 Found 38 hits with Last Name = 'hsung' and Initial = 'rp'
Found 38 hits with Last Name = 'hsung' and Initial = 'rp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50089619
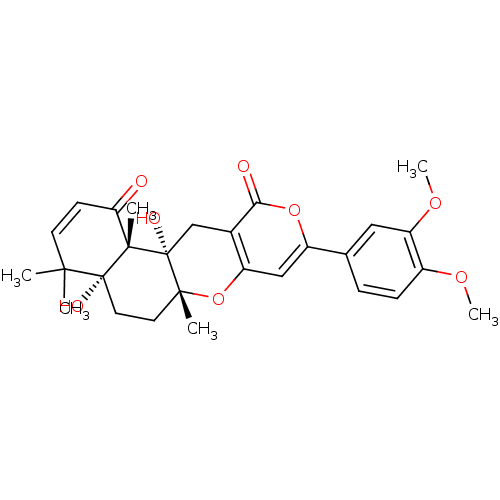
(9-(3,4-Dimethoxy-phenyl)-4a,12a-dihydroxy-4,4,6a,1...)Show SMILES COc1ccc(cc1OC)-c1cc2O[C@]3(C)CC[C@@]4(O)C(C)(C)C=CC(=O)[C@]4(C)[C@@]3(O)Cc2c(=O)o1 |c:24| Show InChI InChI=1S/C28H32O8/c1-24(2)10-9-22(29)26(4)27(24,31)12-11-25(3)28(26,32)15-17-20(36-25)14-19(35-23(17)30)16-7-8-18(33-5)21(13-16)34-6/h7-10,13-14,31-32H,11-12,15H2,1-6H3/t25-,26+,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50089619

(9-(3,4-Dimethoxy-phenyl)-4a,12a-dihydroxy-4,4,6a,1...)Show SMILES COc1ccc(cc1OC)-c1cc2O[C@]3(C)CC[C@@]4(O)C(C)(C)C=CC(=O)[C@]4(C)[C@@]3(O)Cc2c(=O)o1 |c:24| Show InChI InChI=1S/C28H32O8/c1-24(2)10-9-22(29)26(4)27(24,31)12-11-25(3)28(26,32)15-17-20(36-25)14-19(35-23(17)30)16-7-8-18(33-5)21(13-16)34-6/h7-10,13-14,31-32H,11-12,15H2,1-6H3/t25-,26+,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50089619

(9-(3,4-Dimethoxy-phenyl)-4a,12a-dihydroxy-4,4,6a,1...)Show SMILES COc1ccc(cc1OC)-c1cc2O[C@]3(C)CC[C@@]4(O)C(C)(C)C=CC(=O)[C@]4(C)[C@@]3(O)Cc2c(=O)o1 |c:24| Show InChI InChI=1S/C28H32O8/c1-24(2)10-9-22(29)26(4)27(24,31)12-11-25(3)28(26,32)15-17-20(36-25)14-19(35-23(17)30)16-7-8-18(33-5)21(13-16)34-6/h7-10,13-14,31-32H,11-12,15H2,1-6H3/t25-,26+,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of AChE-induced amyloid beta aggregation |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of AChE-induced amyloid beta aggregation |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of AChE-induced amyloid beta aggregation |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50089616
