

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (MOUSE) | CHEMBL5287792 | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | CHEMBL5277326 | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL CHEMBL MCE PC cid PC sid UniChem Similars | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at AT1 receptor in rat aortic rings | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114327 BindingDB Entry DOI: 10.7270/Q2NP28GR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455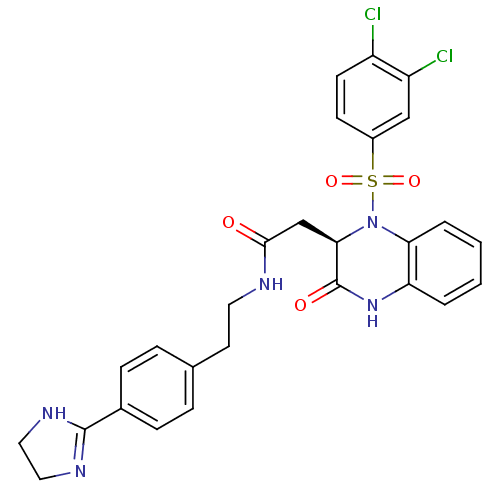 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Lys0-des-Arg9-BK at human bradykinin B1 receptor expressed in HEK293 cells | J Med Chem 53: 5383-99 (2010) Article DOI: 10.1021/jm1000776 BindingDB Entry DOI: 10.7270/Q289162Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123504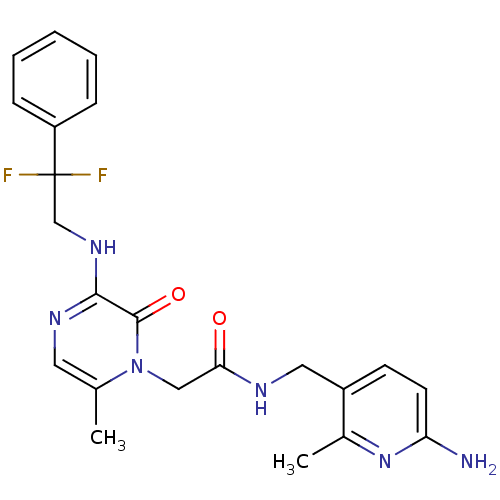 (CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | CHEMBL5270915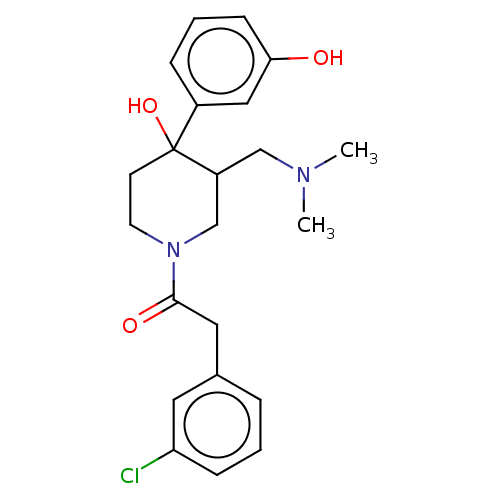 | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50243173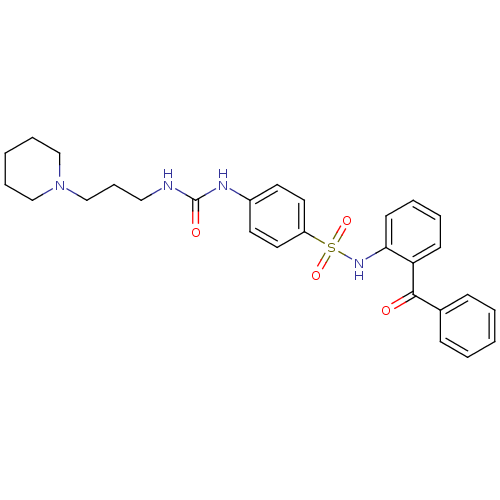 (CHEMBL487445 | N-(2-Benzoyl-phenyl)-4-[3-(3-piperi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Lys0-des-Arg9-BK at human bradykinin B1 receptor expressed in HEK293 cells | J Med Chem 53: 5383-99 (2010) Article DOI: 10.1021/jm1000776 BindingDB Entry DOI: 10.7270/Q289162Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50326710 (7-chloro-2-(3-(9-(pyridin-4-yl)-3,9-diazaspiro[5.5...