

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM153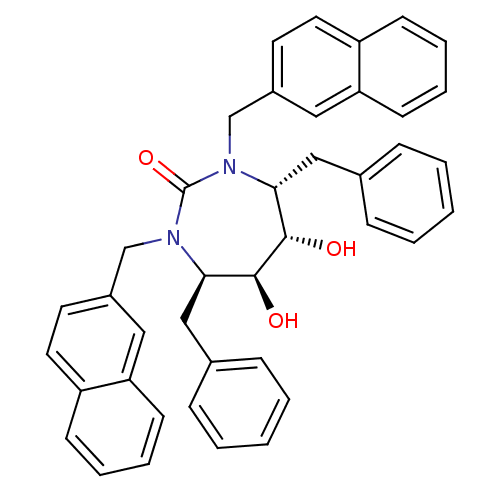 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(n...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 51: 4280-8 (2008) Article DOI: 10.1021/jm800242q BindingDB Entry DOI: 10.7270/Q2S1858S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530711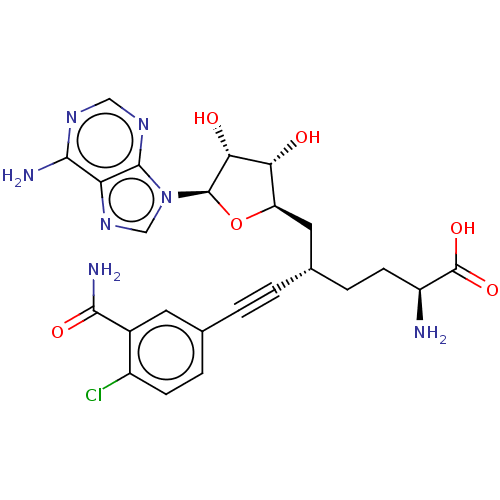 (CHEMBL4553052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530711 (CHEMBL4553052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530731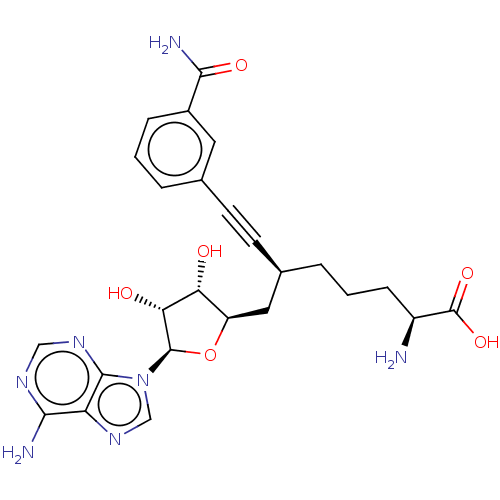 (CHEMBL4580446) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530731 (CHEMBL4580446) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50155999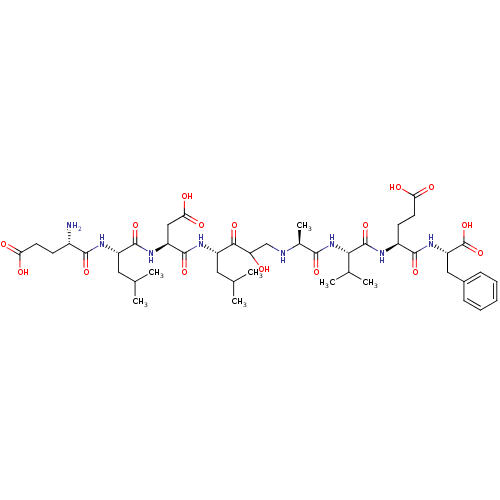 (CHEMBL363255 | Glu-Leu-Asp-Leu-(CHOH-CH2)-Ala-Ala-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards Beta-secretase determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156004 (2-(2-{(2R,4S)-2-Benzyl-5-[2-(2-benzyloxycarbonylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156012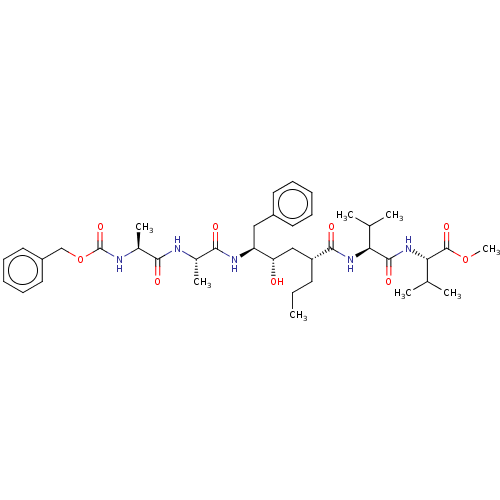 (2-(2-{(2R,4S)-5-[2-(2-Benzyloxycarbonylamino-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530712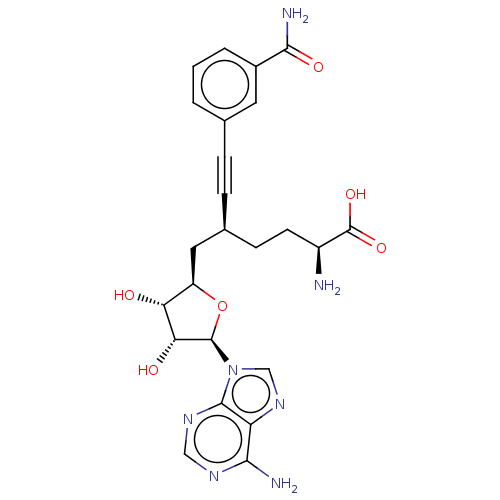 (CHEMBL4591248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530712 (CHEMBL4591248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530712 (CHEMBL4591248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530712 (CHEMBL4591248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156009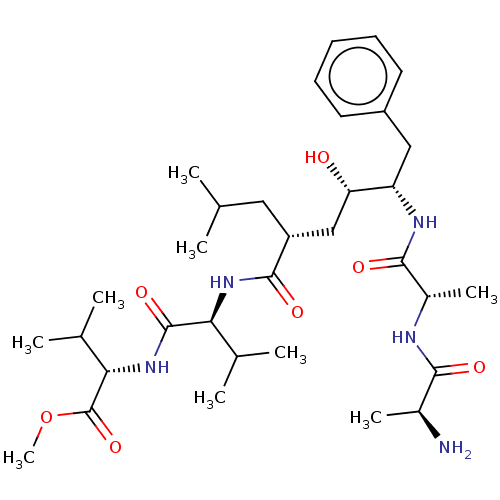 (2-(2-{(2R,4S)-5-[2-(2-Amino-propionylamino)-propio...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156000 (2-{2-[(2R,4S)-2-Benzyl-5-(2-benzyloxycarbonylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50155992 (2-(2-{(2R,4S)-5-[2-(2-Benzyloxycarbonylamino-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50155990 (CHEMBL187379 | {1-[(2S,4R)-1-Benzyl-4-(1-carbamoyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50155991 (2-{2-[(2R,4S)-5-(2-Benzyloxycarbonylamino-propiony...