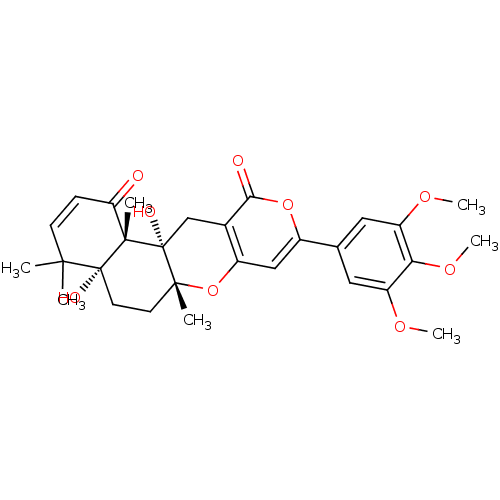
(4a,12a-Dihydroxy-4,4,6a,12b-tetramethyl-9-(3,4,5-t...)Show SMILES COc1cc(cc(OC)c1OC)-c1cc2O[C@]3(C)CC[C@@]4(O)C(C)(C)C=CC(=O)[C@]4(C)[C@@]3(O)Cc2c(=O)o1 |r,c:26| Show InChI InChI=1S/C29H34O9/c1-25(2)9-8-22(30)27(4)28(25,32)11-10-26(3)29(27,33)15-17-19(38-26)14-18(37-24(17)31)16-12-20(34-5)23(36-7)21(13-16)35-6/h8-9,12-14,32-33H,10-11,15H2,1-7H3/t26-,27+,28-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of AChE-induced amyloid beta aggregation |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50130204
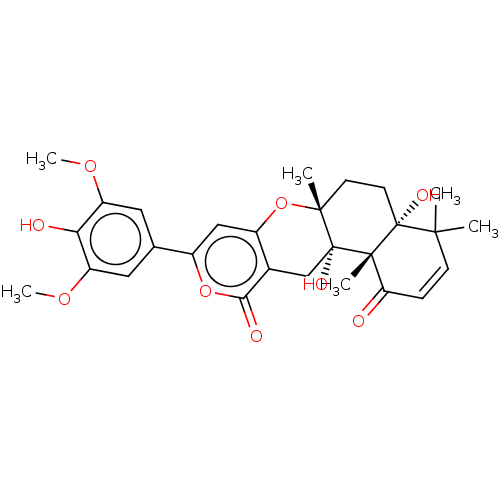
(CHEMBL3632858)Show SMILES COc1cc(cc(OC)c1O)-c1cc2O[C@]3(C)CC[C@@]4(O)C(C)(C)C=CC(=O)[C@]4(C)[C@@]3(O)Cc2c(=O)o1 |r,c:25| Show InChI InChI=1S/C28H32O9/c1-24(2)8-7-21(29)26(4)27(24,32)10-9-25(3)28(26,33)14-16-18(37-25)13-17(36-23(16)31)15-11-19(34-5)22(30)20(12-15)35-6/h7-8,11-13,30,32-33H,9-10,14H2,1-6H3/t25-,26+,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50089616

(4a,12a-Dihydroxy-4,4,6a,12b-tetramethyl-9-(3,4,5-t...)Show SMILES COc1cc(cc(OC)c1OC)-c1cc2O[C@]3(C)CC[C@@]4(O)C(C)(C)C=CC(=O)[C@]4(C)[C@@]3(O)Cc2c(=O)o1 |r,c:26| Show InChI InChI=1S/C29H34O9/c1-25(2)9-8-22(30)27(4)28(25,32)11-10-26(3)29(27,33)15-17-19(38-26)14-18(37-24(17)31)16-12-20(34-5)23(36-7)21(13-16)35-6/h8-9,12-14,32-33H,10-11,15H2,1-7H3/t26-,27+,28-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of BChE |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50089621
