 Found 385 hits with Last Name = 'hubbard' and Initial = 'rd'
Found 385 hits with Last Name = 'hubbard' and Initial = 'rd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor [671-1210,L858R]
(Homo sapiens (Human)) | BDBM27970
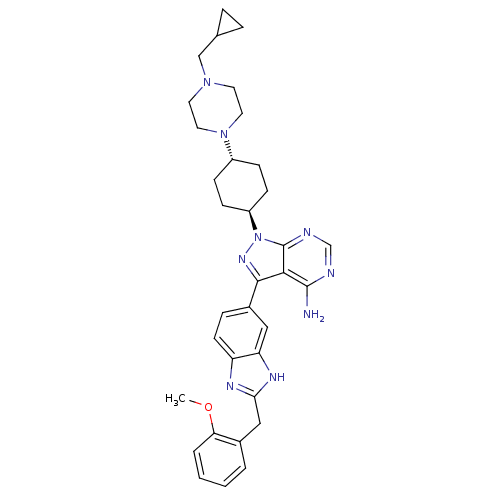
(1-{4-[4-(cyclopropylmethyl)piperazin-1-yl]cyclohex...)Show SMILES COc1ccccc1Cc1nc2ccc(cc2[nH]1)-c1nn([C@H]2CC[C@@H](CC2)N2CCN(CC3CC3)CC2)c2ncnc(N)c12 |r,wU:24.30,wD:21.23,(8.83,13.92,;7.49,13.15,;7.49,11.61,;8.83,10.84,;8.83,9.3,;7.49,8.53,;6.16,9.3,;6.16,10.84,;4.72,11.39,;3.53,10.41,;2.04,10.8,;1.21,9.5,;-.31,9.25,;-.85,7.81,;.12,6.62,;1.64,6.87,;2.19,8.31,;3.62,8.87,;-.35,5.15,;.55,3.91,;-.35,2.66,;.42,1.33,;-.41,.03,;.29,-1.34,;1.83,-1.41,;2.66,-.11,;1.96,1.26,;2.6,-2.74,;1.94,-4.13,;2.81,-5.4,;4.35,-5.28,;5.22,-6.55,;6.75,-6.43,;8.01,-5.54,;8.15,-7.08,;5.01,-3.89,;4.14,-2.62,;-1.82,3.14,;-3.15,2.37,;-4.48,3.14,;-4.48,4.68,;-3.15,5.45,;-3.15,6.99,;-1.82,4.68,)| Show InChI InChI=1S/C34H41N9O/c1-44-29-5-3-2-4-23(29)19-30-38-27-13-8-24(18-28(27)39-30)32-31-33(35)36-21-37-34(31)43(40-32)26-11-9-25(10-12-26)42-16-14-41(15-17-42)20-22-6-7-22/h2-5,8,13,18,21-22,25-26H,6-7,9-12,14-17,19-20H2,1H3,(H,38,39)(H2,35,36,37)/t25-,26- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Abbott Laboratories
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... |
Bioorg Med Chem Lett 19: 1718-21 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.086
BindingDB Entry DOI: 10.7270/Q2CJ8BTG |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50327950
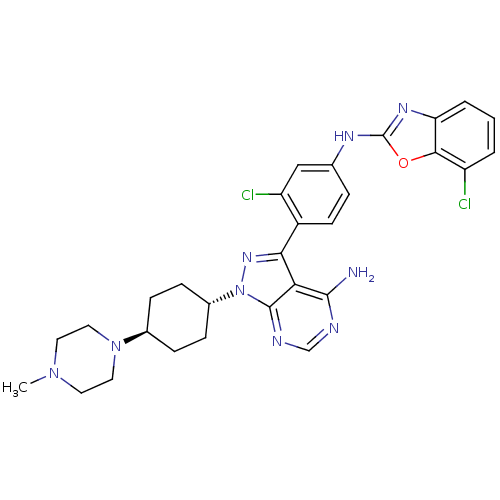
(CHEMBL1256435 | trans-N-(4-(4-amino-1-(4-(4-methyl...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)cc2Cl)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(39.66,-36.63,;39.2,-35.16,;37.69,-34.83,;37.23,-33.37,;38.26,-32.23,;39.77,-32.55,;40.23,-34.02,;37.79,-30.77,;38.82,-29.62,;38.35,-28.15,;36.84,-27.83,;35.81,-28.98,;36.28,-30.44,;36.37,-26.37,;37.27,-25.13,;36.37,-23.88,;37.14,-22.55,;36.36,-21.23,;37.12,-19.89,;38.67,-19.88,;39.44,-18.55,;40.98,-18.54,;41.88,-17.28,;43.34,-17.75,;44.66,-16.98,;45.99,-17.74,;46,-19.28,;44.67,-20.05,;44.68,-21.59,;43.34,-19.29,;41.89,-19.77,;39.44,-21.22,;38.68,-22.55,;39.45,-23.88,;34.9,-24.36,;33.58,-23.59,;33.57,-22.05,;32.24,-24.36,;32.24,-25.9,;33.58,-26.67,;34.9,-25.9,)| Show InChI InChI=1S/C29H31Cl2N9O/c1-38-11-13-39(14-12-38)18-6-8-19(9-7-18)40-28-24(27(32)33-16-34-28)25(37-40)20-10-5-17(15-22(20)31)35-29-36-23-4-2-3-21(30)26(23)41-29/h2-5,10,15-16,18-19H,6-9,11-14H2,1H3,(H,35,36)(H2,32,33,34)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50315895
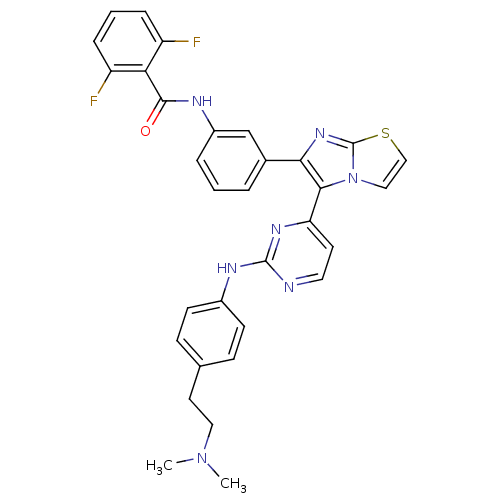
(CHEMBL1090357 | N-(3-(5-(2-(4-(2-(dimethylamino)et...)Show SMILES CN(C)CCc1ccc(Nc2nccc(n2)-c2c(nc3sccn23)-c2cccc(NC(=O)c3c(F)cccc3F)c2)cc1 Show InChI InChI=1S/C32H27F2N7OS/c1-40(2)16-14-20-9-11-22(12-10-20)37-31-35-15-13-26(38-31)29-28(39-32-41(29)17-18-43-32)21-5-3-6-23(19-21)36-30(42)27-24(33)7-4-8-25(27)34/h3-13,15,17-19H,14,16H2,1-2H3,(H,36,42)(H,35,37,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 by time resolved fluorescence assay |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50315905
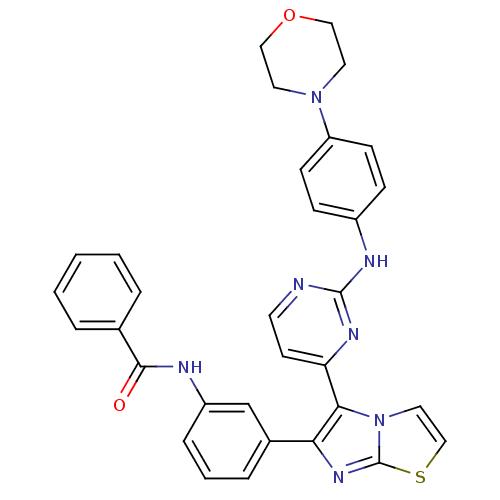
(CHEMBL1090350 | N-(3-(5-(2-(4-morpholinophenylamin...)Show SMILES O=C(Nc1cccc(c1)-c1nc2sccn2c1-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)c1ccccc1 Show InChI InChI=1S/C32H27N7O2S/c40-30(22-5-2-1-3-6-22)34-25-8-4-7-23(21-25)28-29(39-17-20-42-32(39)37-28)27-13-14-33-31(36-27)35-24-9-11-26(12-10-24)38-15-18-41-19-16-38/h1-14,17,20-21H,15-16,18-19H2,(H,34,40)(H,33,35,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 by time resolved fluorescence assay |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50315903

