gamma by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321843 (8-(5-p-tolyloxazol-2-ylamino)-1,2,3,4-tetrahydrona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321846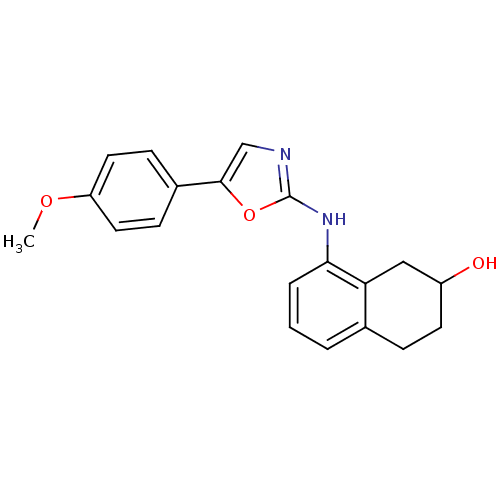 (8-[5-(4-Methoxyphenyl)oxazol-2-ylamino]-1,2,3,4-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from human PPAR gamma by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50179236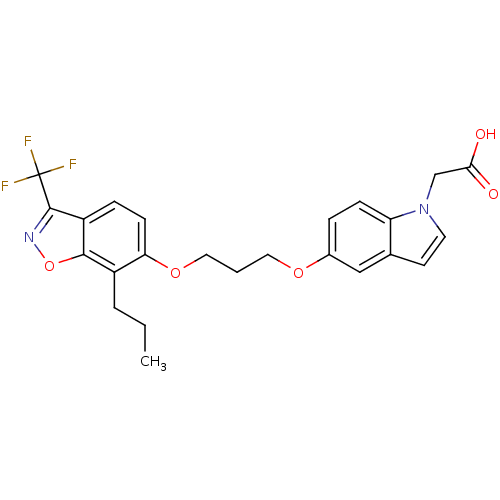 (2-(5-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]L-783,483 from human PPAR delta by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321838 (CHEMBL1173746 | N-(1H-indazol-4-yl)-5-[4-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321847 (8-{5-[4-(Pyrrolidin-1-yl)phenyl]oxazol-2-ylamino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50181911 ((5-{3-[(6-BENZOYL-1-PROPYL-2-NAPHTHYL)OXY]PROPOXY}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321854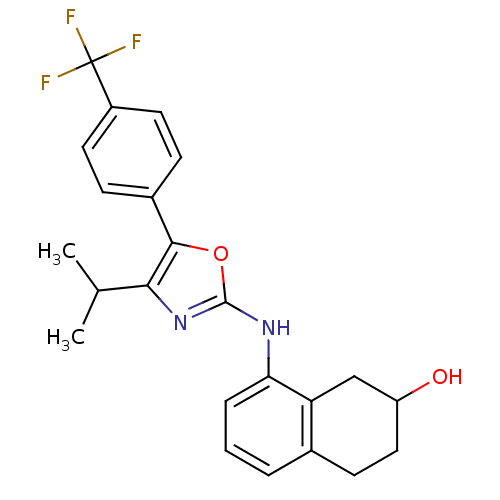 (8-{4-Isopropyl-5-[4-(trifluoromethyl)phenyl]oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50181902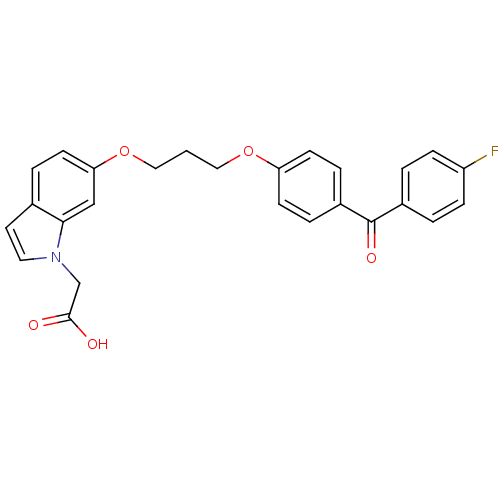 (2-(6-(3-(4-(4-fluorobenzoyl)phenoxy)propoxy)-1H-in...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321834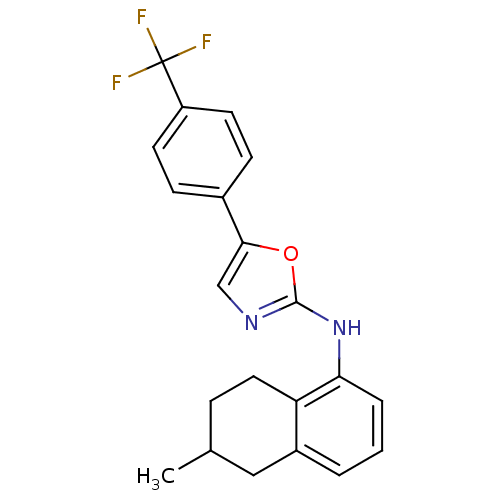 (5-{5-[4-(Trifluoromethyl)phenyl]oxazol-2-ylamino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50181903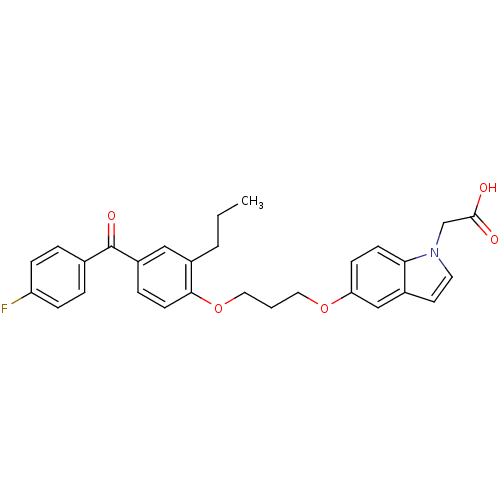 (2-(5-(3-(4-(4-fluorobenzoyl)-2-propylphenoxy)propo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from human PPAR gamma by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321831 (CHEMBL1173093 | N-(7-methoxy-5,6,7,8-tetrahydronap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50179236 (2-(5-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from human PPAR gamma by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321842 (8-(5-m-Tolyloxazol-2-ylamino)-1,2,3,4-tetrahydrona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321840 (8-(5-Phenyloxazol-2-ylamino)-1,2,3,4-tetrahydronap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50181913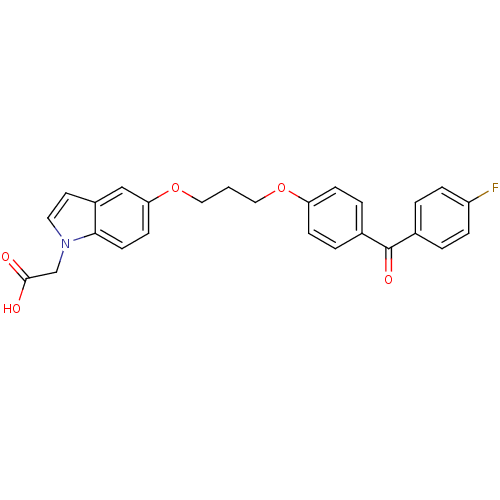 (2-(5-(3-(4-(4-fluorobenzoyl)phenoxy)propoxy)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from human PPAR gamma by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50181911 ((5-{3-[(6-BENZOYL-1-PROPYL-2-NAPHTHYL)OXY]PROPOXY}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]L-783,483 from human PPAR delta by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321837 (CHEMBL1173681 | N-(isoquinolin-5-yl)-5-[4-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321832 (8-[Methyl{5-[4-(trifluoromethyl)phenyl]oxazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321830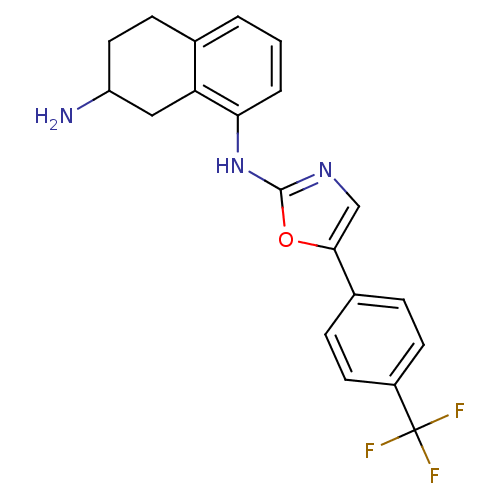 (CHEMBL1173157 | N1-{5-[4-(trifluoromethyl)phenyl]o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 327 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321835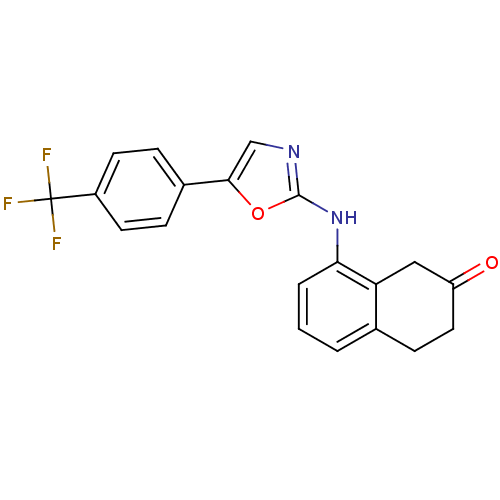 (8-{5-[4-(Trifluoromethyl)phenyl]oxazol-2-ylamino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 374 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50181907 (2-(4-(3-(4-(4-fluorobenzoyl)-2-propylphenoxy)propo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 414 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321841 (8-(5-o-Tolyloxazol-2-ylamino)-1,2,3,4-tetrahydrona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 416 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50181903 (2-(5-(3-(4-(4-fluorobenzoyl)-2-propylphenoxy)propo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50181904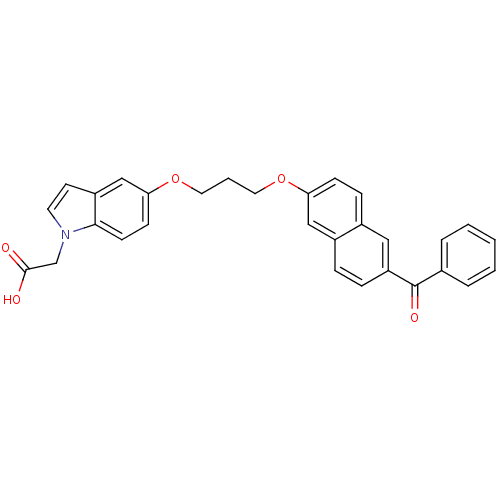 (2-(5-(3-(6-benzoylnaphthalen-2-yloxy)propoxy)-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]L-783,483 from human PPAR alpha by SPA assay | J Med Chem 49: 1212-6 (2006) Article DOI: 10.1021/jm0510373 BindingDB Entry DOI: 10.7270/Q2QV3M3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |