 Found 9272 hits with Last Name = 'huang' and Initial = 'w'
Found 9272 hits with Last Name = 'huang' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human Coagulation factor Xa (trypsin-like serine protease) |
Bioorg Med Chem Lett 12: 1651-5 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RFZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124984
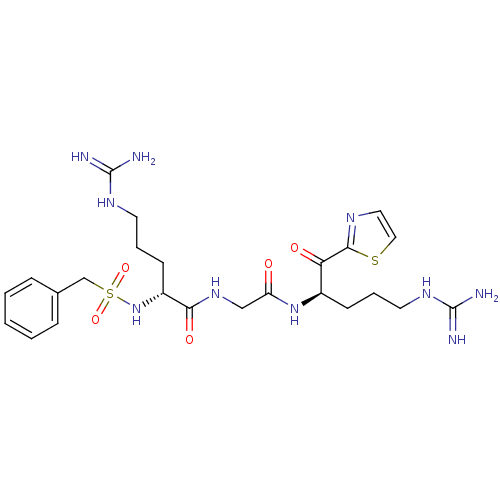
((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...)Show SMILES NC(=N)NCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C24H36N10O5S2/c25-23(26)30-10-4-8-17(20(36)22-29-12-13-40-22)33-19(35)14-32-21(37)18(9-5-11-31-24(27)28)34-41(38,39)15-16-6-2-1-3-7-16/h1-3,6-7,12-13,17-18,34H,4-5,8-11,14-15H2,(H,32,37)(H,33,35)(H4,25,26,30)(H4,27,28,31)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
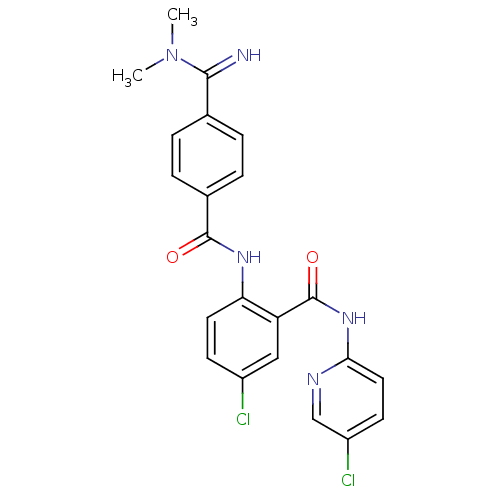
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573891

(A-1282576 | A-1282576.0 | A-12825760 | ABT-493 | G...)Show SMILES CC(C)(C)[C@@H]1NC(=O)O[C@@H]2CCC[C@H]2OC\C=C\C(F)(F)c2nc3ccccc3nc2O[C@@H]2C[C@H](N(C2)C1=O)C(=O)N[C@@]1(C[C@H]1C(F)F)C(=O)NS(=O)(=O)C1(C)CC1 |r,t:17| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM103838
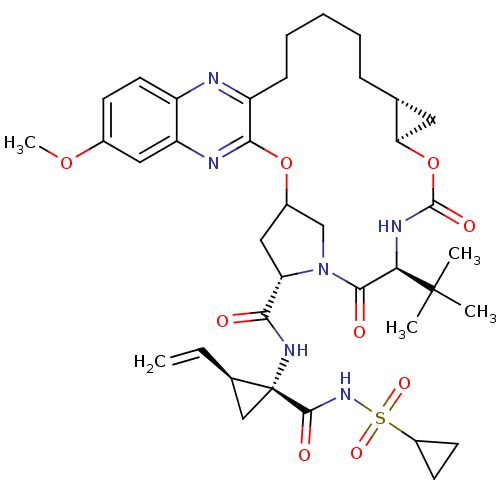
(MK-5172)Show SMILES COc1ccc2nc3CCCCC[C@@H]4C[C@H]4OC(=O)N[C@H](C(=O)N4CC(C[C@H]4C(=O)N[C@@]4(C[C@H]4C=C)C(=O)NS(=O)(=O)C4CC4)Oc3nc2c1)C(C)(C)C |r| Show InChI InChI=1S/C38H50N6O9S/c1-6-22-19-38(22,35(47)43-54(49,50)25-13-14-25)42-32(45)29-18-24-20-44(29)34(46)31(37(2,3)4)41-36(48)53-30-16-21(30)10-8-7-9-11-27-33(52-24)40-28-17-23(51-5)12-15-26(28)39-27/h6,12,15,17,21-22,24-25,29-31H,1,7-11,13-14,16,18-20H2,2-5H3,(H,41,48)(H,42,45)(H,43,47)/t21-,22-,24?,29+,30-,31-,38-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School
| Assay Description
NS3/4A protease was preincubated with increasing concentration of drugs in protease reaction buffer for 1 hour. Inhibition assays were performed in ... |
ACS Chem Biol 8: 1469-78 (2013)
Article DOI: 10.1021/cb400100g
BindingDB Entry DOI: 10.7270/Q2FQ9V7S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249120
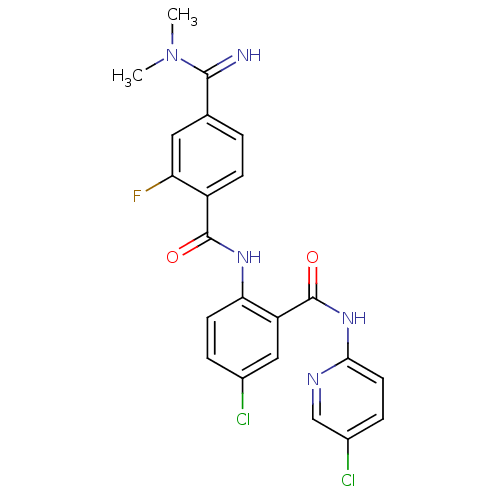
(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50600748
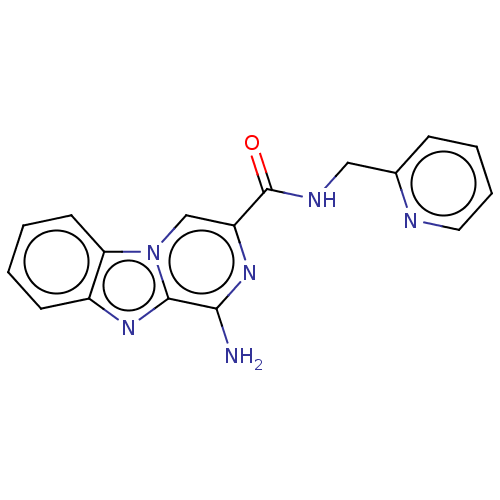
(CHEMBL5176737) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00101
BindingDB Entry DOI: 10.7270/Q2417231 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249423
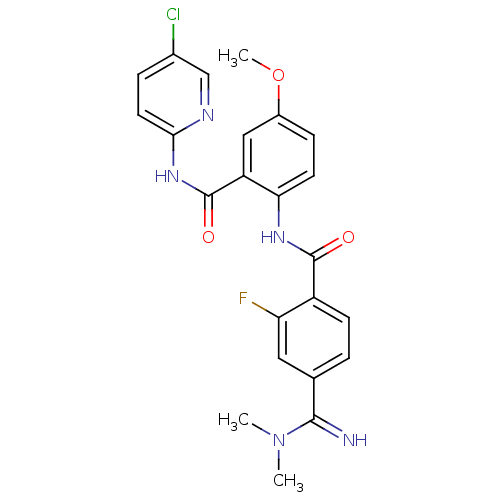
(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249298
