

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apelin receptor (Homo sapiens (Human)) | BDBM50556826 (CHEMBL4745863) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01913 BindingDB Entry DOI: 10.7270/Q2BG2SNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50556827 (CHEMBL4748838) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01913 BindingDB Entry DOI: 10.7270/Q2BG2SNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50556825 (CHEMBL4787784) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01913 BindingDB Entry DOI: 10.7270/Q2BG2SNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50556828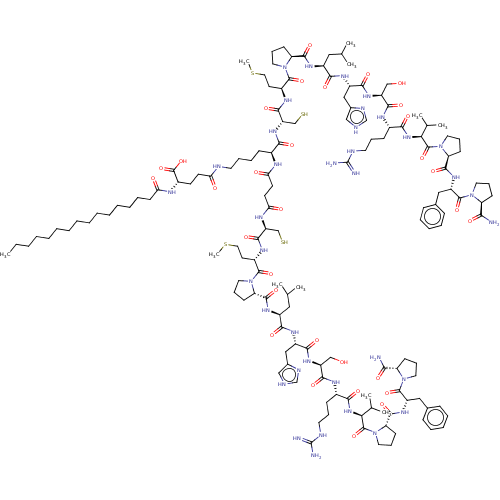 (CHEMBL4798771) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01913 BindingDB Entry DOI: 10.7270/Q2BG2SNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50297420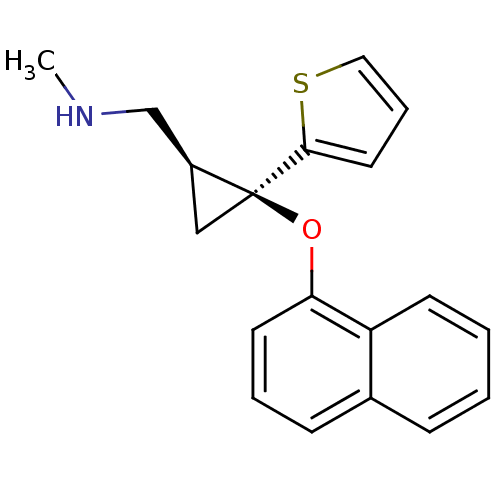 ((Z)-N-Methyl-2-(naphthalen-1-yloxy)-2-(thiophen-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]serotonin reuptake at serotonin transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50297420 ((Z)-N-Methyl-2-(naphthalen-1-yloxy)-2-(thiophen-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]serotonin reuptake at serotonin transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435111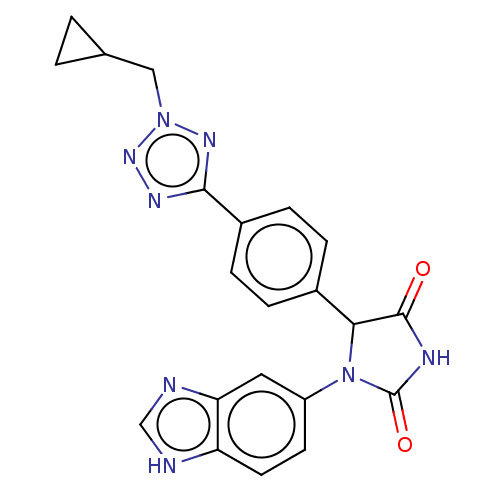 (US10584120, Compound 45) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435105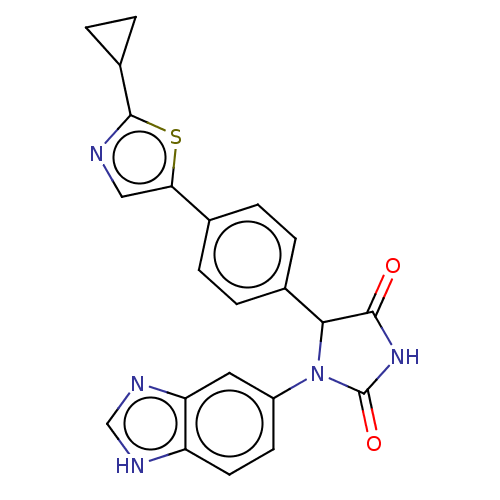 (US10584120, Compound 39) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435104 (US10584120, Compound 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50297420 ((Z)-N-Methyl-2-(naphthalen-1-yloxy)-2-(thiophen-2-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]norepinephrine reuptake at norepinephrine transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50297420 ((Z)-N-Methyl-2-(naphthalen-1-yloxy)-2-(thiophen-2-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]norepinephrine reuptake at norepinephrine transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435108 (US10584120, Compound 42) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22165 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]dopamine reuptake at dopamine transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50556824 (CHEMBL4746142) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01913 BindingDB Entry DOI: 10.7270/Q2BG2SNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 5.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]norepinephrine reuptake at norepinephrine transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50556829 (CHEMBL4743589) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01913 BindingDB Entry DOI: 10.7270/Q2BG2SNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435128 (US10584120, Compound 62) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435114 (US10584120, Compound 47) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435123 (US10584120, Compound 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435126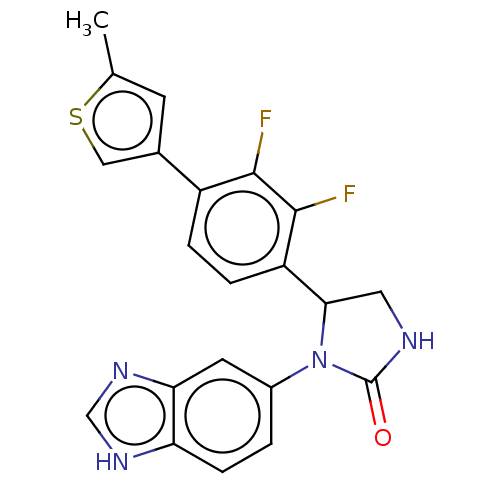 (US10584120, Compound 60) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50297418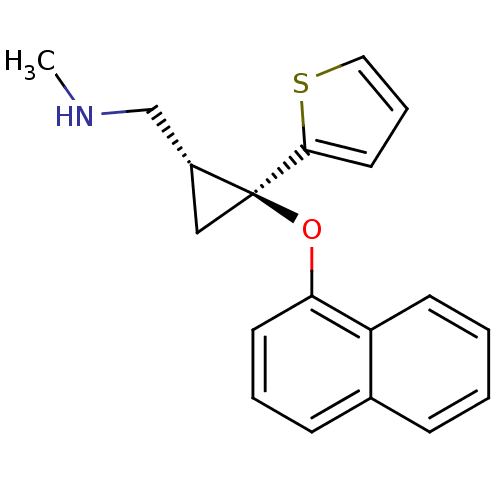 ((E)-N-Methyl-2-(naphthalen-1-yloxy)-2-(thiophen-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]serotonin reuptake at serotonin transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50297418 ((E)-N-Methyl-2-(naphthalen-1-yloxy)-2-(thiophen-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]serotonin reuptake at serotonin transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50010859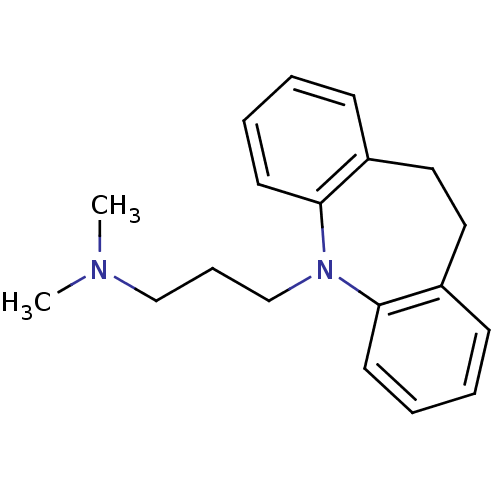 (CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 7.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]serotonin reuptake at serotonin transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435112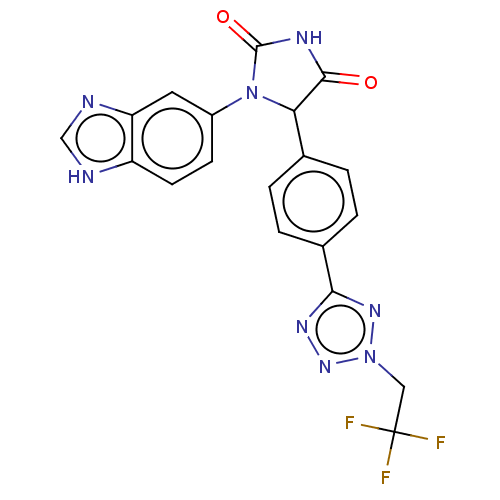 (US10584120, Compound 46) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435109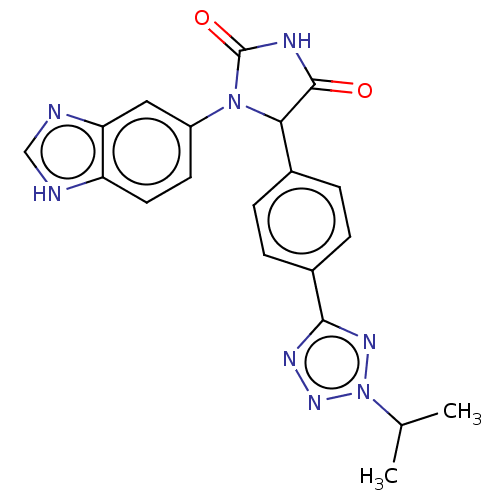 (US10584120, Compound 43) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435106 (US10584120, Compound 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435116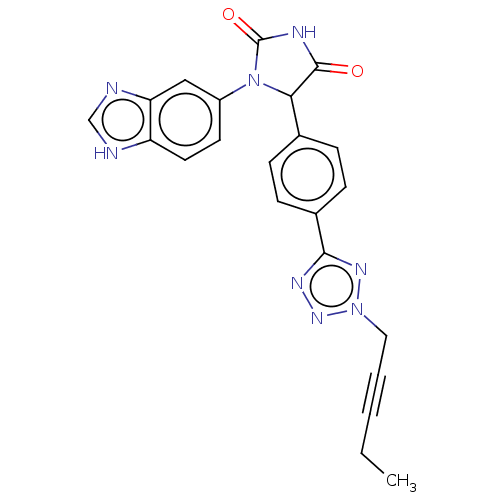 (US10584120, Compound 49) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435115 (US10584120, Compound 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435110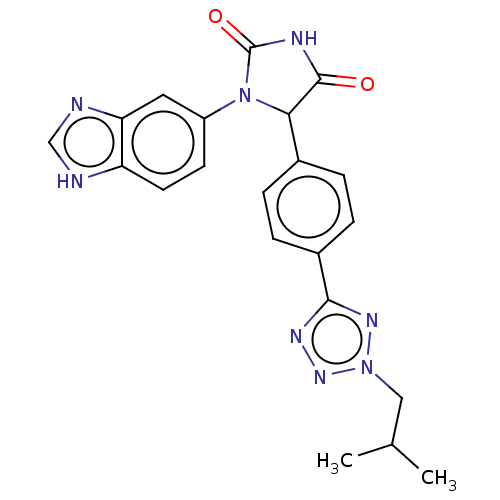 (US10584120, Compound 44) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435129 (US10584120, Compound 63) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435107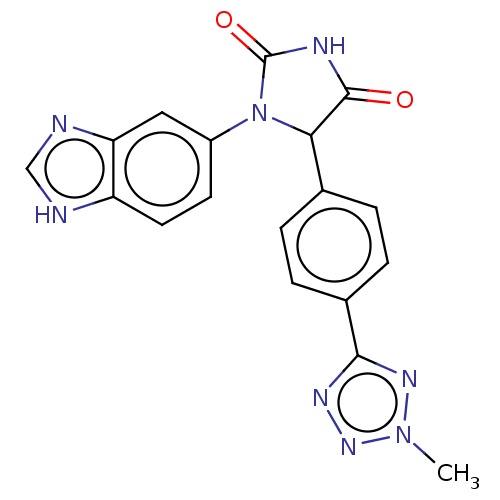 (US10584120, Compound 41) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435130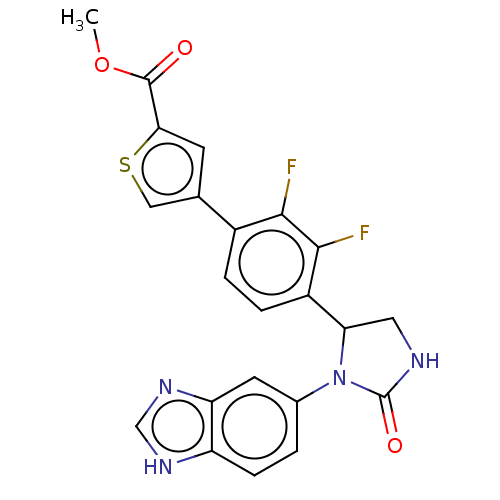 (US10584120, Compound 64) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435117 (US10584120, Compound 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435078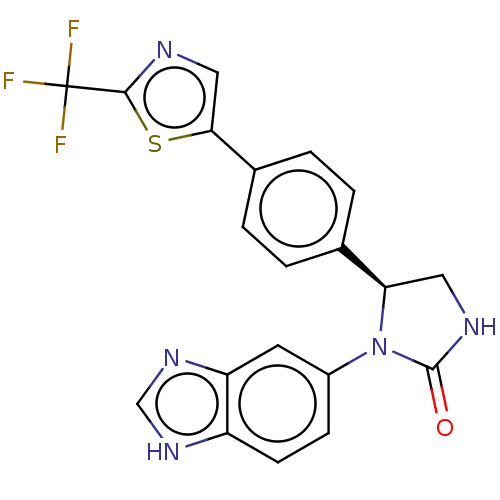 (US10584120, Compound 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435127 (US10584120, Compound 61) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435103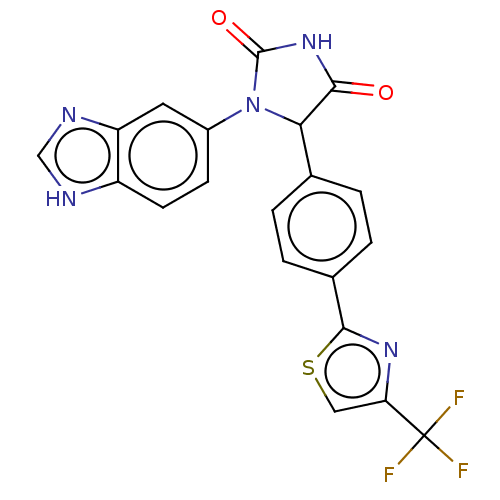 (US10584120, Compound 37) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435125 (US10584120, Compound 59) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435131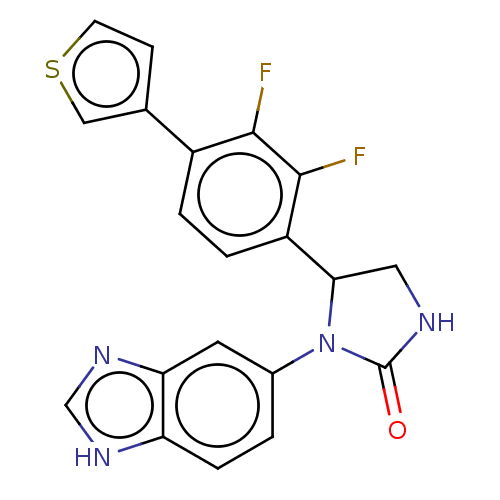 (US10584120, Compound 65) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435075 (US10584120, Compound 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435121 (US10584120, Compound 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435118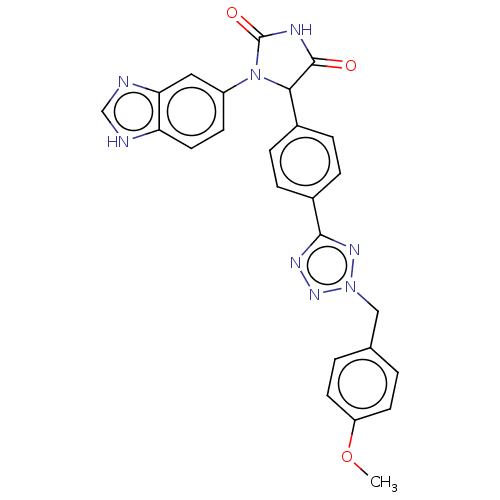 (US10584120, Compound 51) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50297421 (CHEMBL558525 | N-methyl-1-((1R,2R)-2-(naphthalen-1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]norepinephrine reuptake at norepinephrine transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50297421 (CHEMBL558525 | N-methyl-1-((1R,2R)-2-(naphthalen-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]serotonin reuptake at serotonin transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435102 (US10584120, Compound 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50297419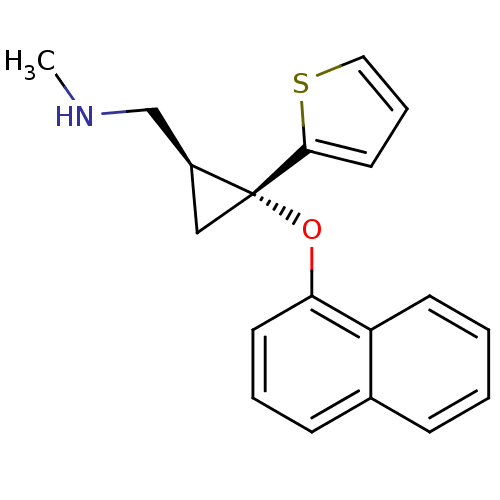 (CHEMBL558065 | N-methyl-1-((1S,2R)-2-(naphthalen-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]serotonin reuptake at serotonin transporter | J Med Chem 52: 5872-9 (2009) Article DOI: 10.1021/jm900847b BindingDB Entry DOI: 10.7270/Q26Q1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435090 (US10584120, Compound 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435097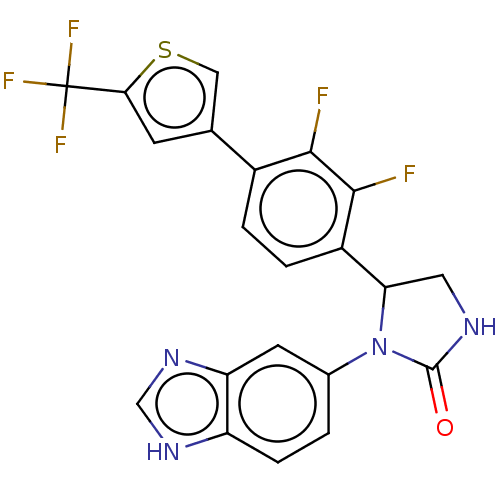 (US10584120, Compound 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435076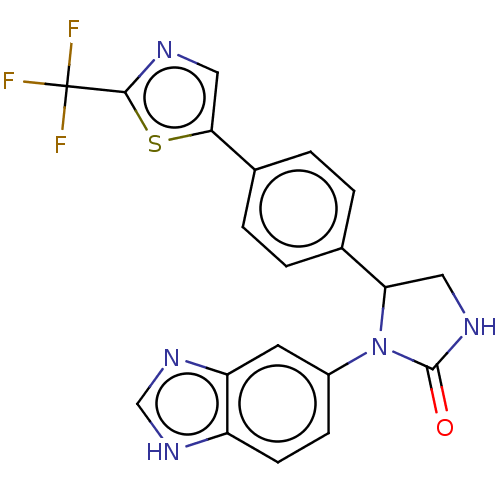 (US10584120, Compound 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435072 (US10584120, Compound 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435089 (US10584120, Compound 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 810 total ) | Next | Last >> |