(CHEMBL1093234 | N-(3-(5-(2-(phenylamino)pyrimidin-...)Show SMILES O=C(Nc1cccc(c1)-c1nc2sccn2c1-c1ccnc(Nc2ccccc2)n1)c1ccccc1 Show InChI InChI=1S/C28H20N6OS/c35-26(19-8-3-1-4-9-19)30-22-13-7-10-20(18-22)24-25(34-16-17-36-28(34)33-24)23-14-15-29-27(32-23)31-21-11-5-2-6-12-21/h1-18H,(H,30,35)(H,29,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 by time resolved fluorescence assay |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM27973
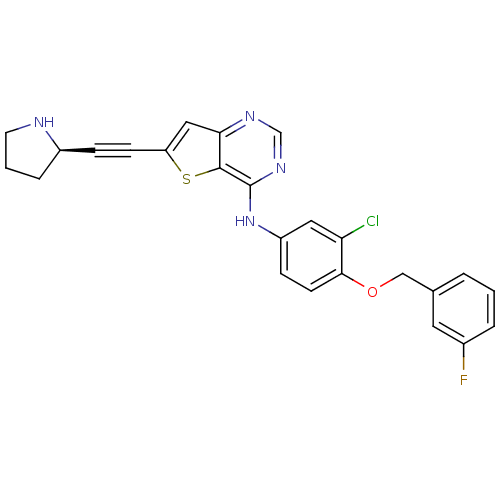
(6-Ethynylthieno[3,2-d]pyrimidine, 8 | N-{3-chloro-...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)C#C[C@H]3CCCN3)cc2Cl)c1 |r| Show InChI InChI=1S/C25H20ClFN4OS/c26-21-12-19(7-9-23(21)32-14-16-3-1-4-17(27)11-16)31-25-24-22(29-15-30-25)13-20(33-24)8-6-18-5-2-10-28-18/h1,3-4,7,9,11-13,15,18,28H,2,5,10,14H2,(H,29,30,31)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR kinase |
Bioorg Med Chem Lett 18: 5738-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.090
BindingDB Entry DOI: 10.7270/Q2H70FTT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50315897
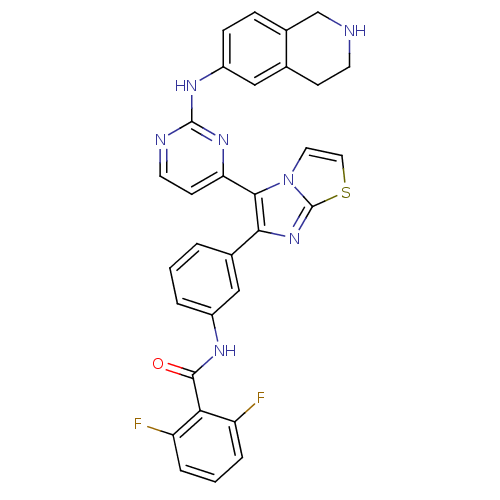
(2,6-difluoro-N-(3-(5-(2-(1,2,3,4-tetrahydroisoquin...)Show SMILES Fc1cccc(F)c1C(=O)Nc1cccc(c1)-c1nc2sccn2c1-c1ccnc(Nc2ccc3CNCCc3c2)n1 Show InChI InChI=1S/C31H23F2N7OS/c32-23-5-2-6-24(33)26(23)29(41)36-21-4-1-3-19(16-21)27-28(40-13-14-42-31(40)39-27)25-10-12-35-30(38-25)37-22-8-7-20-17-34-11-9-18(20)15-22/h1-8,10,12-16,34H,9,11,17H2,(H,36,41)(H,35,37,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 by time resolved fluorescence assay |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50327948
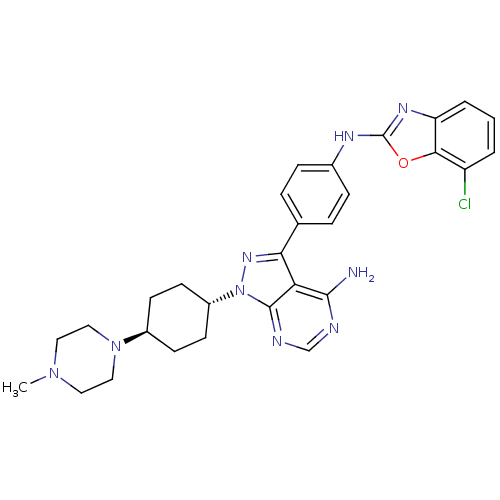
(CHEMBL1257998 | trans-N-(4-(4-amino-1-(4-(4-methyl...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)cc2)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(-3.94,-49.99,;-4.4,-48.53,;-5.91,-48.19,;-6.37,-46.73,;-5.34,-45.59,;-3.83,-45.91,;-3.36,-47.39,;-5.81,-44.13,;-4.78,-42.98,;-5.25,-41.51,;-6.76,-41.19,;-7.79,-42.34,;-7.32,-43.8,;-7.24,-39.73,;-6.33,-38.49,;-7.23,-37.24,;-6.46,-35.9,;-4.92,-35.91,;-4.16,-34.57,;-4.93,-33.24,;-4.16,-31.9,;-2.62,-31.9,;-1.72,-30.64,;-.26,-31.11,;1.06,-30.34,;2.39,-31.1,;2.4,-32.64,;1.07,-33.41,;1.08,-34.95,;-.25,-32.64,;-1.71,-33.13,;-6.48,-33.25,;-7.24,-34.58,;-8.7,-37.71,;-10.03,-36.95,;-10.03,-35.41,;-11.37,-37.72,;-11.37,-39.26,;-10.02,-40.03,;-8.7,-39.26,)| Show InChI InChI=1S/C29H32ClN9O/c1-37-13-15-38(16-14-37)20-9-11-21(12-10-20)39-28-24(27(31)32-17-33-28)25(36-39)18-5-7-19(8-6-18)34-29-35-23-4-2-3-22(30)26(23)40-29/h2-8,17,20-21H,9-16H2,1H3,(H,34,35)(H2,31,32,33)/t20-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50327947
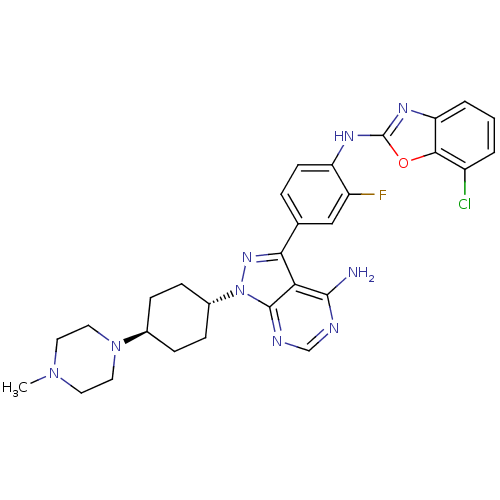
(CHEMBL1256432 | trans-N-(4-(4-amino-1-(4-(4-methyl...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)c(F)c2)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(-3.03,-35.57,;-3.49,-34.1,;-5,-33.77,;-5.46,-32.31,;-4.43,-31.17,;-2.92,-31.49,;-2.45,-32.96,;-4.9,-29.71,;-3.86,-28.56,;-4.33,-27.09,;-5.84,-26.77,;-6.87,-27.92,;-6.4,-29.38,;-6.32,-25.31,;-5.42,-24.07,;-6.32,-22.82,;-5.55,-21.49,;-4.01,-21.49,;-3.24,-20.16,;-4.02,-18.82,;-3.25,-17.49,;-1.71,-17.48,;-.81,-16.22,;.66,-16.69,;1.97,-15.92,;3.3,-16.68,;3.31,-18.22,;1.98,-18.99,;1.99,-20.53,;.66,-18.23,;-.8,-18.71,;-5.56,-18.83,;-6.34,-17.5,;-6.32,-20.17,;-7.78,-23.3,;-9.11,-22.53,;-9.11,-20.99,;-10.45,-23.3,;-10.45,-24.84,;-9.11,-25.61,;-7.78,-24.84,)| Show InChI InChI=1S/C29H31ClFN9O/c1-38-11-13-39(14-12-38)18-6-8-19(9-7-18)40-28-24(27(32)33-16-34-28)25(37-40)17-5-10-22(21(31)15-17)35-29-36-23-4-2-3-20(30)26(23)41-29/h2-5,10,15-16,18-19H,6-9,11-14H2,1H3,(H,35,36)(H2,32,33,34)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50327959