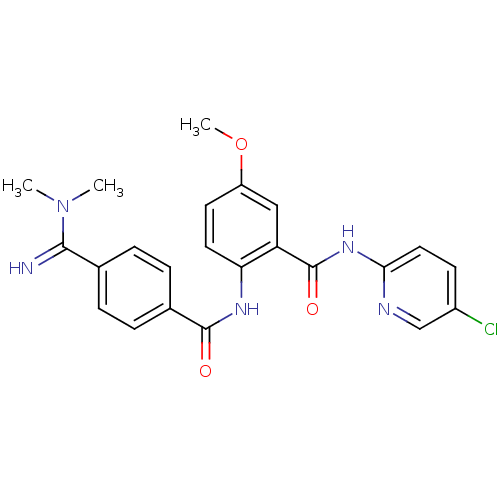
(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124975
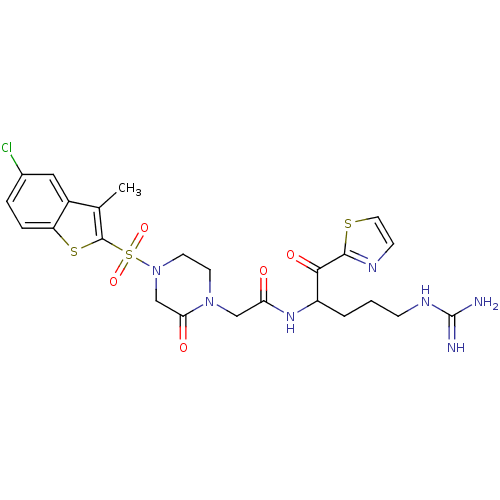
(2-[4-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfon...)Show SMILES Cc1c(sc2ccc(Cl)cc12)S(=O)(=O)N1CCN(CC(=O)NC(CCCNC(N)=N)C(=O)c2nccs2)C(=O)C1 Show InChI InChI=1S/C24H28ClN7O5S3/c1-14-16-11-15(25)4-5-18(16)39-23(14)40(36,37)32-9-8-31(20(34)13-32)12-19(33)30-17(3-2-6-29-24(26)27)21(35)22-28-7-10-38-22/h4-5,7,10-11,17H,2-3,6,8-9,12-13H2,1H3,(H,30,33)(H4,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573903
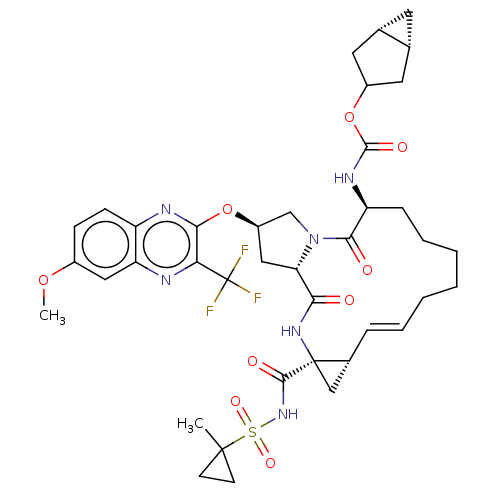
(CHEMBL4852970)Show SMILES [H][C@@]12C[C@]1([H])CC(C2)OC(=O)N[C@H]1CCCCC\C=C\[C@@H]2C[C@]2(NC(=O)[C@@H]2C[C@H](CN2C1=O)Oc1nc2ccc(OC)cc2nc1C(F)(F)F)C(=O)NS(=O)(=O)C1(C)CC1 |r,t:20| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50458698
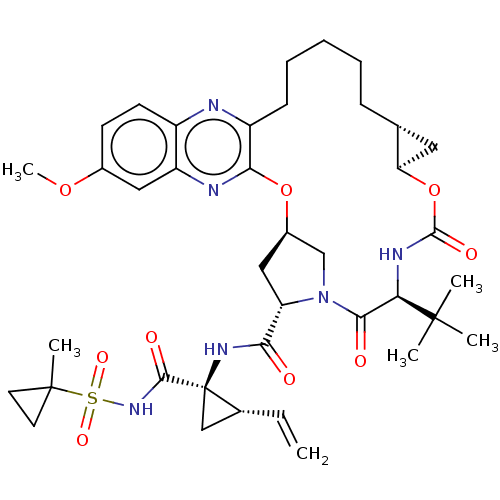
(CHEMBL4203709)Show SMILES COc1ccc2nc3CCCCC[C@@H]4C[C@H]4OC(=O)N[C@H](C(=O)N4C[C@@H](C[C@H]4C(=O)N[C@@]4(C[C@H]4C=C)C(=O)NS(=O)(=O)C4(C)CC4)Oc3nc2c1)C(C)(C)C |r| Show InChI InChI=1S/C39H52N6O9S/c1-7-23-20-39(23,35(48)44-55(50,51)38(5)15-16-38)43-32(46)29-19-25-21-45(29)34(47)31(37(2,3)4)42-36(49)54-30-17-22(30)11-9-8-10-12-27-33(53-25)41-28-18-24(52-6)13-14-26(28)40-27/h7,13-14,18,22-23,25,29-31H,1,8-12,15-17,19-21H2,2-6H3,(H,42,49)(H,43,46)(H,44,48)/t22-,23-,25-,29+,30-,31-,39-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School
Curated by ChEMBL
| Assay Description
Inhibition of wild type HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) using Ac-DE-Dap(QXL 520)-EE-Abu-psi-[COO]AS-C(5-FAMsp... |
ACS Med Chem Lett 9: 691-696 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00150
BindingDB Entry DOI: 10.7270/Q29C7125 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50485492

(Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...)Show SMILES [H][C@@]12C[C@@]1([H])OC(=O)N[C@H](C(=O)N1C[C@@]([H])(C[C@H]1C(=O)N[C@@]1(C[C@H]1C=C)C(=O)NS(=O)(=O)C1CC1)Oc1nc3cc(OC)ccc3nc1CCCCC2)C(C)(C)C |r| Show InChI InChI=1S/C38H50N6O9S/c1-6-22-19-38(22,35(47)43-54(49,50)25-13-14-25)42-32(45)29-18-24-20-44(29)34(46)31(37(2,3)4)41-36(48)53-30-16-21(30)10-8-7-9-11-27-33(52-24)40-28-17-23(51-5)12-15-26(28)39-27/h6,12,15,17,21-22,24-25,29-31H,1,7-11,13-14,16,18-20H2,2-5H3,(H,41,48)(H,42,45)(H,43,47)/t21-,22-,24-,29+,30-,31-,38-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM103837
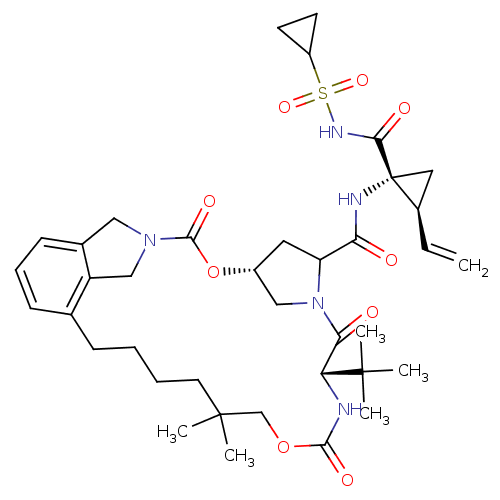