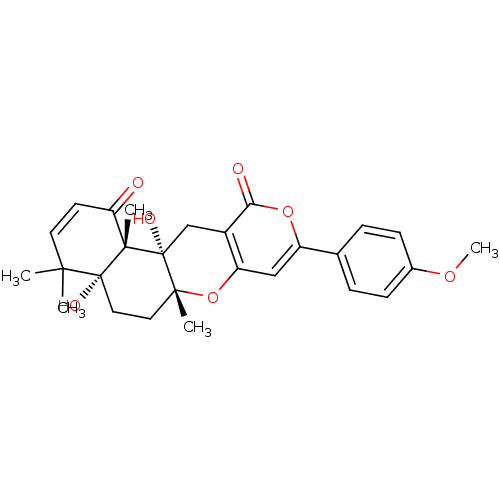
(4a,12a-Dihydroxy-9-(4-methoxy-phenyl)-4,4,6a,12b-t...)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@@]4(O)C(C)(C)C=CC(=O)[C@]4(C)[C@@]3(O)Cc2c(=O)o1 |c:22| Show InChI InChI=1S/C27H30O7/c1-23(2)11-10-21(28)25(4)26(23,30)13-12-24(3)27(25,31)15-18-20(34-24)14-19(33-22(18)29)16-6-8-17(32-5)9-7-16/h6-11,14,30-31H,12-13,15H2,1-5H3/t24-,25+,26-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10620
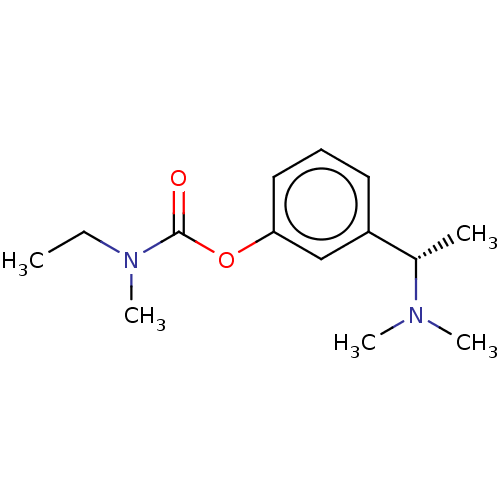
((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of BChE |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of AChE-induced amyloid beta aggregation |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of BChE |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of AChE-induced amyloid beta aggregation |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076634
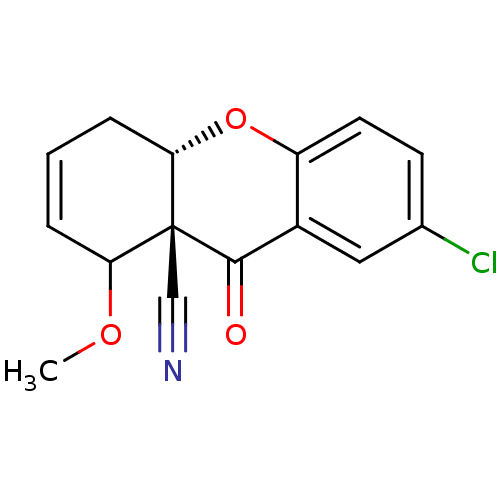
((8R,8aS,10aS)-2-Chloro-8-methoxy-9-oxo-5,10a-dihyd...)Show SMILES COC1C=CC[C@@H]2Oc3ccc(Cl)cc3C(=O)[C@]12C#N |c:3| Show InChI InChI=1S/C15H12ClNO3/c1-19-12-3-2-4-13-15(12,8-17)14(18)10-7-9(16)5-6-11(10)20-13/h2-3,5-7,12-13H,4H2,1H3/t12?,13-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 9: 973-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F91 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of AChE-induced amyloid beta aggregation |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076632

((8aS,10aS)-6-(tert-Butyl-dimethyl-silanyloxy)-8-me...)Show SMILES COC1C=C(C[C@@H]2Oc3ccccc3C(=O)[C@]12C#N)O[Si](C)(C)C(C)(C)C |c:3| Show InChI InChI=1S/C21H27NO4Si/c1-20(2,3)27(5,6)26-14-11-17(24-4)21(13-22)18(12-14)25-16-10-8-7-9-15(16)19(21)23/h7-11,17-18H,12H2,1-6H3/t17?,18-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 9: 973-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F91 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076628
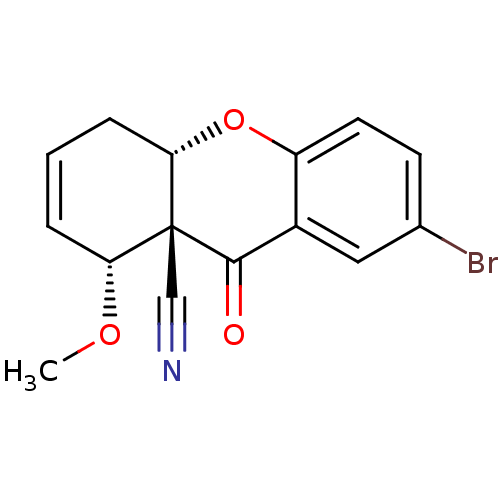
((8R,8aS,10aS)-2-Bromo-8-methoxy-9-oxo-5,10a-dihydr...)Show SMILES CO[C@@H]1C=CC[C@@H]2Oc3ccc(Br)cc3C(=O)[C@]12C#N |c:3| Show InChI InChI=1S/C15H12BrNO3/c1-19-12-3-2-4-13-15(12,8-17)14(18)10-7-9(16)5-6-11(10)20-13/h2-3,5-7,12-13H,4H2,1H3/t12-,13+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 9: 973-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F91 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076636
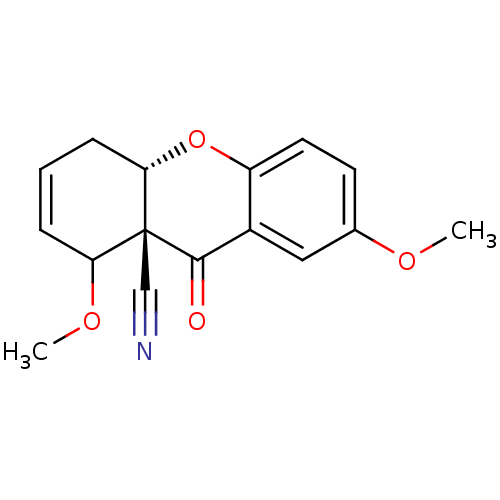
((8aS,10aS)-2,8-Dimethoxy-9-oxo-5,10a-dihydro-8H,9H...)Show SMILES COC1C=CC[C@@H]2Oc3ccc(OC)cc3C(=O)[C@]12C#N |c:3| Show InChI InChI=1S/C16H15NO4/c1-19-10-6-7-12-11(8-10)15(18)16(9-17)13(20-2)4-3-5-14(16)21-12/h3-4,6-8,13-14H,5H2,1-2H3/t13?,14-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 9: 973-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F91 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076629
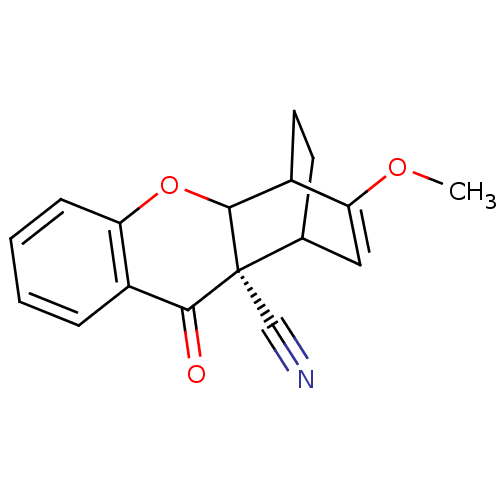