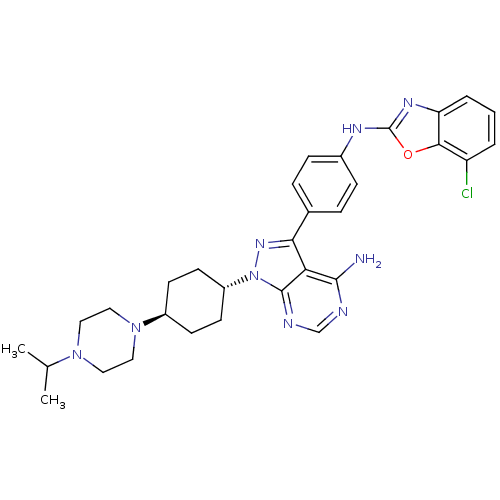
(CHEMBL1256425 | trans-N-(4-(4-amino-1-(4-(4-isopro...)Show SMILES CC(C)N1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)cc2)c2c(N)ncnc12 |r,wU:12.16,wD:9.9,(22.26,-53.97,;23.3,-52.84,;24.8,-53.17,;22.84,-51.37,;21.33,-51.04,;20.87,-49.57,;21.9,-48.44,;23.41,-48.76,;23.87,-50.23,;21.43,-46.97,;22.46,-45.83,;21.99,-44.36,;20.48,-44.04,;19.45,-45.18,;19.92,-46.64,;20.01,-42.58,;20.91,-41.34,;20.01,-40.09,;20.78,-38.75,;22.32,-38.76,;23.08,-37.42,;22.31,-36.09,;23.08,-34.75,;24.62,-34.75,;25.52,-33.49,;26.98,-33.96,;28.3,-33.19,;29.63,-33.95,;29.64,-35.49,;28.31,-36.26,;28.31,-37.8,;26.98,-35.49,;25.53,-35.98,;20.76,-36.1,;20,-37.43,;18.54,-40.56,;17.22,-39.8,;17.22,-38.26,;15.88,-40.57,;15.88,-42.11,;17.22,-42.88,;18.55,-42.11,)| Show InChI InChI=1S/C31H36ClN9O/c1-19(2)39-14-16-40(17-15-39)22-10-12-23(13-11-22)41-30-26(29(33)34-18-35-30)27(38-41)20-6-8-21(9-7-20)36-31-37-25-5-3-4-24(32)28(25)42-31/h3-9,18-19,22-23H,10-17H2,1-2H3,(H,36,37)(H2,33,34,35)/t22-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50293255
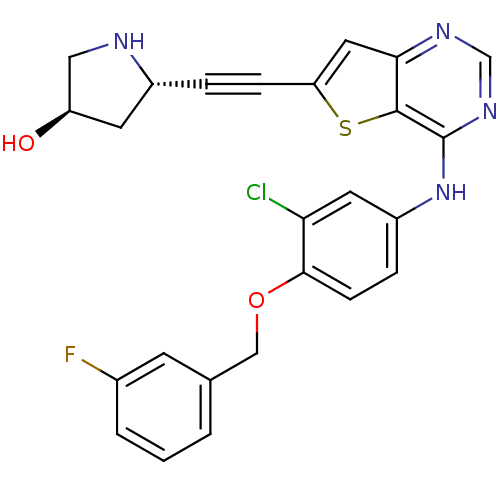
((3R,5S)-5-((4-(3-chloro-4-(3-fluorobenzyloxy)pheny...)Show SMILES O[C@H]1CN[C@@H](C1)C#Cc1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 |r| Show InChI InChI=1S/C25H20ClFN4O2S/c26-21-10-18(5-7-23(21)33-13-15-2-1-3-16(27)8-15)31-25-24-22(29-14-30-25)11-20(34-24)6-4-17-9-19(32)12-28-17/h1-3,5,7-8,10-11,14,17,19,28,32H,9,12-13H2,(H,29,30,31)/t17-,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 kinase |
Bioorg Med Chem Lett 18: 5738-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.090
BindingDB Entry DOI: 10.7270/Q2H70FTT |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50327948

(CHEMBL1257998 | trans-N-(4-(4-amino-1-(4-(4-methyl...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)cc2)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(-3.94,-49.99,;-4.4,-48.53,;-5.91,-48.19,;-6.37,-46.73,;-5.34,-45.59,;-3.83,-45.91,;-3.36,-47.39,;-5.81,-44.13,;-4.78,-42.98,;-5.25,-41.51,;-6.76,-41.19,;-7.79,-42.34,;-7.32,-43.8,;-7.24,-39.73,;-6.33,-38.49,;-7.23,-37.24,;-6.46,-35.9,;-4.92,-35.91,;-4.16,-34.57,;-4.93,-33.24,;-4.16,-31.9,;-2.62,-31.9,;-1.72,-30.64,;-.26,-31.11,;1.06,-30.34,;2.39,-31.1,;2.4,-32.64,;1.07,-33.41,;1.08,-34.95,;-.25,-32.64,;-1.71,-33.13,;-6.48,-33.25,;-7.24,-34.58,;-8.7,-37.71,;-10.03,-36.95,;-10.03,-35.41,;-11.37,-37.72,;-11.37,-39.26,;-10.02,-40.03,;-8.7,-39.26,)| Show InChI InChI=1S/C29H32ClN9O/c1-37-13-15-38(16-14-37)20-9-11-21(12-10-20)39-28-24(27(31)32-17-33-28)25(36-39)18-5-7-19(8-6-18)34-29-35-23-4-2-3-22(30)26(23)40-29/h2-8,17,20-21H,9-16H2,1H3,(H,34,35)(H2,31,32,33)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM27970

(1-{4-[4-(cyclopropylmethyl)piperazin-1-yl]cyclohex...)Show SMILES COc1ccccc1Cc1nc2ccc(cc2[nH]1)-c1nn([C@H]2CC[C@@H](CC2)N2CCN(CC3CC3)CC2)c2ncnc(N)c12 |r,wU:24.30,wD:21.23,(8.83,13.92,;7.49,13.15,;7.49,11.61,;8.83,10.84,;8.83,9.3,;7.49,8.53,;6.16,9.3,;6.16,10.84,;4.72,11.39,;3.53,10.41,;2.04,10.8,;1.21,9.5,;-.31,9.25,;-.85,7.81,;.12,6.62,;1.64,6.87,;2.19,8.31,;3.62,8.87,;-.35,5.15,;.55,3.91,;-.35,2.66,;.42,1.33,;-.41,.03,;.29,-1.34,;1.83,-1.41,;2.66,-.11,;1.96,1.26,;2.6,-2.74,;1.94,-4.13,;2.81,-5.4,;4.35,-5.28,;5.22,-6.55,;6.75,-6.43,;8.01,-5.54,;8.15,-7.08,;5.01,-3.89,;4.14,-2.62,;-1.82,3.14,;-3.15,2.37,;-4.48,3.14,;-4.48,4.68,;-3.15,5.45,;-3.15,6.99,;-1.82,4.68,)| Show InChI InChI=1S/C34H41N9O/c1-44-29-5-3-2-4-23(29)19-30-38-27-13-8-24(18-28(27)39-30)32-31-33(35)36-21-37-34(31)43(40-32)26-11-9-25(10-12-26)42-16-14-41(15-17-42)20-22-6-7-22/h2-5,8,13,18,21-22,25-26H,6-7,9-12,14-17,19-20H2,1H3,(H,38,39)(H2,35,36,37)/t25-,26- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Abbott Laboratories