(Dan-mcP2P4)Show SMILES CC(C)(C)[C@@H]1NC(=O)OCC(C)(C)CCCCc2cccc3CN(Cc23)C(=O)O[C@@H]2CC(N(C2)C1=O)C(=O)N[C@@]1(C[C@H]1C=C)C(=O)NS(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C38H53N5O9S/c1-7-25-18-38(25,33(46)41-53(49,50)27-14-15-27)40-31(44)29-17-26-20-43(29)32(45)30(36(2,3)4)39-34(47)51-22-37(5,6)16-9-8-11-23-12-10-13-24-19-42(21-28(23)24)35(48)52-26/h7,10,12-13,25-27,29-30H,1,8-9,11,14-22H2,2-6H3,(H,39,47)(H,40,44)(H,41,46)/t25-,26-,29?,30-,38-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School
| Assay Description
NS3/4A protease was preincubated with increasing concentration of drugs in protease reaction buffer for 1 hour. Inhibition assays were performed in ... |
ACS Chem Biol 8: 1469-78 (2013)
Article DOI: 10.1021/cb400100g
BindingDB Entry DOI: 10.7270/Q2FQ9V7S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140394
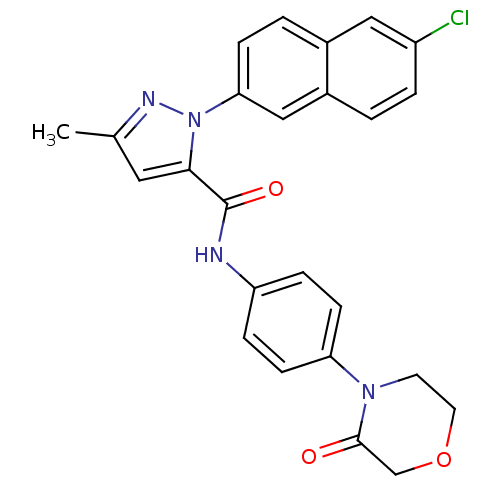
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)N2CCOCC2=O)n(n1)-c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H21ClN4O3/c1-16-12-23(30(28-16)22-7-3-17-13-19(26)4-2-18(17)14-22)25(32)27-20-5-8-21(9-6-20)29-10-11-33-15-24(29)31/h2-9,12-14H,10-11,15H2,1H3,(H,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
Bioorg Med Chem Lett 14: 1221-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.053
BindingDB Entry DOI: 10.7270/Q2VD6XV5 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573887
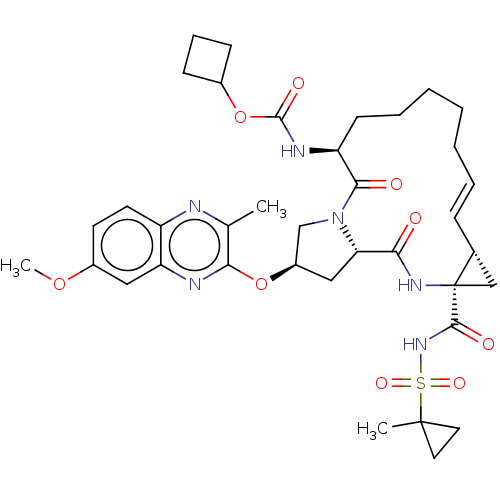
(CHEMBL4879085)Show SMILES COc1ccc2nc(C)c(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C\[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3(C)CC3)NC(=O)OC3CCC3)nc2c1 |r,t:25| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50600748

(CHEMBL5176737) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00101
BindingDB Entry DOI: 10.7270/Q2417231 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
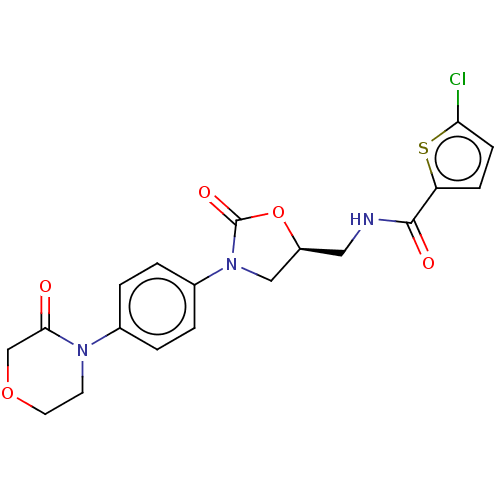
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM103836
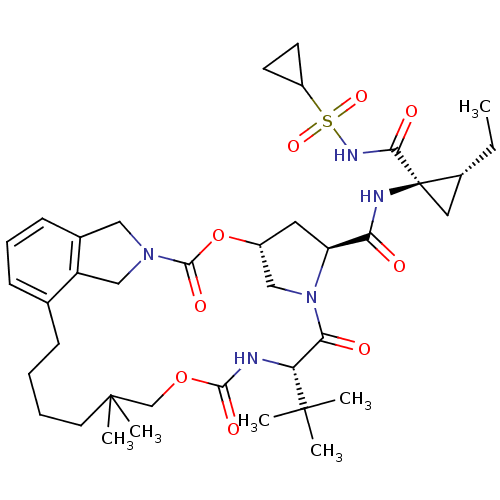
(Vaniprevir)Show SMILES CC[C@@H]1C[C@]1(NC(=O)[C@@H]1C[C@@H]2CN1C(=O)[C@@H](NC(=O)OCC(C)(C)CCCCc1cccc3CN(Cc13)C(=O)O2)C(C)(C)C)C(=O)NS(=O)(=O)C1CC1 Show InChI InChI=1S/C38H55N5O9S/c1-7-25-18-38(25,33(46)41-53(49,50)27-14-15-27)40-31(44)29-17-26-20-43(29)32(45)30(36(2,3)4)39-34(47)51-22-37(5,6)16-9-8-11-23-12-10-13-24-19-42(21-28(23)24)35(48)52-26/h10,12-13,25-27,29-30H,7-9,11,14-22H2,1-6H3,(H,39,47)(H,40,44)(H,41,46)/t25-,26-,29+,30-,38-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School
| Assay Description