(13-methoxy-3-oxo-(2S)-10-oxatetracyclo[10.2.2.02,1...)Show SMILES COC1=CC2CCC1C1Oc3ccccc3C(=O)[C@]21C#N |t:2,TLB:9:8:2.3:6.5,THB:16:18:2.3:6.5,19:18:2.3:6.5,1:2:18.8:6.5| Show InChI InChI=1S/C17H15NO3/c1-20-14-8-10-6-7-12(14)16-17(10,9-18)15(19)11-4-2-3-5-13(11)21-16/h2-5,8,10,12,16H,6-7H2,1H3/t10?,12?,16?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 9: 973-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F91 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076633
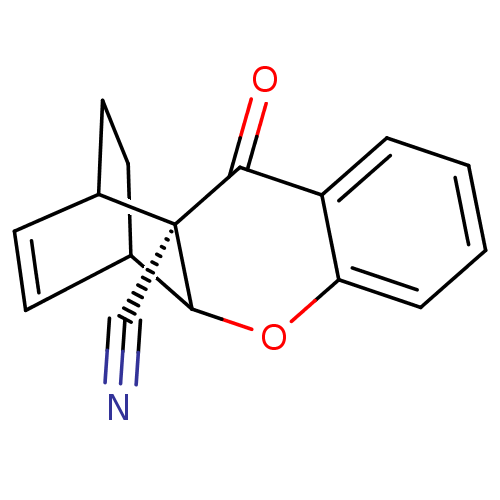
(3-oxo-(2S)-10-oxatetracyclo[10.2.2.02,11.04,9]hexa...)Show SMILES O=C1c2ccccc2OC2C3CCC(C=C3)[C@@]12C#N |c:15,TLB:1:16:14.15:12.11,17:16:14.15:12.11,THB:8:9:14.15:12.11| Show InChI InChI=1S/C16H13NO2/c17-9-16-11-7-5-10(6-8-11)15(16)19-13-4-2-1-3-12(13)14(16)18/h1-5,7,10-11,15H,6,8H2/t10?,11?,15?,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 9: 973-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F91 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076635
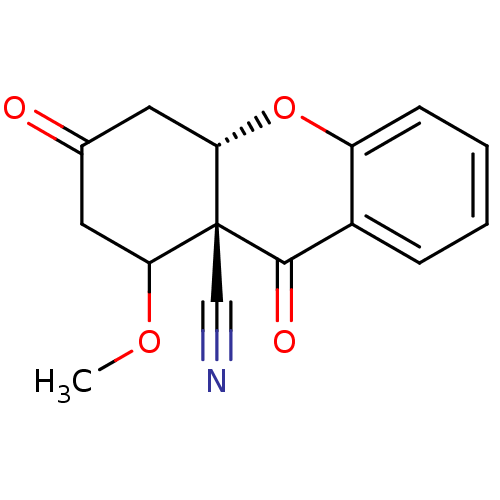
((8aR,10aS)-8-Methoxy-6,9-dioxo-5,7,8,10a-tetrahydr...)Show InChI InChI=1S/C15H13NO4/c1-19-12-6-9(17)7-13-15(12,8-16)14(18)10-4-2-3-5-11(10)20-13/h2-5,12-13H,6-7H2,1H3/t12?,13-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 9: 973-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F91 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076627

((8aS,10aS)-8-Methoxy-2-methyl-9-oxo-5,10a-dihydro-...)Show SMILES COC1C=CC[C@@H]2Oc3ccc(C)cc3C(=O)[C@]12C#N |c:3| Show InChI InChI=1S/C16H15NO3/c1-10-6-7-12-11(8-10)15(18)16(9-17)13(19-2)4-3-5-14(16)20-12/h3-4,6-8,13-14H,5H2,1-2H3/t13?,14-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 9: 973-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F91 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076630