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Lys0-des-Arg9-BK at human bradykinin B1 receptor expressed in HEK293 cells | J Med Chem 53: 5383-99 (2010) Article DOI: 10.1021/jm1000776 BindingDB Entry DOI: 10.7270/Q289162Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50326708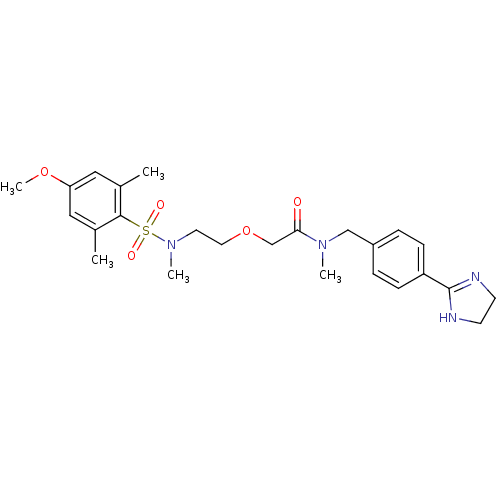 (CHEMBL1254771 | N-(4-(4,5-dihydro-1H-imidazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Lys0-des-Arg9-BK at human bradykinin B1 receptor expressed in HEK293 cells | J Med Chem 53: 5383-99 (2010) Article DOI: 10.1021/jm1000776 BindingDB Entry DOI: 10.7270/Q289162Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377618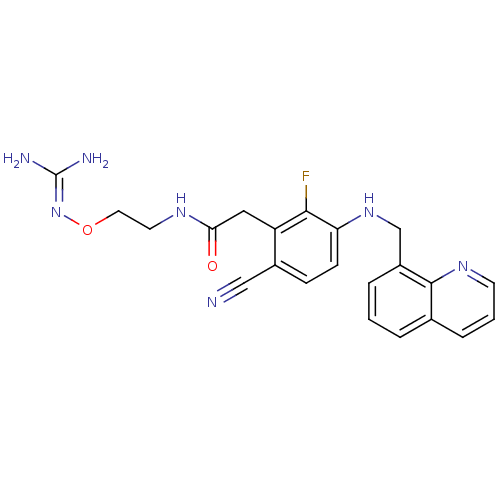 (CHEMBL254353) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50463297 (CHEMBL4246433) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463294 (CHEMBL4249256) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50506108 (CHEMBL4449252) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting | J Med Chem 62: 11054-11070 (2019) Article DOI: 10.1021/acs.jmedchem.9b00857 BindingDB Entry DOI: 10.7270/Q2VD72RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377625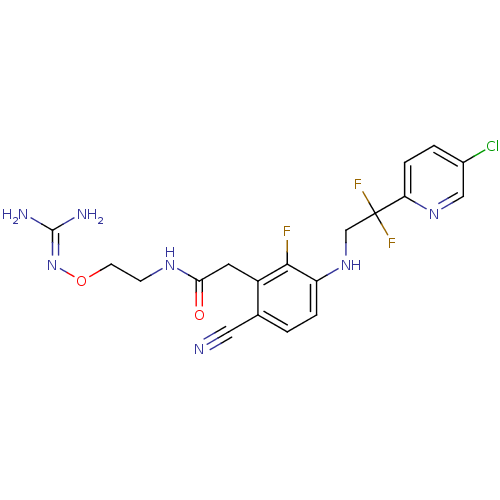 (CHEMBL254557) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50326709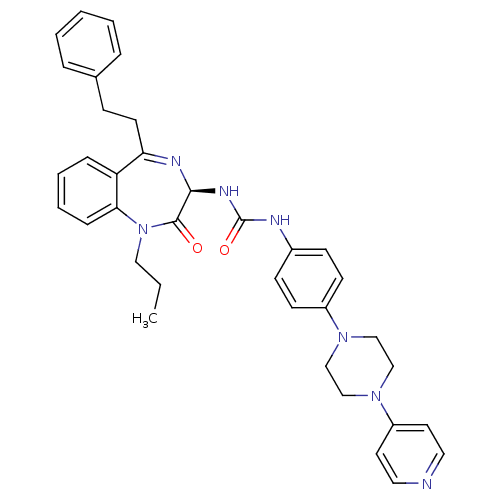 ((R)-1-(2-oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-b...