NS3/4A protease was preincubated with increasing concentration of drugs in protease reaction buffer for 1 hour. Inhibition assays were performed in ... |
ACS Chem Biol 8: 1469-78 (2013)
Article DOI: 10.1021/cb400100g
BindingDB Entry DOI: 10.7270/Q2FQ9V7S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM294039
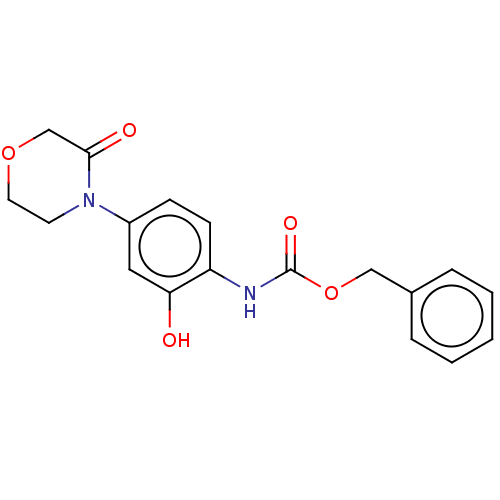
(Process 1 | US10106557, Compound 1)Show InChI InChI=1S/C18H18N2O5/c21-16-10-14(20-8-9-24-12-17(20)22)6-7-15(16)19-18(23)25-11-13-4-2-1-3-5-13/h1-7,10,21H,8-9,11-12H2,(H,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD.
US Patent
| Assay Description
The activity of the compound to be tested against prothrombinase was determined by the production of thrombin. In summary, 12.5 μL human factor ... |
US Patent US10106557 (2018)
BindingDB Entry DOI: 10.7270/Q2X63Q0W |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140388
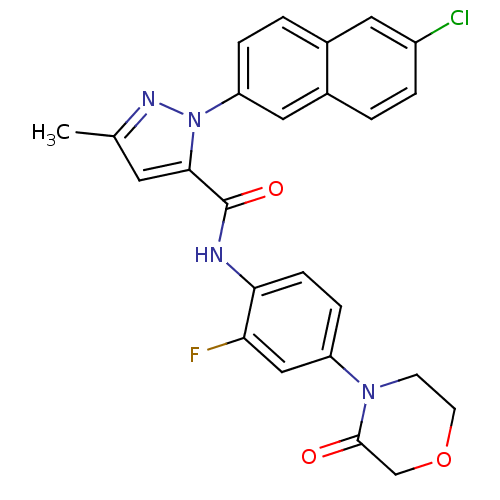
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)N2CCOCC2=O)n(n1)-c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H20ClFN4O3/c1-15-10-23(31(29-15)20-5-3-16-11-18(26)4-2-17(16)12-20)25(33)28-22-7-6-19(13-21(22)27)30-8-9-34-14-24(30)32/h2-7,10-13H,8-9,14H2,1H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
Bioorg Med Chem Lett 14: 1221-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.053
BindingDB Entry DOI: 10.7270/Q2VD6XV5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140371
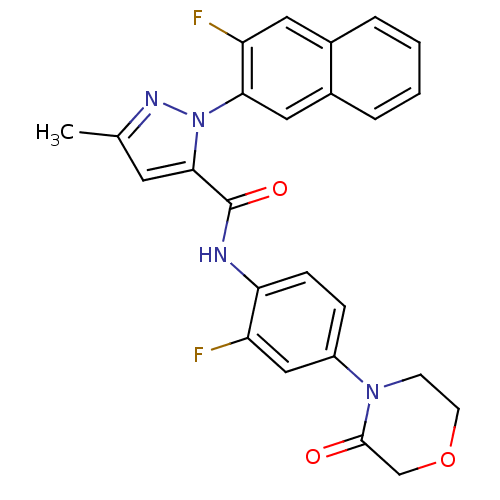
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)N2CCOCC2=O)n(n1)-c1cc2ccccc2cc1F Show InChI InChI=1S/C25H20F2N4O3/c1-15-10-23(31(29-15)22-12-17-5-3-2-4-16(17)11-20(22)27)25(33)28-21-7-6-18(13-19(21)26)30-8-9-34-14-24(30)32/h2-7,10-13H,8-9,14H2,1H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
Bioorg Med Chem Lett 14: 1221-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.053
BindingDB Entry DOI: 10.7270/Q2VD6XV5 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573891

(A-1282576 | A-1282576.0 | A-12825760 | ABT-493 | G...)Show SMILES CC(C)(C)[C@@H]1NC(=O)O[C@@H]2CCC[C@H]2OC\C=C\C(F)(F)c2nc3ccccc3nc2O[C@@H]2C[C@H](N(C2)C1=O)C(=O)N[C@@]1(C[C@H]1C(F)F)C(=O)NS(=O)(=O)C1(C)CC1 |r,t:17| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-3a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573886
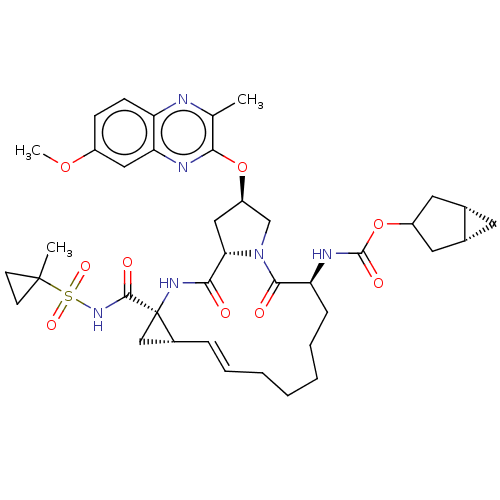
(CHEMBL4848452)Show SMILES [H][C@@]12C[C@]1([H])CC(C2)OC(=O)N[C@H]1CCCCC\C=C\[C@@H]2C[C@]2(NC(=O)[C@@H]2C[C@H](CN2C1=O)Oc1nc2cc(OC)ccc2nc1C)C(=O)NS(=O)(=O)C1(C)CC1 |r,t:20| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573919