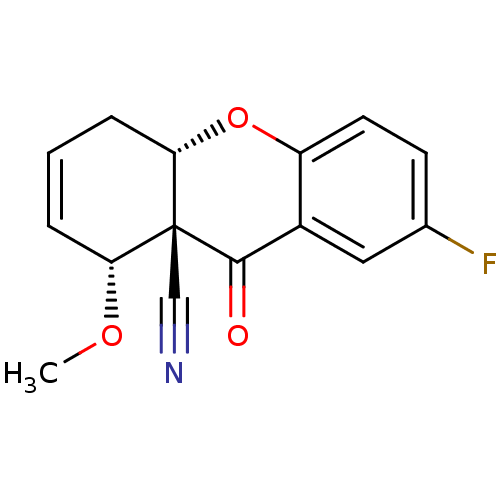
((8R,8aS,10aS)-2-Fluoro-8-methoxy-9-oxo-5,10a-dihyd...)Show SMILES CO[C@@H]1C=CC[C@@H]2Oc3ccc(F)cc3C(=O)[C@]12C#N |c:3| Show InChI InChI=1S/C15H12FNO3/c1-19-12-3-2-4-13-15(12,8-17)14(18)10-7-9(16)5-6-11(10)20-13/h2-3,5-7,12-13H,4H2,1H3/t12-,13+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 9: 973-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F91 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076631
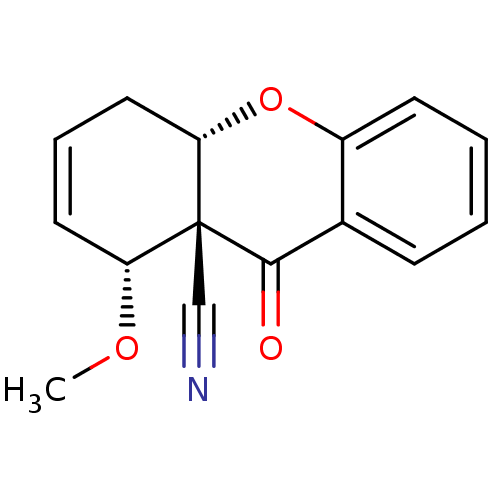
((8R,8aS,10aS)-8-Methoxy-9-oxo-5,10a-dihydro-8H,9H-...)Show SMILES CO[C@@H]1C=CC[C@@H]2Oc3ccccc3C(=O)[C@]12C#N |c:3| Show InChI InChI=1S/C15H13NO3/c1-18-12-7-4-8-13-15(12,9-16)14(17)10-5-2-3-6-11(10)19-13/h2-7,12-13H,8H2,1H3/t12-,13+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 9: 973-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F91 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50130210
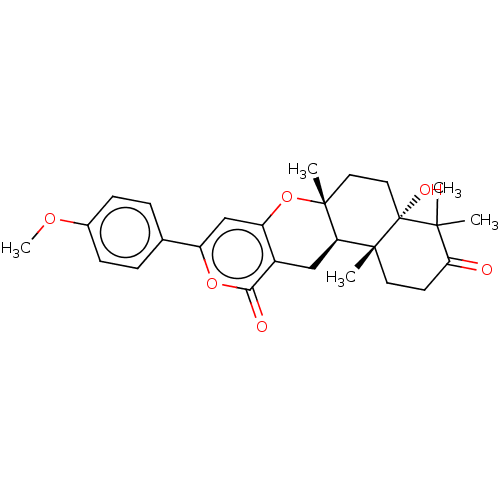
(CHEMBL3632852)Show SMILES [H][C@]12Cc3c(O[C@]1(C)CC[C@@]1(O)C(C)(C)C(=O)CC[C@]21C)cc(oc3=O)-c1ccc(OC)cc1 |r| Show InChI InChI=1S/C27H32O6/c1-24(2)22(28)10-11-25(3)21-14-18-20(33-26(21,4)12-13-27(24,25)30)15-19(32-23(18)29)16-6-8-17(31-5)9-7-16/h6-9,15,21,30H,10-14H2,1-5H3/t21-,25-,26-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50130209
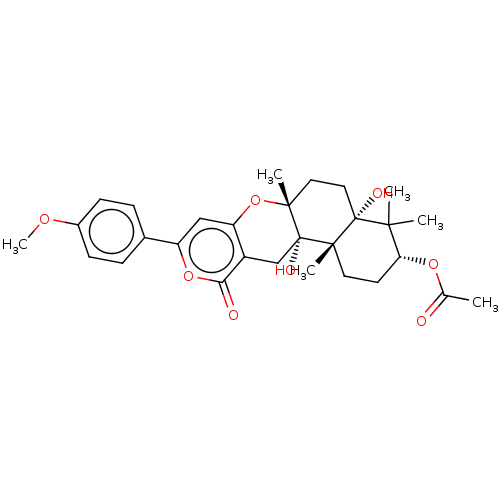