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109106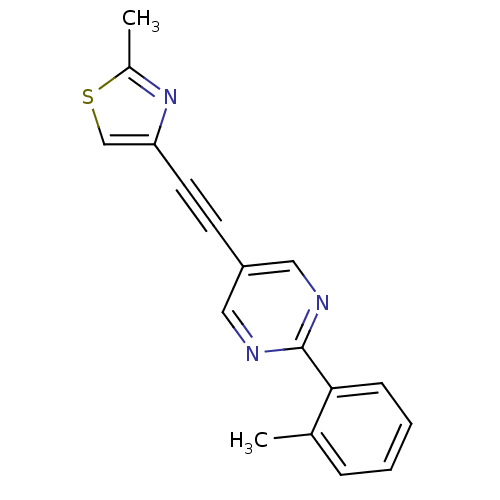 (US8609852, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109136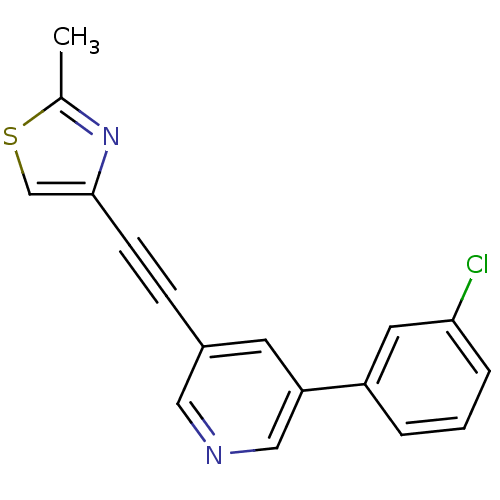 (US8609852, 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.650 | -52.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109101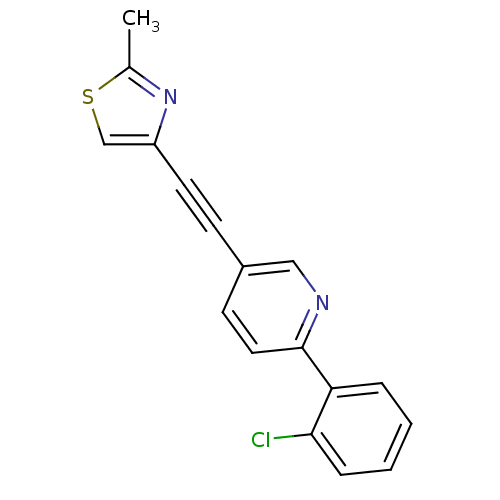 (US8609852, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109157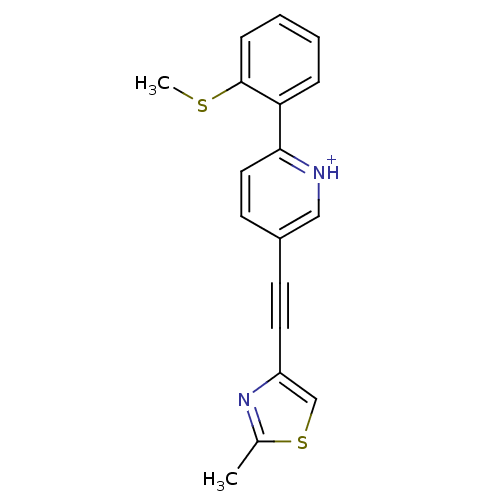 (US8609852, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50155984 (2-(2-{(2R,4S)-5-[2-(2-Benzyloxycarbonylamino-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109102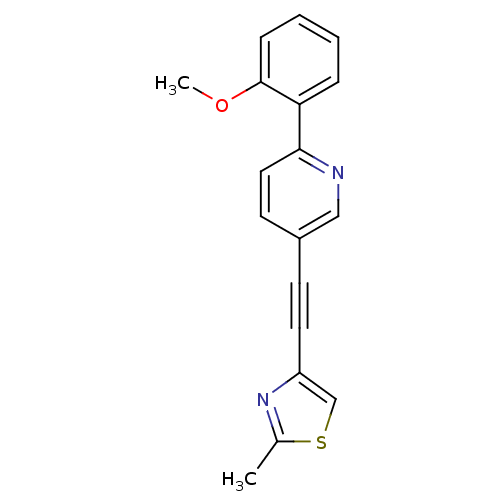 (US8609852, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109121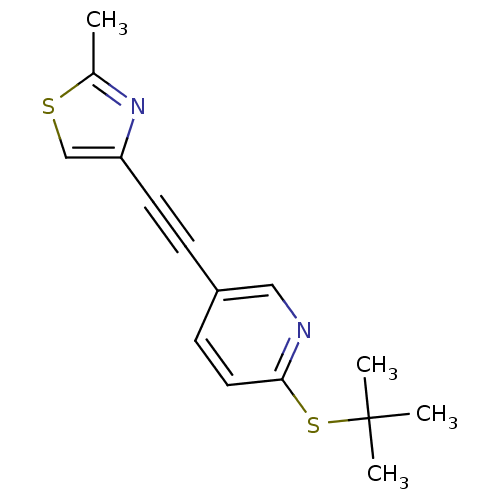 (US8609852, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109115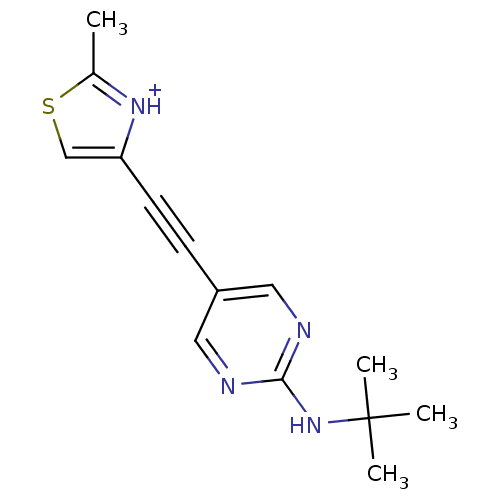 (US8609852, 117 | US8609852, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156011 (2-{2-[(2R,4S)-5-(2-Benzyloxycarbonylamino-propiony...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109120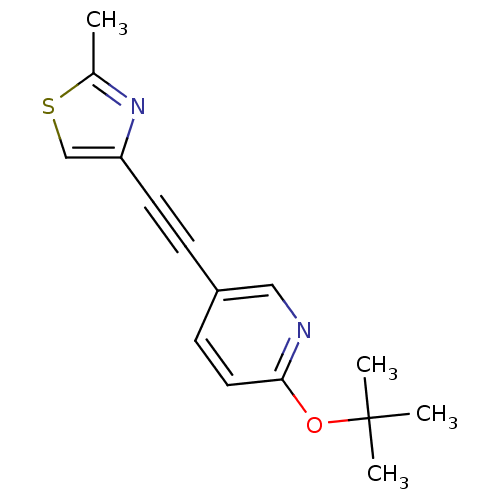 (US8609852, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109115 (US8609852, 117 | US8609852, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109182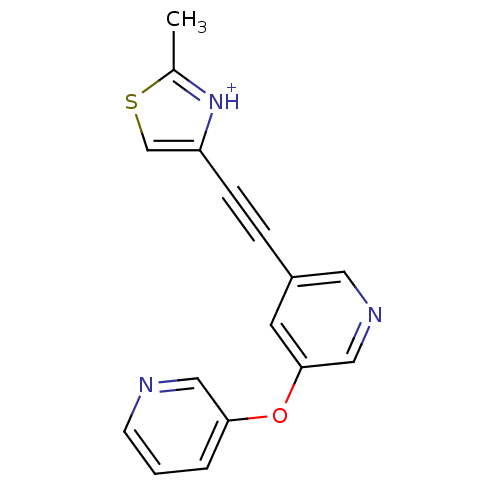 (US8609852, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109099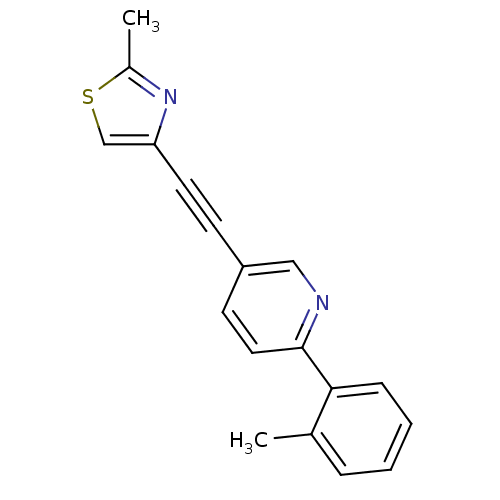 (US8609852, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109176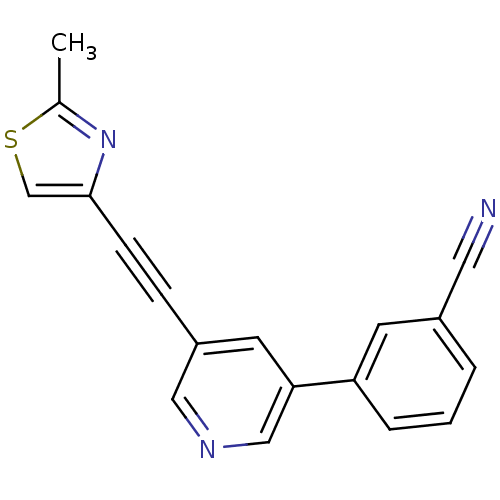 (US8609852, 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50149792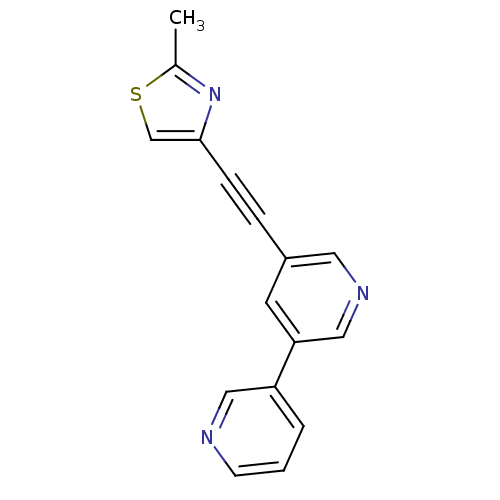 (5-(2-Methyl-thiazol-4-ylethynyl)-[3,3'']bipyridiny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement by compound of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat cortical membranes | Bioorg Med Chem Lett 14: 3993-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.037 BindingDB Entry DOI: 10.7270/Q2T43SKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109105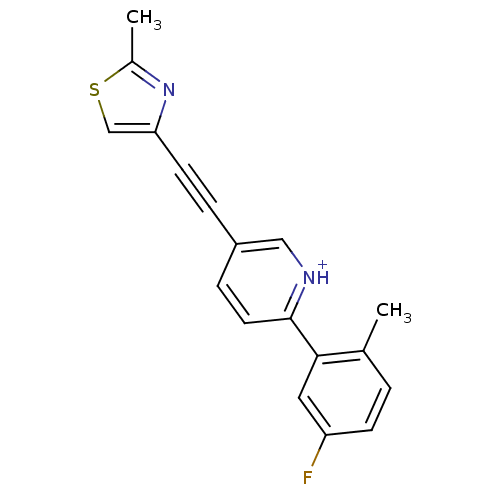 (US8609852, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109100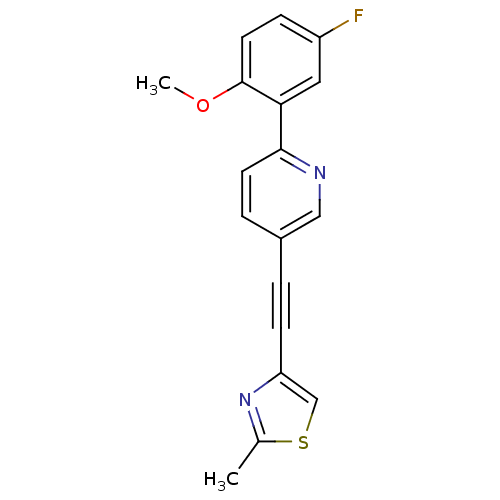 (US8609852, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50155985 (2-(2-{(2R,4S)-5-[2-(2-Amino-propionylamino)-propio...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50181753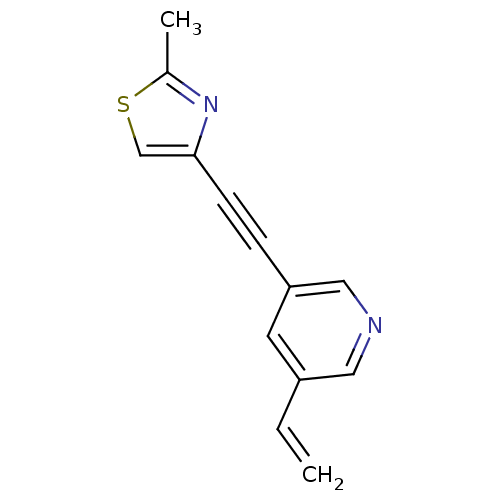 (3-[(2-methyl-4-thiazolyl)ethynyl]-5-vinylpyridine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50155993 (CHEMBL364773 | [(2S,4R)-1-Benzyl-4-(1-carbamoyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109113 (US8609852, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109153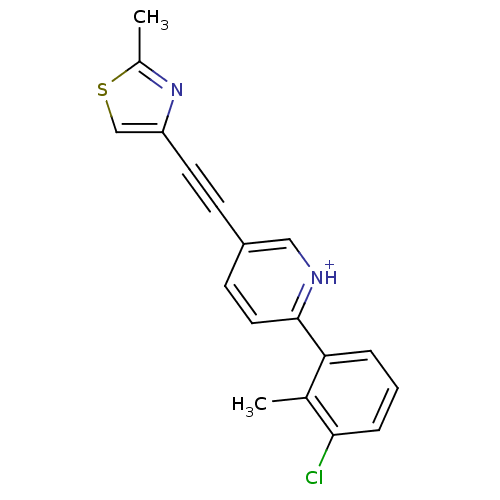 (US8609852, 60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109151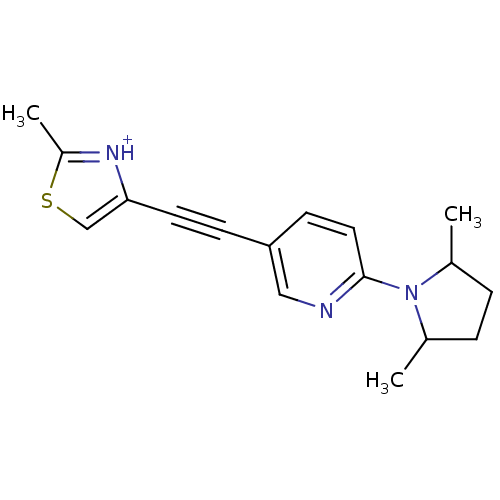 (US8609852, 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109147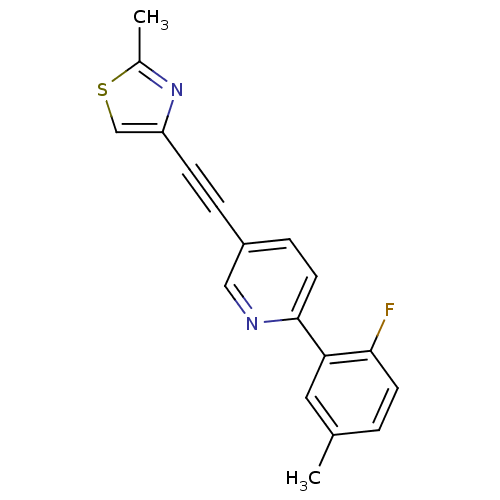 (US8609852, 54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109114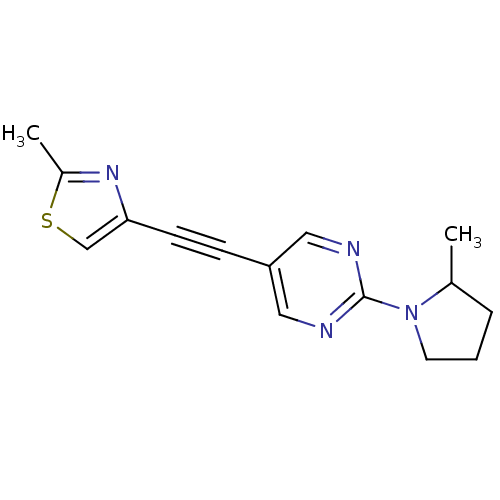 (US8609852, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50155988 ((S)-4-{(S)-2-[(S)-2-((S)-4-{(S)-2-[(S)-2-((S)-2-Am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards Beta-secretase determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109116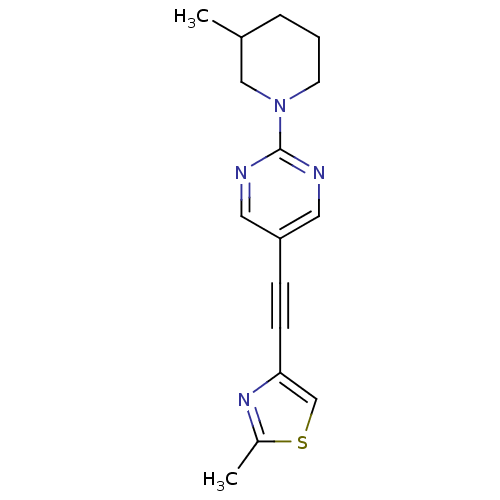 (US8609852, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | -50.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109194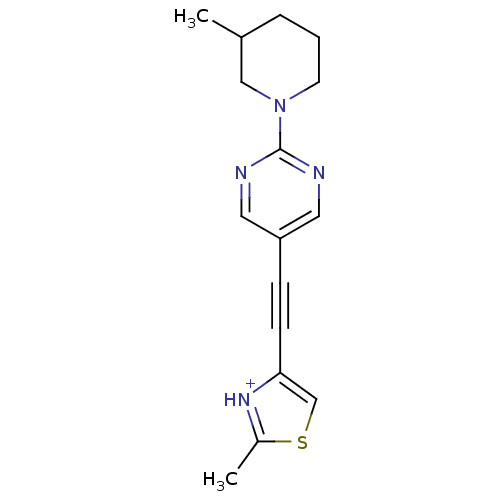 (US8609852, 103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | -50.