(CHEMBL4854503)Show SMILES COc1ccc2nc(C)c(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C\[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3(C)CC3)NC(=O)OC3CC(F)(F)C3)nc2c1 |r,t:25| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573915

(CHEMBL4864092)Show SMILES COc1ccc2nc(C)c(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C\[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3(C)CC3)NC(=O)O[C@@H](C)C(F)(F)F)nc2c1 |r,t:25| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140424
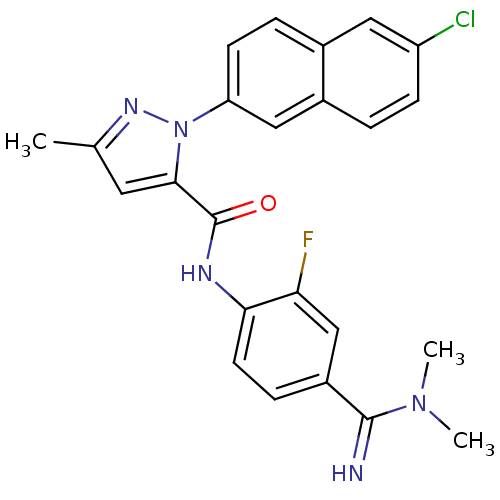
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C24H21ClFN5O/c1-14-10-22(24(32)28-21-9-6-17(13-20(21)26)23(27)30(2)3)31(29-14)19-8-5-15-11-18(25)7-4-16(15)12-19/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140413
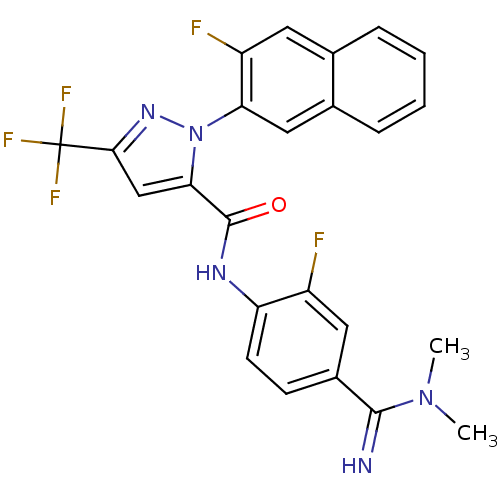
(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18F5N5O/c1-33(2)22(30)15-7-8-18(16(25)10-15)31-23(35)20-12-21(24(27,28)29)32-34(20)19-11-14-6-4-3-5-13(14)9-17(19)26/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573914
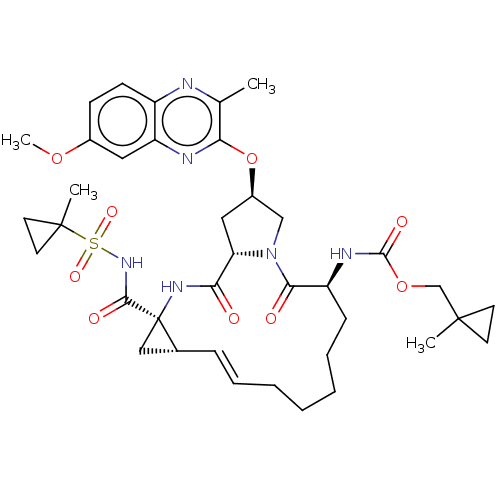
(CHEMBL4875061)Show SMILES COc1ccc2nc(C)c(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C\[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3(C)CC3)NC(=O)OCC3(C)CC3)nc2c1 |r,t:25| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140443
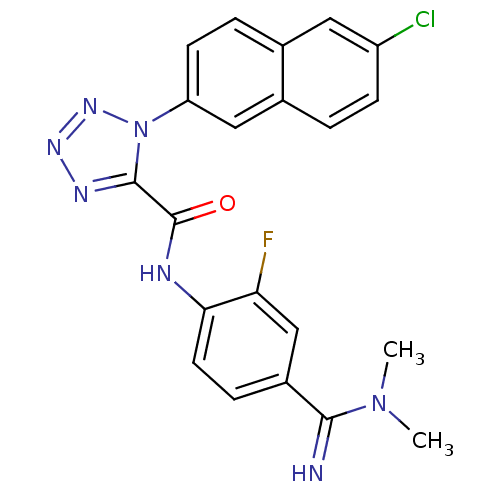
(1-(6-Chloro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2nnnn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C21H17ClFN7O/c1-29(2)19(24)14-5-8-18(17(23)11-14)25-21(31)20-26-27-28-30(20)16-7-4-12-9-15(22)6-3-13(12)10-16/h3-11,24H,1-2H3,(H,25,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123431
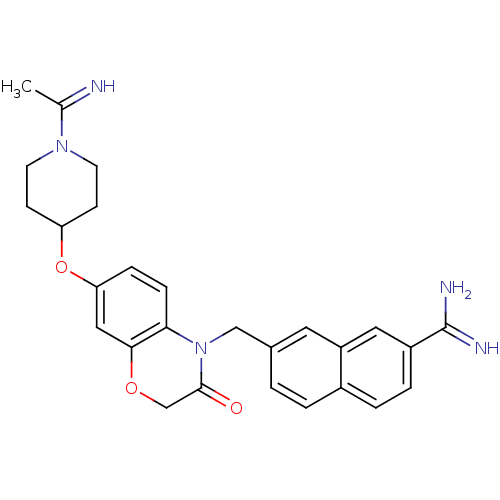
(7-{7-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-3-oxo-2...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2N(Cc3ccc4ccc(cc4c3)C(N)=N)C(=O)COc2c1 Show InChI InChI=1S/C27H29N5O3/c1-17(28)31-10-8-22(9-11-31)35-23-6-7-24-25(14-23)34-16-26(33)32(24)15-18-2-3-19-4-5-20(27(29)30)13-21(19)12-18/h2-7,12-14,22,28H,8-11,15-16H2,1H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards factor Xa |
Bioorg Med Chem Lett 13: 561-6 (2003)
BindingDB Entry DOI: 10.7270/Q2DB816X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140370
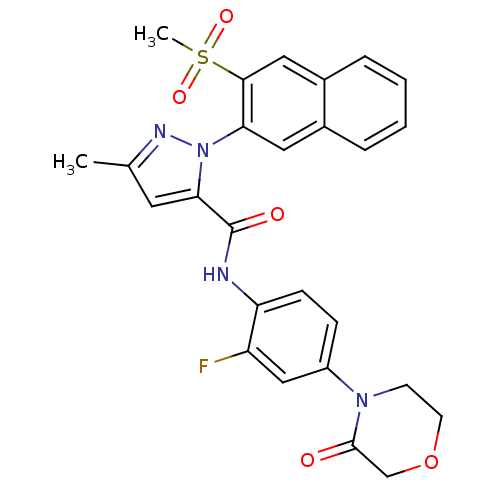