(CHEMBL3632853)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@@]4(O)C(C)(C)[C@@H](CC[C@]4(C)[C@@]3(O)Cc2c(=O)o1)OC(C)=O |r| Show InChI InChI=1S/C29H36O8/c1-17(30)35-23-11-12-26(4)28(32,25(23,2)3)14-13-27(5)29(26,33)16-20-22(37-27)15-21(36-24(20)31)18-7-9-19(34-6)10-8-18/h7-10,15,23,32-33H,11-14,16H2,1-6H3/t23-,26+,27-,28-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of BChE |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50089619

(9-(3,4-Dimethoxy-phenyl)-4a,12a-dihydroxy-4,4,6a,1...)Show SMILES COc1ccc(cc1OC)-c1cc2O[C@]3(C)CC[C@@]4(O)C(C)(C)C=CC(=O)[C@]4(C)[C@@]3(O)Cc2c(=O)o1 |c:24| Show InChI InChI=1S/C28H32O8/c1-24(2)10-9-22(29)26(4)27(24,31)12-11-25(3)28(26,32)15-17-20(36-25)14-19(35-23(17)30)16-7-8-18(33-5)21(13-16)34-6/h7-10,13-14,31-32H,11-12,15H2,1-6H3/t25-,26+,27-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of BChE |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of BChE |
Bioorg Med Chem Lett 21: 2687-91 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.041
BindingDB Entry DOI: 10.7270/Q27W6CH9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50130207
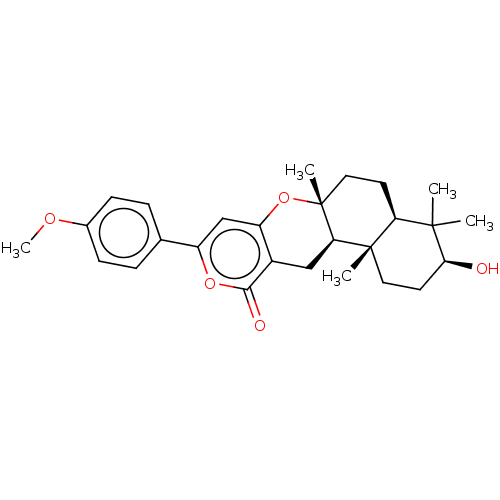
(CHEMBL3632855)Show SMILES [H][C@@]12CC[C@@]3(C)Oc4cc(oc(=O)c4C[C@]3([H])[C@@]1(C)CC[C@H](O)C2(C)C)-c1ccc(OC)cc1 |r| Show InChI InChI=1S/C27H34O5/c1-25(2)21-10-13-27(4)22(26(21,3)12-11-23(25)28)14-18-20(32-27)15-19(31-24(18)29)16-6-8-17(30-5)9-7-16/h6-9,15,21-23,28H,10-14H2,1-5H3/t21-,22+,23-,26-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50130206