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Lys0-des-Arg9-BK at human bradykinin B1 receptor expressed in HEK293 cells | J Med Chem 53: 5383-99 (2010) Article DOI: 10.1021/jm1000776 BindingDB Entry DOI: 10.7270/Q289162Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463297 (CHEMBL4246433) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50536712 (CHEMBL4571075) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5A transfected in HEK293 cell membranes after 60 mins by microbeta liquid scintillation counting analysis | Bioorg Med Chem Lett 26: 4165-9 (2016) Article DOI: 10.1016/j.bmcl.2016.07.065 BindingDB Entry DOI: 10.7270/Q2HX1H5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377615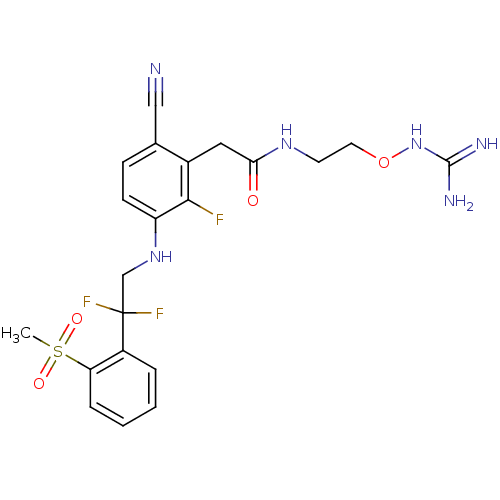 (CHEMBL254962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377623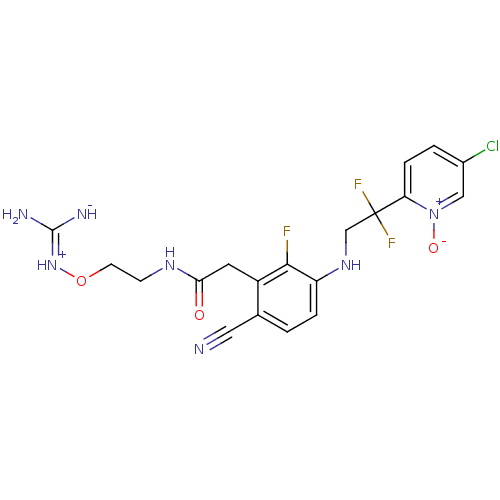 (CHEMBL254759) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50243301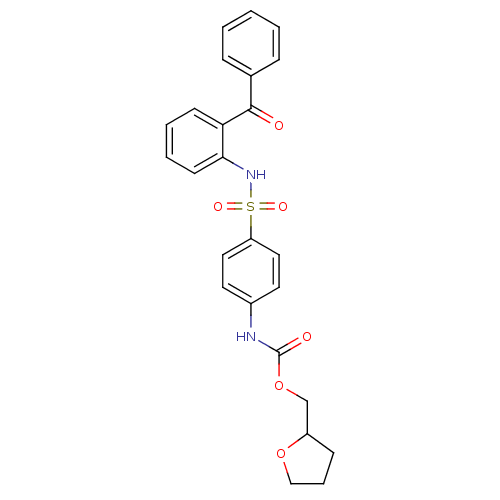 (CHEMBL454085 | Tetrahydrofuran-2-ylmethyl 4-{[(2-b...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Lys0-des-Arg9-BK at human bradykinin B1 receptor expressed in HEK293 cells | J Med Chem 53: 5383-99 (2010) Article DOI: 10.1021/jm1000776 BindingDB Entry DOI: 10.7270/Q289162Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | CHEMBL5274117 | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377611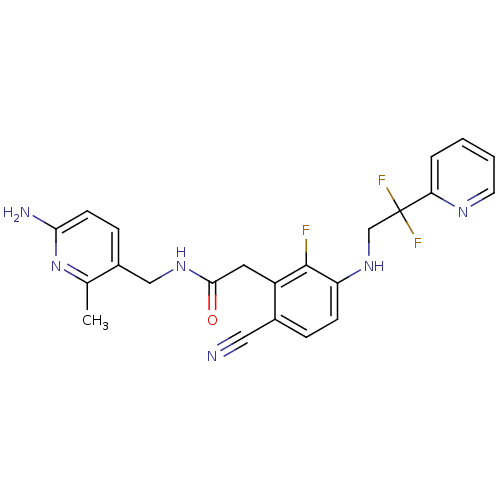 (CHEMBL258018) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377619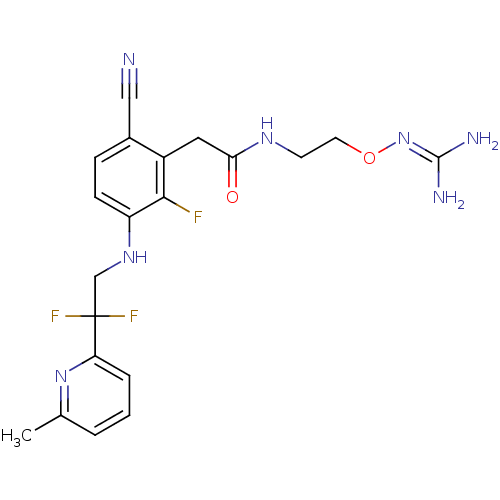 (CHEMBL402758) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21130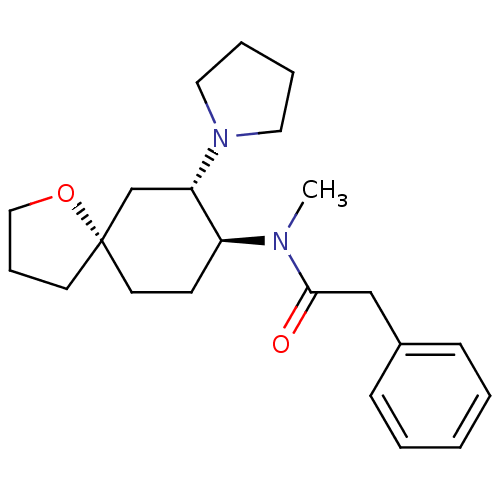 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at oxytocin receptor in Wistar rat uterus assessed as inhibition of oxytocin-induced uterotonic activity treated 1 min prior to o... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from human kappa opioid receptor expressed in CHO cells incubated for 30 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112070 BindingDB Entry DOI: 10.7270/Q2JW8JHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (RAT) | BDBM50326709 ((R)-1-(2-oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-b...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to rat bradykinin B1 receptor | J Med Chem 53: 5383-99 (2010) Article DOI: 10.1021/jm1000776 BindingDB Entry DOI: 10.7270/Q289162Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551170 (CHEMBL4786173) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-DAMGO from rat mu opioid receptor expressed in CHO cells incubated for 30 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112070 BindingDB Entry DOI: 10.7270/Q2JW8JHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377620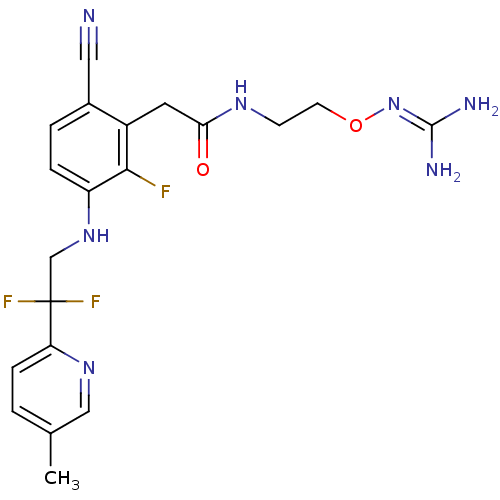 (CHEMBL254784) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377622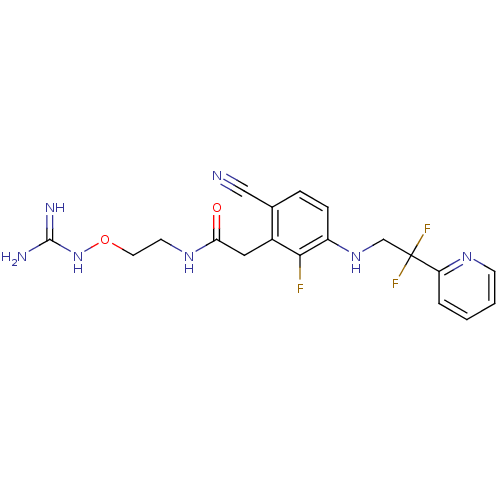 (CHEMBL257543) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377614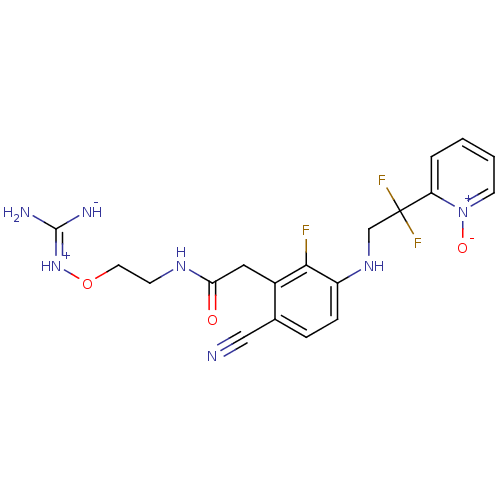 (CHEMBL401655) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM50326392 ((2S)-1-({(2S,4S)-4-[2-(1,3-Dihydro-2H-isoindol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant FAP expressed in Hi5 