(2-(3-Methanesulfonyl-naphthalen-2-yl)-5-methyl-2H-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)N2CCOCC2=O)n(n1)-c1cc2ccccc2cc1S(C)(=O)=O Show InChI InChI=1S/C26H23FN4O5S/c1-16-11-23(26(33)28-21-8-7-19(14-20(21)27)30-9-10-36-15-25(30)32)31(29-16)22-12-17-5-3-4-6-18(17)13-24(22)37(2,34)35/h3-8,11-14H,9-10,15H2,1-2H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
Bioorg Med Chem Lett 14: 1221-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.053
BindingDB Entry DOI: 10.7270/Q2VD6XV5 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50458698

(CHEMBL4203709)Show SMILES COc1ccc2nc3CCCCC[C@@H]4C[C@H]4OC(=O)N[C@H](C(=O)N4C[C@@H](C[C@H]4C(=O)N[C@@]4(C[C@H]4C=C)C(=O)NS(=O)(=O)C4(C)CC4)Oc3nc2c1)C(C)(C)C |r| Show InChI InChI=1S/C39H52N6O9S/c1-7-23-20-39(23,35(48)44-55(50,51)38(5)15-16-38)43-32(46)29-19-25-21-45(29)34(47)31(37(2,3)4)42-36(49)54-30-17-22(30)11-9-8-10-12-27-33(53-25)41-28-18-24(52-6)13-14-26(28)40-27/h7,13-14,18,22-23,25,29-31H,1,8-12,15-17,19-21H2,2-6H3,(H,42,49)(H,43,46)(H,44,48)/t22-,23-,25-,29+,30-,31-,39-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 1a NS3/4A protease R155K mutant expressed in Escherichia coli BL21(DE3) using Ac-DE-Dap(QXL 520)-EE-Abu-psi-[COO]AS-C(5-FA... |
ACS Med Chem Lett 9: 691-696 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00150
BindingDB Entry DOI: 10.7270/Q29C7125 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50600757
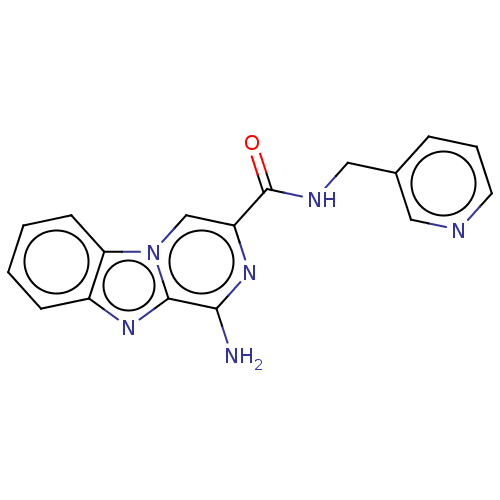
(CHEMBL5208415) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00101
BindingDB Entry DOI: 10.7270/Q2417231 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140410
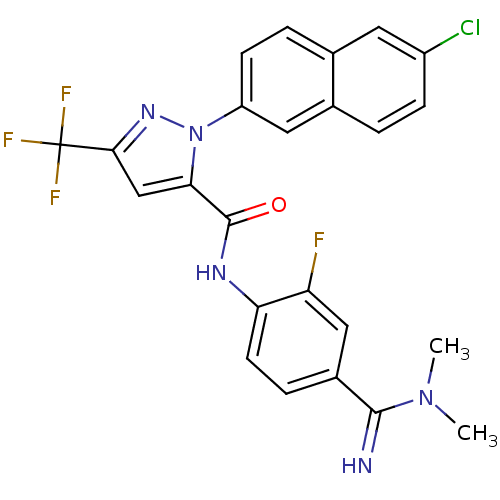
(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2ccc3cc(Cl)ccc3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18ClF4N5O/c1-33(2)22(30)15-5-8-19(18(26)11-15)31-23(35)20-12-21(24(27,28)29)32-34(20)17-7-4-13-9-16(25)6-3-14(13)10-17/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573902
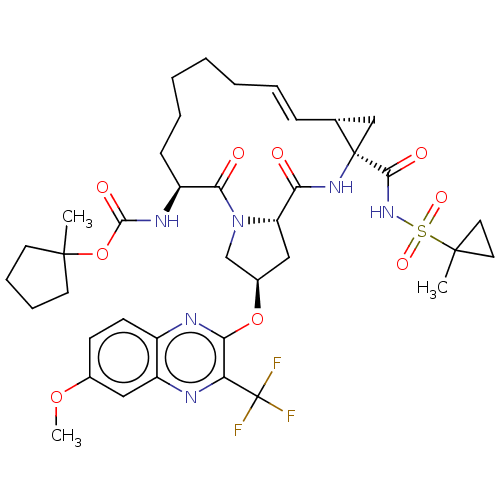
(CHEMBL4860811)Show SMILES COc1ccc2nc(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C\[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3(C)CC3)NC(=O)OC3(C)CCCC3)c(nc2c1)C(F)(F)F |r,t:23| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM294042
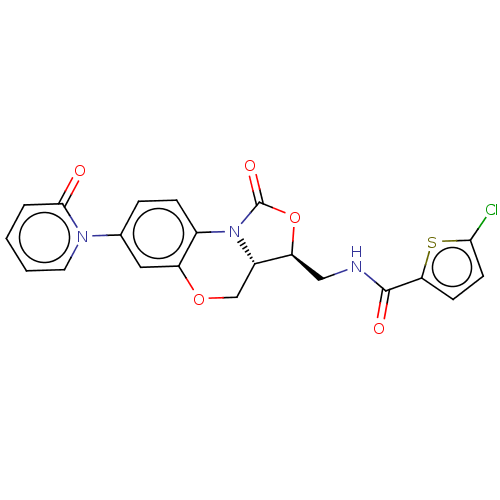
(US10106557, Compound 8)Show SMILES Clc1ccc(s1)C(=O)NC[C@@H]1OC(=O)N2[C@H]1COc1cc(ccc21)-n1ccccc1=O |r| Show InChI InChI=1S/C21H16ClN3O5S/c22-18-7-6-17(31-18)20(27)23-10-16-14-11-29-15-9-12(24-8-2-1-3-19(24)26)4-5-13(15)25(14)21(28)30-16/h1-9,14,16H,10-11H2,(H,23,27)/t14-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD.