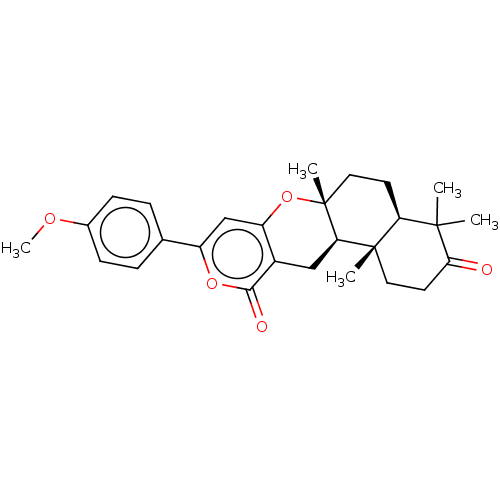
(CHEMBL3632856)Show SMILES [H][C@@]12CC[C@@]3(C)Oc4cc(oc(=O)c4C[C@]3([H])[C@@]1(C)CCC(=O)C2(C)C)-c1ccc(OC)cc1 |r| Show InChI InChI=1S/C27H32O5/c1-25(2)21-10-13-27(4)22(26(21,3)12-11-23(25)28)14-18-20(32-27)15-19(31-24(18)29)16-6-8-17(30-5)9-7-16/h6-9,15,21-22H,10-14H2,1-5H3/t21-,22+,26-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
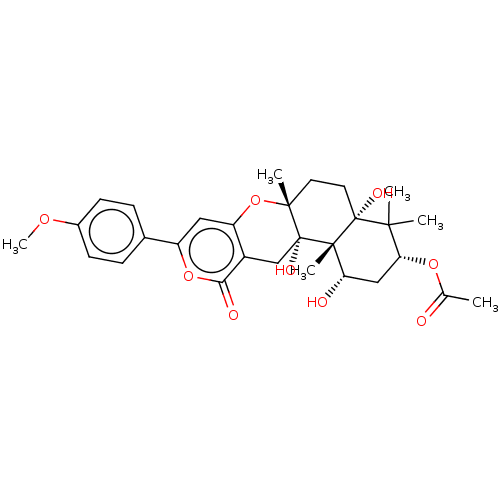
(Homo sapiens (Human)) | BDBM50130205
(CHEMBL3632857)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@@]4(O)C(C)(C)[C@@H](C[C@H](O)[C@]4(C)[C@@]3(O)Cc2c(=O)o1)OC(C)=O |r| Show InChI InChI=1S/C29H36O9/c1-16(30)36-23-14-22(31)27(5)28(33,25(23,2)3)12-11-26(4)29(27,34)15-19-21(38-26)13-20(37-24(19)32)17-7-9-18(35-6)10-8-17/h7-10,13,22-23,31,33-34H,11-12,14-15H2,1-6H3/t22-,23+,26+,27-,28+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
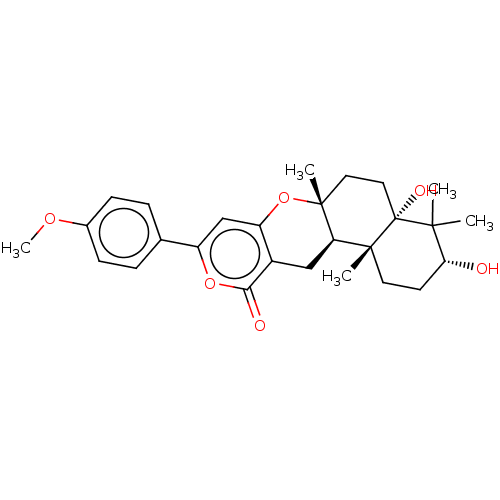
(Homo sapiens (Human)) | BDBM50130208
(CHEMBL3632854)Show SMILES [H][C@]12Cc3c(O[C@]1(C)CC[C@@]1(O)C(C)(C)[C@H](O)CC[C@]21C)cc(oc3=O)-c1ccc(OC)cc1 |r| Show InChI InChI=1S/C27H34O6/c1-24(2)22(28)10-11-25(3)21-14-18-20(33-26(21,4)12-13-27(24,25)30)15-19(32-23(18)29)16-6-8-17(31-5)9-7-16/h6-9,15,21-22,28,30H,10-14H2,1-5H3/t21-,22-,25-,26-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemical Research, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem Lett 25: 4848-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.047
BindingDB Entry DOI: 10.7270/Q2668G1T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data