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... |
Bioorg Med Chem Lett 19: 1718-21 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.086
BindingDB Entry DOI: 10.7270/Q2CJ8BTG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM27973

(6-Ethynylthieno[3,2-d]pyrimidine, 8 | N-{3-chloro-...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)C#C[C@H]3CCCN3)cc2Cl)c1 |r| Show InChI InChI=1S/C25H20ClFN4OS/c26-21-12-19(7-9-23(21)32-14-16-3-1-4-17(27)11-16)31-25-24-22(29-15-30-25)13-20(33-24)8-6-18-5-2-10-28-18/h1,3-4,7,9,11-13,15,18,28H,2,5,10,14H2,(H,29,30,31)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 kinase |
Bioorg Med Chem Lett 18: 5738-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.090
BindingDB Entry DOI: 10.7270/Q2H70FTT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50327952
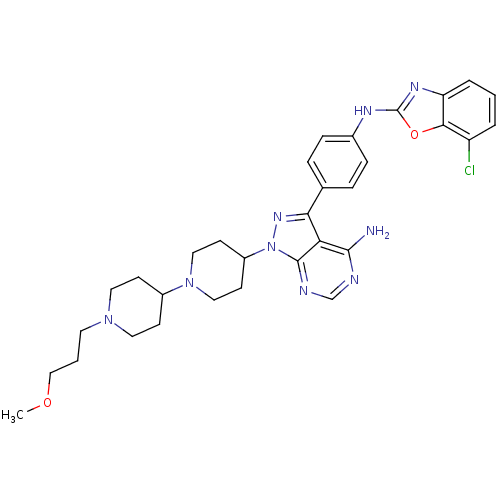
(CHEMBL1256431 | N-(4-(4-amino-1-(1'-(3-methoxyprop...)Show SMILES COCCCN1CCC(CC1)N1CCC(CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C32H38ClN9O2/c1-43-19-3-14-40-15-10-23(11-16-40)41-17-12-24(13-18-41)42-31-27(30(34)35-20-36-31)28(39-42)21-6-8-22(9-7-21)37-32-38-26-5-2-4-25(33)29(26)44-32/h2,4-9,20,23-24H,3,10-19H2,1H3,(H,37,38)(H2,34,35,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50293255

((3R,5S)-5-((4-(3-chloro-4-(3-fluorobenzyloxy)pheny...)Show SMILES O[C@H]1CN[C@@H](C1)C#Cc1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 |r| Show InChI InChI=1S/C25H20ClFN4O2S/c26-21-10-18(5-7-23(21)33-13-15-2-1-3-16(27)8-15)31-25-24-22(29-14-30-25)11-20(34-24)6-4-17-9-19(32)12-28-17/h1-3,5,7-8,10-11,14,17,19,28,32H,9,12-13H2,(H,29,30,31)/t17-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR kinase |
Bioorg Med Chem Lett 18: 5738-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.090
BindingDB Entry DOI: 10.7270/Q2H70FTT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50315896

(CHEMBL1090358 | N-(3-(5-(2-(4-(4-ethylpiperazin-1-...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2nccc(n2)-c2c(nc3sccn23)-c2cccc(NC(=O)c3c(F)cccc3F)c2)cc1 Show InChI InChI=1S/C34H30F2N8OS/c1-2-42-15-17-43(18-16-42)25-11-9-23(10-12-25)39-33-37-14-13-28(40-33)31-30(41-34-44(31)19-20-46-34)22-5-3-6-24(21-22)38-32(45)29-26(35)7-4-8-27(29)36/h3-14,19-21H,2,15-18H2,1H3,(H,38,45)(H,37,39,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 by time resolved fluorescence assay |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50327954
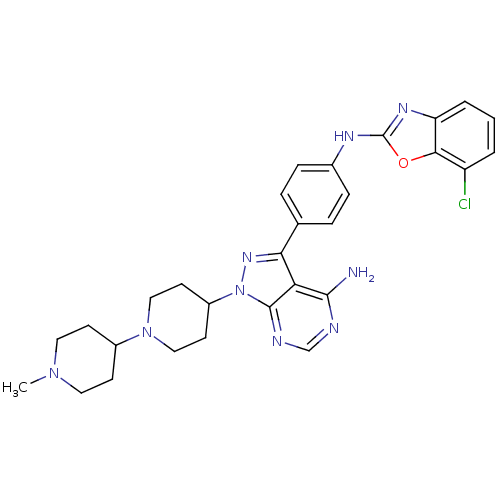
(CHEMBL1258231 | N-(4-(4-amino-1-(1'-methyl-1,4'-bi...)Show SMILES CN1CCC(CC1)N1CCC(CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C29H32ClN9O/c1-37-13-9-20(10-14-37)38-15-11-21(12-16-38)39-28-24(27(31)32-17-33-28)25(36-39)18-5-7-19(8-6-18)34-29-35-23-4-2-3-22(30)26(23)40-29/h2-8,17,20-21H,9-16H2,1H3,(H,34,35)(H2,31,32,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50327953
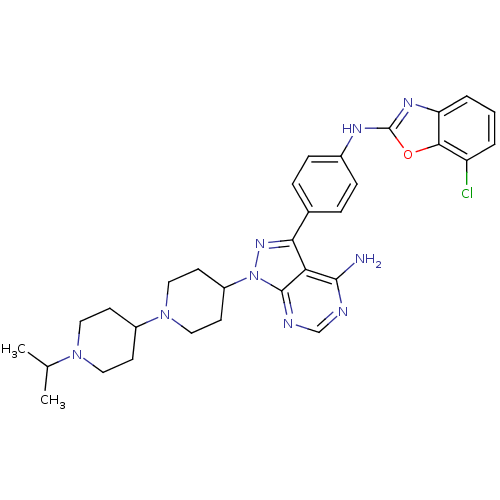
(CHEMBL1256430 | N-(4-(4-amino-1-(1'-isopropyl-1,4'...)Show SMILES CC(C)N1CCC(CC1)N1CCC(CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C31H36ClN9O/c1-19(2)39-14-10-22(11-15-39)40-16-12-23(13-17-40)41-30-26(29(33)34-18-35-30)27(38-41)20-6-8-21(9-7-20)36-31-37-25-5-3-4-24(32)28(25)42-31/h3-9,18-19,22-23H,10-17H2,1-2H3,(H,36,37)(H2,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50327950