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156006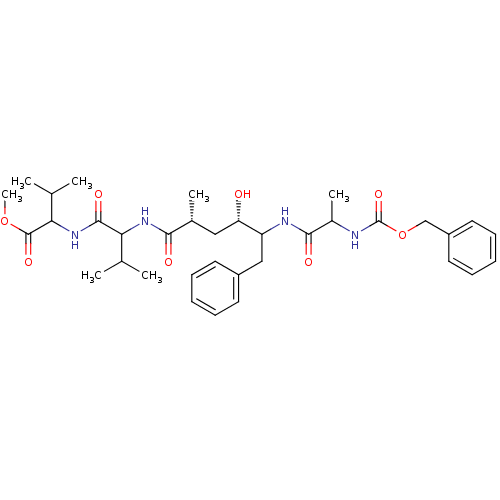 (2-{2-[(2R,4S)-5-(2-Benzyloxycarbonylamino-propiony...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards human immunodeficiency virus type 1 protease determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50154997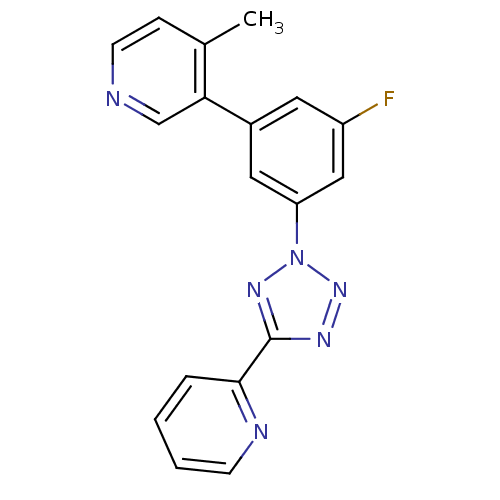 (3-[3-Fluoro-5-(5-pyridin-2-yl-tetrazol-2-yl)-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Metabotropic glutamate receptor was determined by displacing [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat cortica... | Bioorg Med Chem Lett 14: 5481-4 (2004) Article DOI: 10.1016/j.bmcl.2004.09.018 BindingDB Entry DOI: 10.7270/Q25M657V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530723 (CHEMBL4455627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530723 (CHEMBL4455627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109096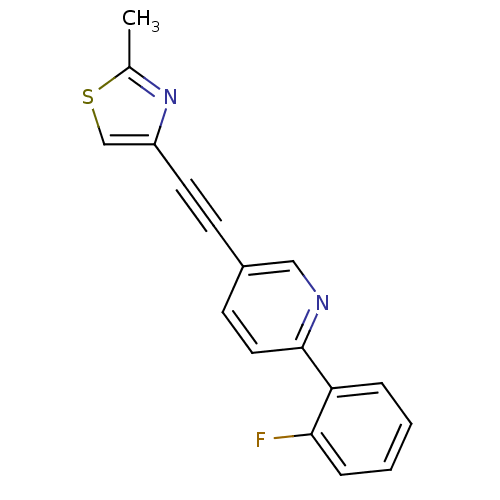 (US8609852, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4168 total ) | Next | Last >> |