insect cells by Lineweaver-Burke plot analysis | J Med Chem 53: 6572-83 (2010) Article DOI: 10.1021/jm1002556 BindingDB Entry DOI: 10.7270/Q23F4QM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50536717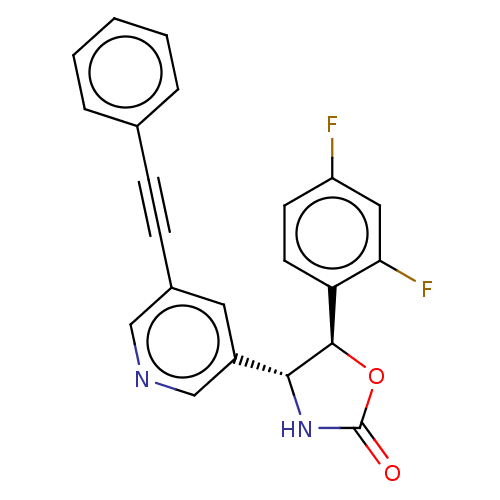 (CHEMBL4574977) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5A transfected in HEK293 cell membranes after 60 mins by microbeta liquid scintillation counting analysis | Bioorg Med Chem Lett 26: 4165-9 (2016) Article DOI: 10.1016/j.bmcl.2016.07.065 BindingDB Entry DOI: 10.7270/Q2HX1H5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50536718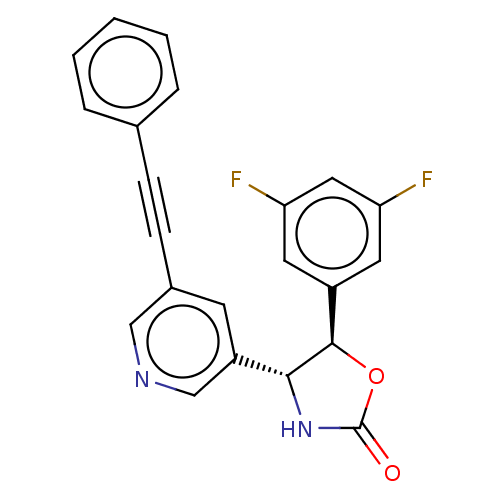 (CHEMBL4545607) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5A transfected in HEK293 cell membranes after 60 mins by microbeta liquid scintillation counting analysis | Bioorg Med Chem Lett 26: 4165-9 (2016) Article DOI: 10.1016/j.bmcl.2016.07.065 BindingDB Entry DOI: 10.7270/Q2HX1H5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50536704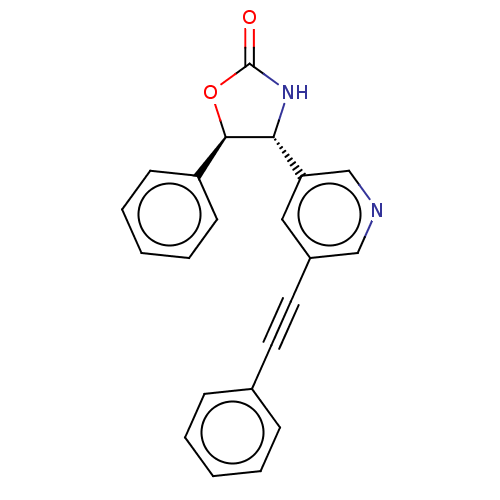 (CHEMBL4580610) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5A transfected in HEK293 cell membranes after 60 mins by microbeta liquid scintillation counting analysis | Bioorg Med Chem Lett 26: 4165-9 (2016) Article DOI: 10.1016/j.bmcl.2016.07.065 BindingDB Entry DOI: 10.7270/Q2HX1H5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50169541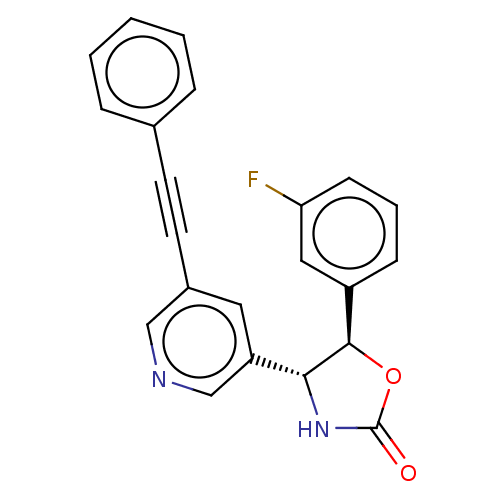 (CHEMBL3805542) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]MPEPgamma in mGlu5 receptor (unknown origin) incubated for 60 mins by liquid scintillation counting method | ACS Med Chem Lett 7: 289-93 (2016) Article DOI: 10.1021/acsmedchemlett.5b00450 BindingDB Entry DOI: 10.7270/Q28G8NMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | CHEMBL5275873 | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL CHEMBL KEGG PC cid PC sid UniChem Similars | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | CHEMBL5272258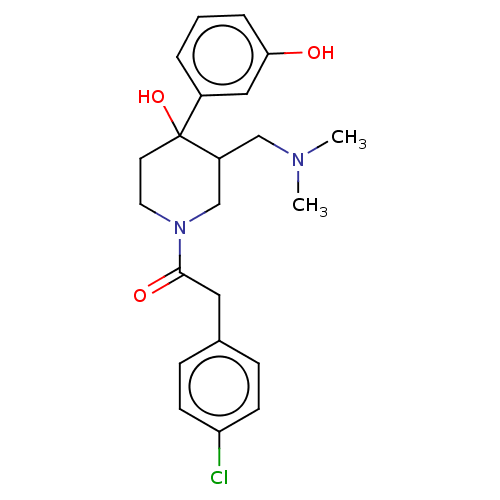 | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50169541 (CHEMBL3805542) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5A transfected in HEK293 cell membranes after 60 mins by microbeta liquid scintillation counting analysis | Bioorg Med Chem Lett 26: 4165-9 (2016) Article DOI: 10.1016/j.bmcl.2016.07.065 BindingDB Entry DOI: 10.7270/Q2HX1H5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50169542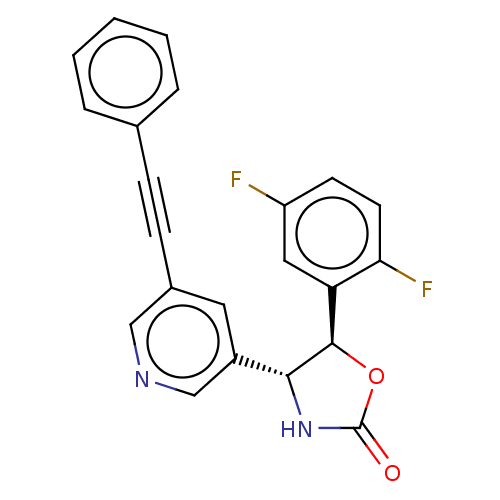 (CHEMBL3804846) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5A transfected in HEK293 cell membranes after 60 mins by microbeta liquid scintillation counting analysis | Bioorg Med Chem Lett 26: 4165-9 (2016) Article DOI: 10.1016/j.bmcl.2016.07.065 BindingDB Entry DOI: 10.7270/Q2HX1H5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50169542 (CHEMBL3804846) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]MPEPgamma in mGlu5 receptor (unknown origin) incubated for 60 mins by liquid scintillation counting method | ACS Med Chem Lett 7: 289-93 (2016) Article DOI: 