(CHEMBL1256435 | trans-N-(4-(4-amino-1-(4-(4-methyl...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)cc2Cl)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(39.66,-36.63,;39.2,-35.16,;37.69,-34.83,;37.23,-33.37,;38.26,-32.23,;39.77,-32.55,;40.23,-34.02,;37.79,-30.77,;38.82,-29.62,;38.35,-28.15,;36.84,-27.83,;35.81,-28.98,;36.28,-30.44,;36.37,-26.37,;37.27,-25.13,;36.37,-23.88,;37.14,-22.55,;36.36,-21.23,;37.12,-19.89,;38.67,-19.88,;39.44,-18.55,;40.98,-18.54,;41.88,-17.28,;43.34,-17.75,;44.66,-16.98,;45.99,-17.74,;46,-19.28,;44.67,-20.05,;44.68,-21.59,;43.34,-19.29,;41.89,-19.77,;39.44,-21.22,;38.68,-22.55,;39.45,-23.88,;34.9,-24.36,;33.58,-23.59,;33.57,-22.05,;32.24,-24.36,;32.24,-25.9,;33.58,-26.67,;34.9,-25.9,)| Show InChI InChI=1S/C29H31Cl2N9O/c1-38-11-13-39(14-12-38)18-6-8-19(9-7-18)40-28-24(27(32)33-16-34-28)25(37-40)20-10-5-17(15-22(20)31)35-29-36-23-4-2-3-21(30)26(23)41-29/h2-5,10,15-16,18-19H,6-9,11-14H2,1H3,(H,35,36)(H2,32,33,34)/t18-,19- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM28511
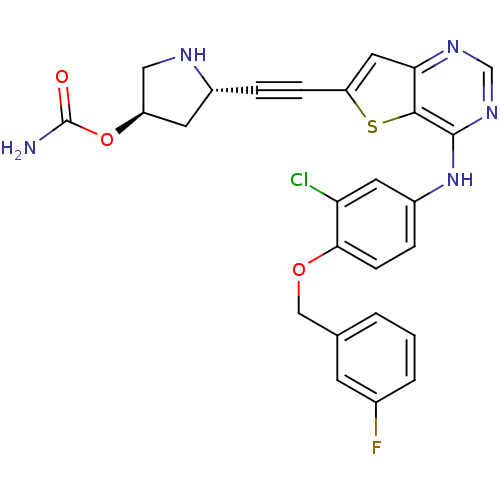
((3R,5S)-5-{2-[4-({3-chloro-4-[(3-fluorophenyl)meth...)Show SMILES NC(=O)O[C@H]1CN[C@@H](C1)C#Cc1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 |r| Show InChI InChI=1S/C26H21ClFN5O3S/c27-21-10-18(5-7-23(21)35-13-15-2-1-3-16(28)8-15)33-25-24-22(31-14-32-25)11-20(37-24)6-4-17-9-19(12-30-17)36-26(29)34/h1-3,5,7-8,10-11,14,17,19,30H,9,12-13H2,(H2,29,34)(H,31,32,33)/t17-,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Assays were performed in 96-well polystyrene round-bottomed plates in reaction buffer containing biotinylated substrate and purified kinase in the pr... |
Bioorg Med Chem Lett 19: 21-6 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.023
BindingDB Entry DOI: 10.7270/Q2NV9GKZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50327947

(CHEMBL1256432 | trans-N-(4-(4-amino-1-(4-(4-methyl...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)c(F)c2)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(-3.03,-35.57,;-3.49,-34.1,;-5,-33.77,;-5.46,-32.31,;-4.43,-31.17,;-2.92,-31.49,;-2.45,-32.96,;-4.9,-29.71,;-3.86,-28.56,;-4.33,-27.09,;-5.84,-26.77,;-6.87,-27.92,;-6.4,-29.38,;-6.32,-25.31,;-5.42,-24.07,;-6.32,-22.82,;-5.55,-21.49,;-4.01,-21.49,;-3.24,-20.16,;-4.02,-18.82,;-3.25,-17.49,;-1.71,-17.48,;-.81,-16.22,;.66,-16.69,;1.97,-15.92,;3.3,-16.68,;3.31,-18.22,;1.98,-18.99,;1.99,-20.53,;.66,-18.23,;-.8,-18.71,;-5.56,-18.83,;-6.34,-17.5,;-6.32,-20.17,;-7.78,-23.3,;-9.11,-22.53,;-9.11,-20.99,;-10.45,-23.3,;-10.45,-24.84,;-9.11,-25.61,;-7.78,-24.84,)| Show InChI InChI=1S/C29H31ClFN9O/c1-38-11-13-39(14-12-38)18-6-8-19(9-7-18)40-28-24(27(32)33-16-34-28)25(37-40)17-5-10-22(21(31)15-17)35-29-36-23-4-2-3-20(30)26(23)41-29/h2-5,10,15-16,18-19H,6-9,11-14H2,1H3,(H,35,36)(H2,32,33,34)/t18-,19- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM27968
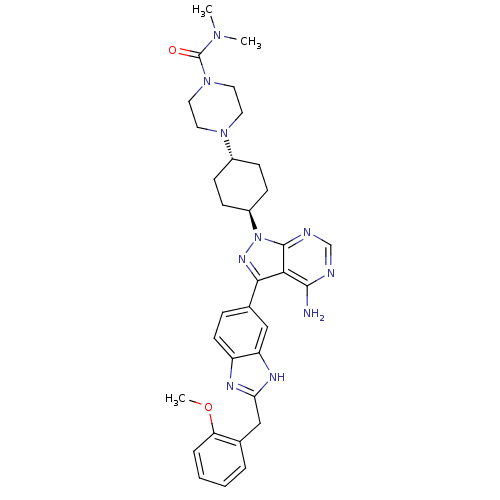
(4-[4-(4-amino-3-{2-[(2-methoxyphenyl)methyl]-1H-1,...)Show SMILES COc1ccccc1Cc1nc2ccc(cc2[nH]1)-c1nn([C@H]2CC[C@@H](CC2)N2CCN(CC2)C(=O)N(C)C)c2ncnc(N)c12 |r,wU:24.30,wD:21.23,(8.83,13.92,;7.49,13.15,;7.49,11.61,;8.83,10.84,;8.83,9.3,;7.49,8.53,;6.16,9.3,;6.16,10.84,;4.72,11.39,;3.53,10.41,;2.04,10.8,;1.21,9.5,;-.31,9.25,;-.85,7.81,;.12,6.62,;1.64,6.87,;2.19,8.31,;3.62,8.87,;-.35,5.15,;.55,3.91,;-.35,2.66,;.42,1.33,;-.41,.03,;.29,-1.34,;1.83,-1.41,;2.66,-.11,;1.96,1.26,;2.6,-2.74,;1.94,-4.13,;2.81,-5.4,;4.35,-5.28,;5.01,-3.89,;4.14,-2.62,;5.22,-6.55,;6.75,-6.43,;4.55,-7.94,;4.55,-9.48,;3.05,-7.6,;-1.82,3.14,;-3.15,2.37,;-4.48,3.14,;-4.48,4.68,;-3.15,5.45,;-3.15,6.99,;-1.82,4.68,)| Show InChI InChI=1S/C33H40N10O2/c1-40(2)33(44)42-16-14-41(15-17-42)23-9-11-24(12-10-23)43-32-29(31(34)35-20-36-32)30(39-43)22-8-13-25-26(18-22)38-28(37-25)19-21-6-4-5-7-27(21)45-3/h4-8,13,18,20,23-24H,9-12,14-17,19H2,1-3H3,(H,37,38)(H2,34,35,36)/t23-,24- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Abbott Laboratories