US Patent
| Assay Description
The activity of the compound to be tested against prothrombinase was determined by the production of thrombin. In summary, 12.5 μL human factor ... |
US Patent US10106557 (2018)
BindingDB Entry DOI: 10.7270/Q2X63Q0W |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573884
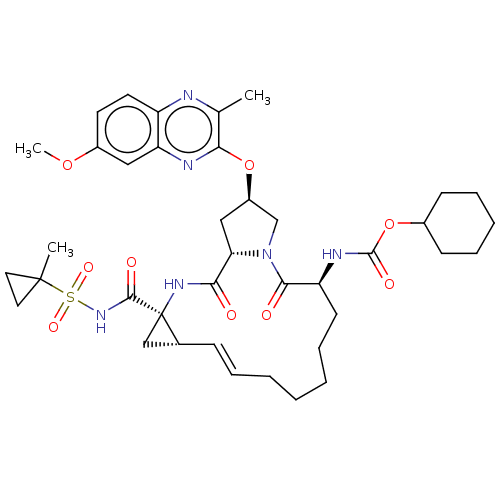
(CHEMBL4863541)Show SMILES COc1ccc2nc(C)c(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C\[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3(C)CC3)NC(=O)OC3CCCCC3)nc2c1 |r,t:25| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573881
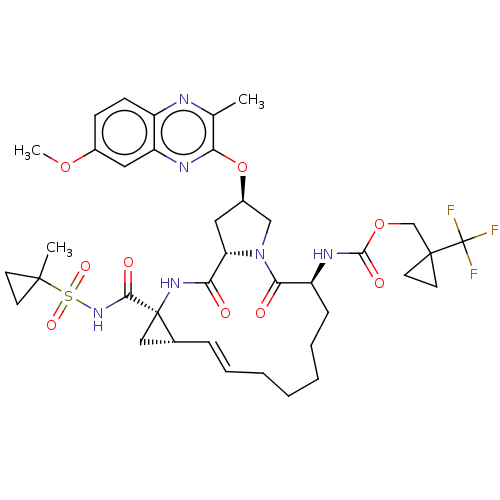
(CHEMBL4848772)Show SMILES COc1ccc2nc(C)c(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C\[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3(C)CC3)NC(=O)OCC3(CC3)C(F)(F)F)nc2c1 |r,t:25| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | |
Peptidylprolyl isomerase
(Gallus gallus) | BDBM50068597
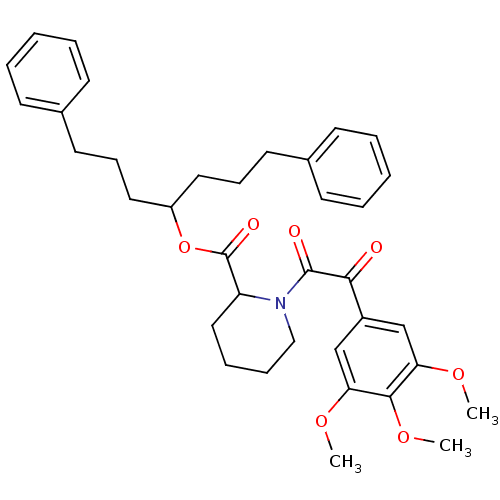
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCCc1ccccc1 Show InChI InChI=1S/C36H43NO7/c1-41-31-24-28(25-32(42-2)34(31)43-3)33(38)35(39)37-23-11-10-22-30(37)36(40)44-29(20-12-18-26-14-6-4-7-15-26)21-13-19-27-16-8-5-9-17-27/h4-9,14-17,24-25,29-30H,10-13,18-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding to FKBP12 receptor |
Bioorg Med Chem Lett 7: 1785-1790 (1997)
Article DOI: 10.1016/S0960-894X(97)00304-1
BindingDB Entry DOI: 10.7270/Q2PZ58TF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140422
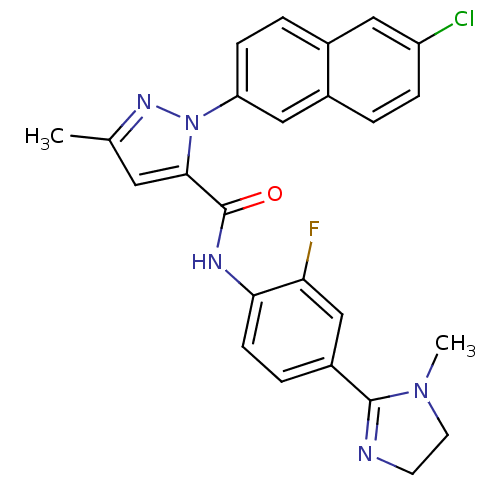
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 |c:4| Show InChI InChI=1S/C25H21ClFN5O/c1-15-11-23(32(30-15)20-7-4-16-12-19(26)6-3-17(16)13-20)25(33)29-22-8-5-18(14-21(22)27)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM294039

(Process 1 | US10106557, Compound 1)Show InChI InChI=1S/C18H18N2O5/c21-16-10-14(20-8-9-24-12-17(20)22)6-7-15(16)19-18(23)25-11-13-4-2-1-3-5-13/h1-7,10,21H,8-9,11-12H2,(H,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD.