10.1021/acsmedchemlett.5b00450 BindingDB Entry DOI: 10.7270/Q28G8NMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50152162 (CHEMBL188844 | CHEMBL2165204 | [3-(6-Dimethylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Binding affinity to CRFR1 by autoradiography | Bioorg Med Chem Lett 22: 6651-5 (2012) Article DOI: 10.1016/j.bmcl.2012.08.112 BindingDB Entry DOI: 10.7270/Q20C4WWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50395823 (CHEMBL2165205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Binding affinity to CRFR1 by autoradiography | Bioorg Med Chem Lett 22: 6651-5 (2012) Article DOI: 10.1016/j.bmcl.2012.08.112 BindingDB Entry DOI: 10.7270/Q20C4WWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50536710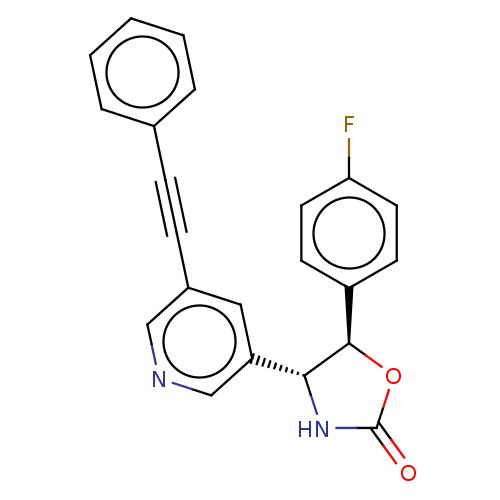 (CHEMBL4560002) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5A transfected in HEK293 cell membranes after 60 mins by microbeta liquid scintillation counting analysis | Bioorg Med Chem Lett 26: 4165-9 (2016) Article DOI: 10.1016/j.bmcl.2016.07.065 BindingDB Entry DOI: 10.7270/Q2HX1H5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377617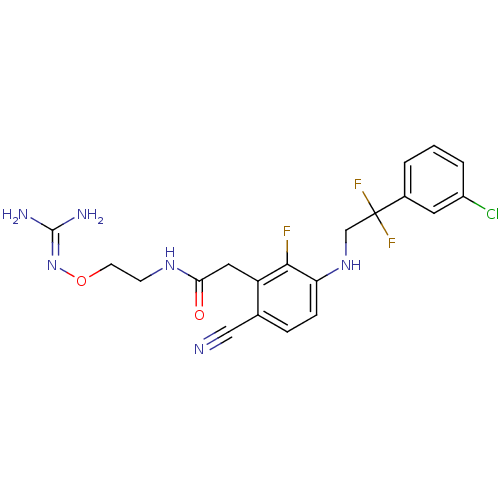 (CHEMBL403359) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM86192 (CAS_197801-88-0 | CHEMBL2165203 | SN003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Binding affinity to CRFR1 by autoradiography | Bioorg Med Chem Lett 22: 6651-5 (2012) Article DOI: 10.1016/j.bmcl.2012.08.112 BindingDB Entry DOI: 10.7270/Q20C4WWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | CHEMBL5279890 | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at histamine H1 receptor in guinea pig ileum assessed as histamine-induced contractions | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50536719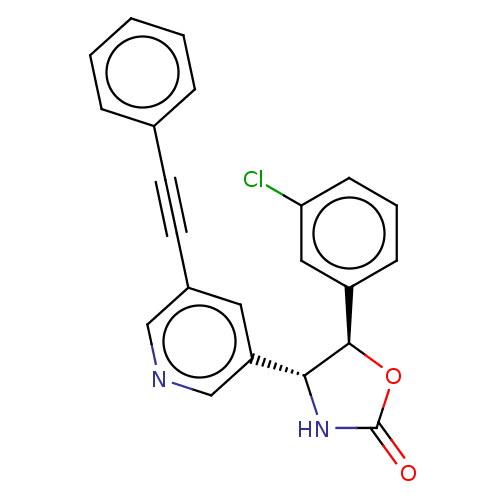 (CHEMBL4517787) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5A transfected in HEK293 cell membranes after 60 mins by microbeta liquid scintillation counting analysis | Bioorg Med Chem Lett 26: 4165-9 (2016) Article DOI: 10.1016/j.bmcl.2016.07.065 BindingDB Entry DOI: 10.7270/Q2HX1H5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377624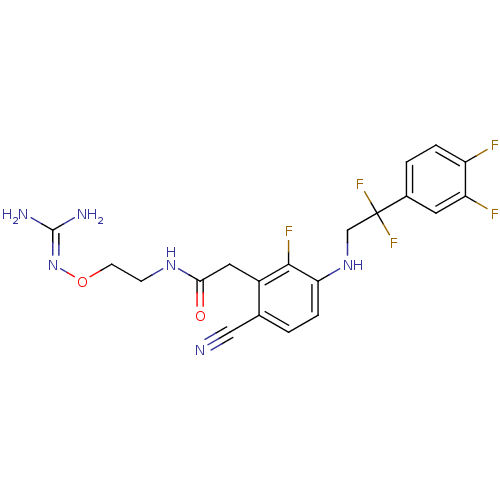 (CHEMBL403310) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50536702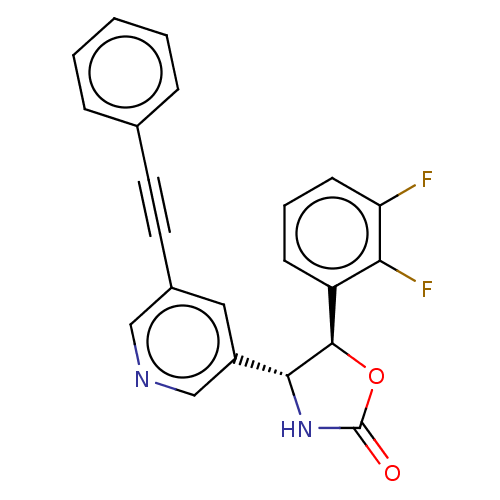 (CHEMBL4554515) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5A transfected in HEK293 cell membranes after 60 mins by microbeta liquid scintillation counting analysis | Bioorg Med Chem Lett 26: 4165-9 (2016) Article DOI: 10.1016/j.bmcl.2016.07.065 BindingDB Entry DOI: 10.7270/Q2HX1H5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50536709 (CHEMBL4537236) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5A transfected in HEK293 cell membranes after 60 mins by microbeta liquid scintillation counting analysis | Bioorg Med Chem Lett 26: 4165-9 (2016) Article DOI: 10.1016/j.bmcl.2016.07.065 BindingDB Entry DOI: 10.7270/Q2HX1H5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 13996 total ) | Next | Last >> |