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... |
Bioorg Med Chem Lett 19: 1718-21 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.086
BindingDB Entry DOI: 10.7270/Q2CJ8BTG |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887
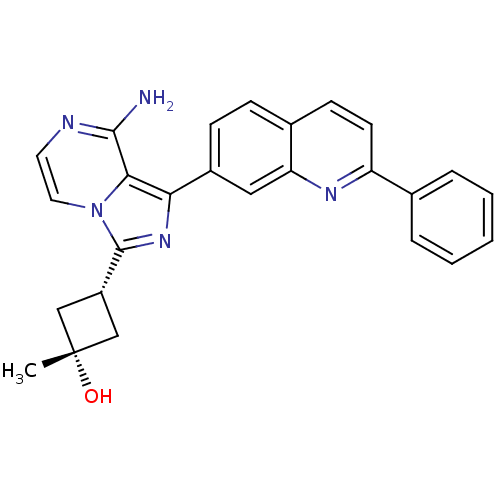
((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IGF1R expressed in Sf21 cells by time resolved fluorescence assay |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50315888

(CHEMBL1090360 | N-(3-(5-(2-(3-morpholinophenylamin...)Show SMILES O=C(Cc1ccccc1)Nc1cccc(c1)-c1nc2sccn2c1-c1ccnc(Nc2cccc(c2)N2CCOCC2)n1 Show InChI InChI=1S/C33H29N7O2S/c41-29(20-23-6-2-1-3-7-23)35-25-9-4-8-24(21-25)30-31(40-16-19-43-33(40)38-30)28-12-13-34-32(37-28)36-26-10-5-11-27(22-26)39-14-17-42-18-15-39/h1-13,16,19,21-22H,14-15,17-18,20H2,(H,35,41)(H,34,36,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErRB4 |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM28512
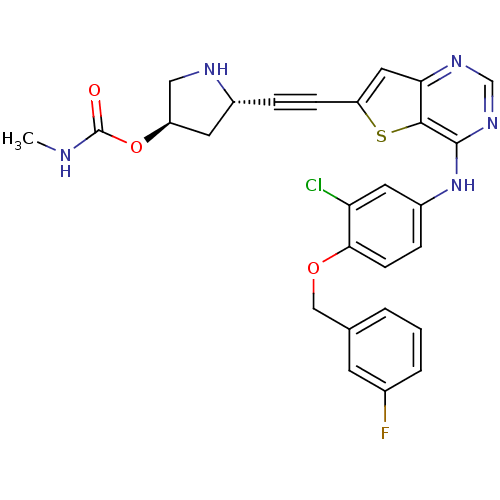
((3R,5S)-5-{2-[4-({3-chloro-4-[(3-fluorophenyl)meth...)Show SMILES CNC(=O)O[C@H]1CN[C@@H](C1)C#Cc1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 |r| Show InChI InChI=1S/C27H23ClFN5O3S/c1-30-27(35)37-20-10-18(31-13-20)5-7-21-12-23-25(38-21)26(33-15-32-23)34-19-6-8-24(22(28)11-19)36-14-16-3-2-4-17(29)9-16/h2-4,6,8-9,11-12,15,18,20,31H,10,13-14H2,1H3,(H,30,35)(H,32,33,34)/t18-,20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Assays were performed in 96-well polystyrene round-bottomed plates in reaction buffer containing biotinylated substrate and purified kinase in the pr... |
Bioorg Med Chem Lett 19: 21-6 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.023
BindingDB Entry DOI: 10.7270/Q2NV9GKZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM28518
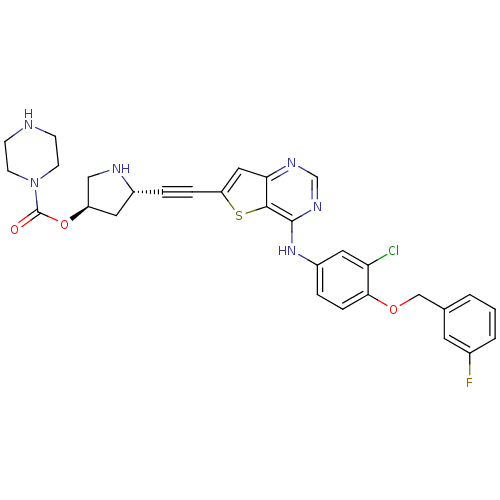
((3R,5S)-5-{2-[4-({3-chloro-4-[(3-fluorophenyl)meth...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)C#C[C@@H]3C[C@H](CN3)OC(=O)N3CCNCC3)cc2Cl)c1 |r| Show InChI InChI=1S/C30H28ClFN6O3S/c31-25-14-22(5-7-27(25)40-17-19-2-1-3-20(32)12-19)37-29-28-26(35-18-36-29)15-24(42-28)6-4-21-13-23(16-34-21)41-30(39)38-10-8-33-9-11-38/h1-3,5,7,12,14-15,18,21,23,33-34H,8-11,13,16-17H2,(H,35,36,37)/t21-,23-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Assays were performed in 96-well polystyrene round-bottomed plates in reaction buffer containing biotinylated substrate and purified kinase in the pr... |
Bioorg Med Chem Lett 19: 21-6 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.023
BindingDB Entry DOI: 10.7270/Q2NV9GKZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM28526

((3R,5R)-5-{2-[4-({3-chloro-4-[(3-fluorophenyl)meth...)Show SMILES CCNC(=O)O[C@H]1CN[C@H](C1)C#Cc1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 |r| Show InChI InChI=1S/C28H25ClFN5O3S/c1-2-31-28(36)38-21-11-19(32-14-21)6-8-22-13-24-26(39-22)27(34-16-33-24)35-20-7-9-25(23(29)12-20)37-15-17-4-3-5-18(30)10-17/h3-5,7,9-10,12-13,16,19,21,32H,2,11,14-15H2,1H3,(H,31,36)(H,33,34,35)/t19-,21+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Assays were performed in 96-well polystyrene round-bottomed plates in reaction buffer containing biotinylated substrate and purified kinase in the pr... |
Bioorg Med Chem Lett 19: 21-6 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.023