US Patent
| Assay Description
The inhibitory activity on coagulation factor Xa activity in human was measured by using Tris-HCl buffer (50 mM, pH 8.3, 150 mM NaCl). A buffer of 50... |
US Patent US10106557 (2018)
BindingDB Entry DOI: 10.7270/Q2X63Q0W |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140363
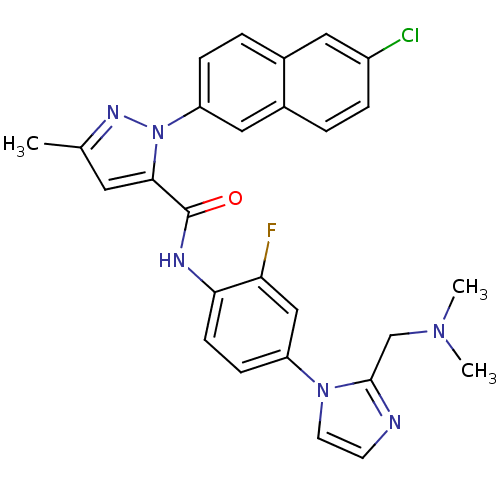
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C27H24ClFN6O/c1-17-12-25(35(32-17)22-7-5-18-13-20(28)6-4-19(18)14-22)27(36)31-24-9-8-21(15-23(24)29)34-11-10-30-26(34)16-33(2)3/h4-15H,16H2,1-3H3,(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
Bioorg Med Chem Lett 14: 1221-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.053
BindingDB Entry DOI: 10.7270/Q2VD6XV5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140387
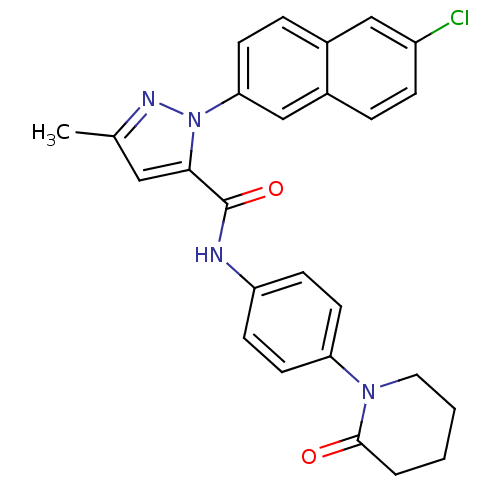
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)N2CCCCC2=O)n(n1)-c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C26H23ClN4O2/c1-17-14-24(31(29-17)23-10-6-18-15-20(27)7-5-19(18)16-23)26(33)28-21-8-11-22(12-9-21)30-13-3-2-4-25(30)32/h5-12,14-16H,2-4,13H2,1H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
Bioorg Med Chem Lett 14: 1221-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.053
BindingDB Entry DOI: 10.7270/Q2VD6XV5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140384
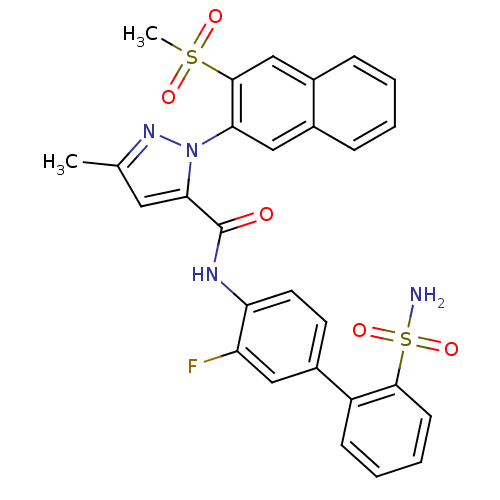
(2-(3-Methanesulfonyl-naphthalen-2-yl)-5-methyl-2H-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)-c2ccccc2S(N)(=O)=O)n(n1)-c1cc2ccccc2cc1S(C)(=O)=O Show InChI InChI=1S/C28H23FN4O5S2/c1-17-13-25(33(32-17)24-15-18-7-3-4-8-19(18)16-27(24)39(2,35)36)28(34)31-23-12-11-20(14-22(23)29)21-9-5-6-10-26(21)40(30,37)38/h3-16H,1-2H3,(H,31,34)(H2,30,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 1221-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.053
BindingDB Entry DOI: 10.7270/Q2VD6XV5 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573880
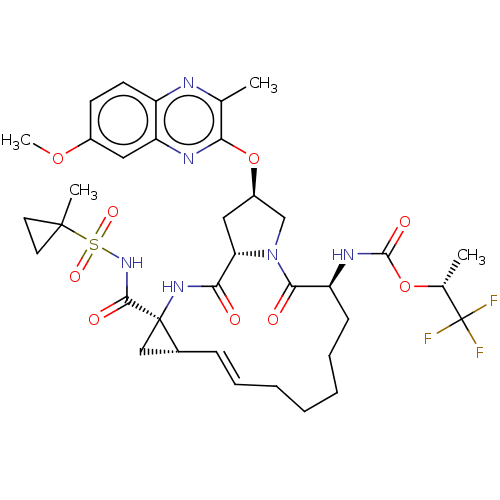
(CHEMBL4847539)Show SMILES COc1ccc2nc(C)c(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C\[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3(C)CC3)NC(=O)O[C@H](C)C(F)(F)F)nc2c1 |r,t:25| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | |
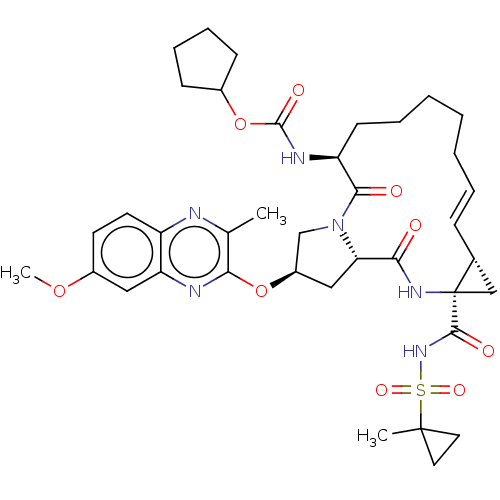
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50573883
(CHEMBL4863232)Show SMILES COc1ccc2nc(C)c(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C\[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3(C)CC3)NC(=O)OC3CCCC3)nc2c1 |r,t:25| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00554
BindingDB Entry DOI: 10.7270/Q2377DH9 |
More data for this
Ligand-Target Pair | |
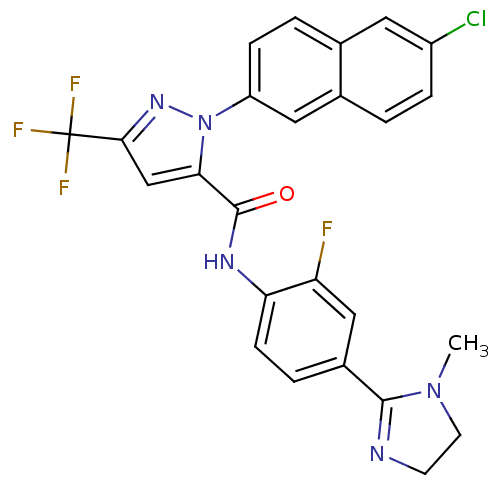
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140415
(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(nn2-c2ccc3cc(Cl)ccc3c2)C(F)(F)F)c(F)c1 |c:4| Show InChI InChI=1S/C25H18ClF4N5O/c1-34-9-8-31-23(34)16-4-7-20(19(27)12-16)32-24(36)21-13-22(25(28,29)30)33-35(21)18-6-3-14-10-17(26)5-2-15(14)11-18/h2-7,10-13H,8-9H2,1H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50600736

(CHEMBL5169483) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00101
BindingDB Entry DOI: 10.7270/Q2417231 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data