BindingDB Entry DOI: 10.7270/Q2NV9GKZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM28512

((3R,5S)-5-{2-[4-({3-chloro-4-[(3-fluorophenyl)meth...)Show SMILES CNC(=O)O[C@H]1CN[C@@H](C1)C#Cc1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 |r| Show InChI InChI=1S/C27H23ClFN5O3S/c1-30-27(35)37-20-10-18(31-13-20)5-7-21-12-23-25(38-21)26(33-15-32-23)34-19-6-8-24(22(28)11-19)36-14-16-3-2-4-17(29)9-16/h2-4,6,8-9,11-12,15,18,20,31H,10,13-14H2,1H3,(H,30,35)(H,32,33,34)/t18-,20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 kinase |
Bioorg Med Chem Lett 18: 5738-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.090
BindingDB Entry DOI: 10.7270/Q2H70FTT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50327957
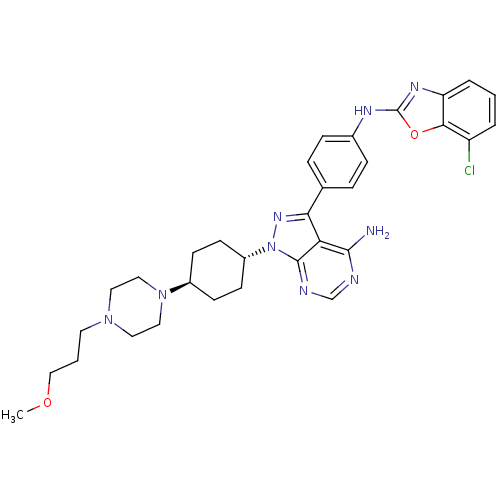
(CHEMBL1256427 | trans-N-(4-(4-amino-1-(4-(4-(3-met...)Show SMILES COCCCN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)cc2)c2c(N)ncnc12 |r,wU:14.18,wD:11.11,(57.12,-57.29,;56.65,-55.82,;55.15,-55.49,;54.69,-54.02,;53.18,-53.69,;52.72,-52.22,;51.21,-51.88,;50.75,-50.42,;51.78,-49.29,;53.29,-49.61,;53.76,-51.08,;51.31,-47.82,;52.35,-46.68,;51.88,-45.2,;50.37,-44.89,;49.34,-46.03,;49.81,-47.49,;49.89,-43.42,;50.79,-42.19,;49.89,-40.94,;50.66,-39.6,;52.2,-39.61,;52.97,-38.27,;52.19,-36.94,;52.96,-35.6,;54.5,-35.6,;55.4,-34.34,;56.87,-34.81,;58.18,-34.04,;59.51,-34.79,;59.52,-36.34,;58.19,-37.11,;58.2,-38.65,;56.87,-36.34,;55.41,-36.83,;50.65,-36.95,;49.89,-38.28,;48.43,-41.41,;47.1,-40.65,;47.1,-39.11,;45.76,-41.42,;45.76,-42.95,;47.1,-43.72,;48.43,-42.95,)| Show InChI InChI=1S/C32H38ClN9O2/c1-43-19-3-14-40-15-17-41(18-16-40)23-10-12-24(13-11-23)42-31-27(30(34)35-20-36-31)28(39-42)21-6-8-22(9-7-21)37-32-38-26-5-2-4-25(33)29(26)44-32/h2,4-9,20,23-24H,3,10-19H2,1H3,(H,37,38)(H2,34,35,36)/t23-,24- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50327955
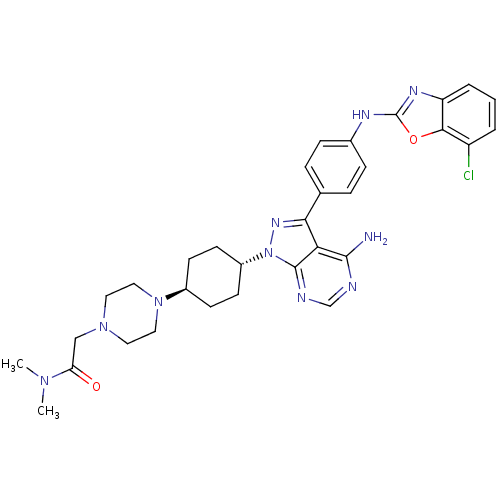
(CHEMBL1256429 | trans-2-(4-(4-(4-amino-3-(4-(7-chl...)Show SMILES CN(C)C(=O)CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)cc2)c2c(N)ncnc12 |r,wU:15.19,wD:12.12,(13.59,-15.08,;14.63,-13.94,;16.13,-14.28,;14.16,-12.48,;15.2,-11.34,;12.66,-12.14,;12.2,-10.68,;10.69,-10.34,;10.23,-8.88,;11.26,-7.74,;12.77,-8.06,;13.23,-9.54,;10.79,-6.28,;11.82,-5.14,;11.35,-3.66,;9.84,-3.35,;8.81,-4.49,;9.28,-5.95,;9.37,-1.88,;10.27,-.65,;9.37,.6,;10.14,1.94,;11.68,1.94,;12.44,3.27,;11.67,4.6,;12.44,5.94,;13.98,5.95,;14.88,7.2,;16.34,6.73,;17.66,7.51,;18.99,6.75,;19,5.21,;17.67,4.44,;17.67,2.9,;16.34,5.2,;14.89,4.71,;10.12,4.6,;9.36,3.26,;7.9,.13,;6.58,.9,;6.58,2.44,;5.24,.12,;5.24,-1.41,;6.58,-2.18,;7.91,-1.41,)| Show InChI InChI=1S/C32H37ClN10O2/c1-40(2)26(44)18-41-14-16-42(17-15-41)22-10-12-23(13-11-22)43-31-27(30(34)35-19-36-31)28(39-43)20-6-8-21(9-7-20)37-32-38-25-5-3-4-24(33)29(25)45-32/h3-9,19,22-23H,10-18H2,1-2H3,(H,37,38)(H2,34,35,36)/t22-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50327949
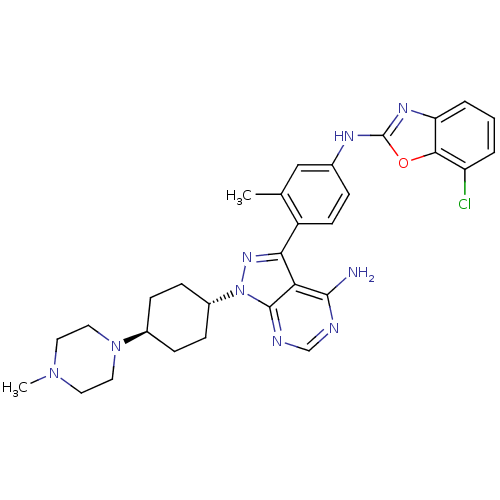
(CHEMBL1256436 | trans-N-(4-(4-amino-1-(4-(4-methyl...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)cc2C)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(58.49,-34.78,;58.03,-33.31,;56.53,-32.98,;56.06,-31.51,;57.09,-30.38,;58.6,-30.7,;59.07,-32.17,;56.62,-28.91,;57.66,-27.77,;57.19,-26.3,;55.68,-25.98,;54.65,-27.12,;55.12,-28.58,;55.2,-24.52,;56.11,-23.28,;55.2,-22.03,;55.97,-20.69,;55.2,-19.37,;55.96,-18.04,;57.51,-18.03,;58.27,-16.69,;59.81,-16.69,;60.71,-15.43,;62.18,-15.9,;63.5,-15.13,;64.82,-15.89,;64.83,-17.43,;63.51,-18.2,;63.51,-19.74,;62.18,-17.43,;60.72,-17.92,;58.28,-19.36,;57.51,-20.7,;58.28,-22.03,;53.74,-22.5,;52.41,-21.74,;52.41,-20.2,;51.07,-22.51,;51.07,-24.05,;52.41,-24.82,;53.74,-24.05,)| Show InChI InChI=1S/C30H34ClN9O/c1-18-16-19(35-30-36-24-5-3-4-23(31)27(24)41-30)6-11-22(18)26-25-28(32)33-17-34-29(25)40(37-26)21-9-7-20(8-10-21)39-14-12-38(2)13-15-39/h3-6,11,16-17,20-21H,7-10,12-15H2,1-2H3,(H,35,36)(H2,32,33,34)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27962
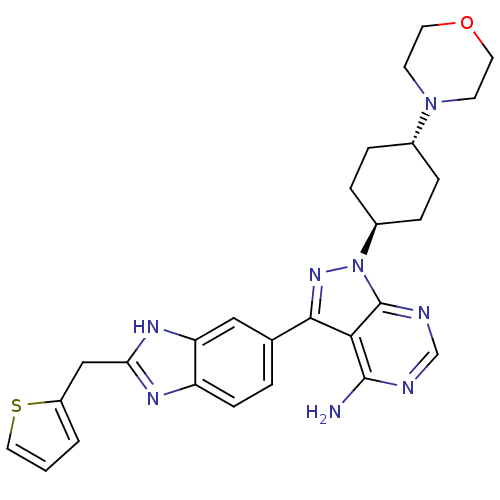
(1-((1r,4r)-4-morpholinocyclohexyl)-3-(2-(thiophen-...)Show SMILES Nc1ncnc2n(nc(-c3ccc4nc(Cc5cccs5)[nH]c4c3)c12)[C@H]1CC[C@@H](CC1)N1CCOCC1 |r,wU:28.36,wD:25.29,(-3.15,6.99,;-3.15,5.45,;-4.48,4.68,;-4.48,3.14,;-3.15,2.37,;-1.82,3.14,;-.35,2.66,;.55,3.91,;-.35,5.15,;.12,6.62,;-.85,7.81,;-.31,9.25,;1.21,9.5,;2.04,10.8,;3.53,10.41,;4.72,11.39,;6.16,10.84,;7.59,11.42,;8.58,10.23,;7.76,8.93,;6.26,9.31,;3.62,8.87,;2.19,8.31,;1.64,6.87,;-1.82,4.68,;.42,1.33,;-.41,.03,;.29,-1.34,;1.83,-1.41,;2.66,-.11,;1.96,1.26,;2.6,-2.74,;1.94,-4.13,;2.81,-5.4,;4.35,-5.28,;5.01,-3.89,;4.14,-2.62,)| Show InChI InChI=1S/C27H30N8OS/c28-26-24-25(17-3-8-21-22(14-17)32-23(31-21)15-20-2-1-13-37-20)33-35(27(24)30-16-29-26)19-6-4-18(5-7-19)34-9-11-36-12-10-34/h1-3,8,13-14,16,18-19H,4-7,9-12,15H2,(H,31,32)(H2,28,29,30)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in Sf21 cells by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 5406-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.037
BindingDB Entry DOI: 10.7270/Q20P0ZRZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27962

(1-((1r,4r)-4-morpholinocyclohexyl)-3-(2-(thiophen-...)Show SMILES Nc1ncnc2n(nc(-c3ccc4nc(Cc5cccs5)[nH]c4c3)c12)[C@H]1CC[C@@H](CC1)N1CCOCC1 |r,wU:28.36,wD:25.29,(-3.15,6.99,;-3.15,5.45,;-4.48,4.68,;-4.48,3.14,;-3.15,2.37,;-1.82,3.14,;-.35,2.66,;.55,3.91,;-.35,5.15,;.12,6.62,;-.85,7.81,;-.31,9.25,;1.21,9.5,;2.04,10.8,;3.53,10.41,;4.72,11.39,;6.16,10.84,;7.59,11.42,;8.58,10.23,;7.76,8.93,;6.26,9.31,;3.62,8.87,;2.19,8.31,;1.64,6.87,;-1.82,4.68,;.42,1.33,;-.41,.03,;.29,-1.34,;1.83,-1.41,;2.66,-.11,;1.96,1.26,;2.6,-2.74,;1.94,-4.13,;2.81,-5.4,;4.35,-5.28,;5.01,-3.89,;4.14,-2.62,)| Show InChI InChI=1S/C27H30N8OS/c28-26-24-25(17-3-8-21-22(14-17)32-23(31-21)15-20-2-1-13-37-20)33-35(27(24)30-16-29-26)19-6-4-18(5-7-19)34-9-11-36-12-10-34/h1-3,8,13-14,16,18-19H,4-7,9-12,15H2,(H,31,32)(H2,28,29,30)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... |
Bioorg Med Chem Lett 19: 1718-21 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.086
BindingDB Entry DOI: 10.7270/Q2CJ8BTG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [671-1210,L858R]
(Homo sapiens (Human)) | BDBM27968

(4-[4-(4-amino-3-{2-[(2-methoxyphenyl)methyl]-1H-1,...)Show SMILES COc1ccccc1Cc1nc2ccc(cc2[nH]1)-c1nn([C@H]2CC[C@@H](CC2)N2CCN(CC2)C(=O)N(C)C)c2ncnc(N)c12 |r,wU:24.30,wD:21.23,(8.83,13.92,;7.49,13.15,;7.49,11.61,;8.83,10.84,;8.83,9.3,;7.49,8.53,;6.16,9.3,;6.16,10.84,;4.72,11.39,;3.53,10.41,;2.04,10.8,;1.21,9.5,;-.31,9.25,;-.85,7.81,;.12,6.62,;1.64,6.87,;2.19,8.31,;3.62,8.87,;-.35,5.15,;.55,3.91,;-.35,2.66,;.42,1.33,;-.41,.03,;.29,-1.34,;1.83,-1.41,;2.66,-.11,;1.96,1.26,;2.6,-2.74,;1.94,-4.13,;2.81,-5.4,;4.35,-5.28,;5.01,-3.89,;4.14,-2.62,;5.22,-6.55,;6.75,-6.43,;4.55,-7.94,;4.55,-9.48,;3.05,-7.6,;-1.82,3.14,;-3.15,2.37,;-4.48,3.14,;-4.48,4.68,;-3.15,5.45,;-3.15,6.99,;-1.82,4.68,)| Show InChI InChI=1S/C33H40N10O2/c1-40(2)33(44)42-16-14-41(15-17-42)23-9-11-24(12-10-23)43-32-29(31(34)35-20-36-32)30(39-43)22-8-13-25-26(18-22)38-28(37-25)19-21-6-4-5-7-27(21)45-3/h4-8,13,18,20,23-24H,9-12,14-17,19H2,1-3H3,(H,37,38)(H2,34,35,36)/t23-,24- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Abbott Laboratories
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... |
Bioorg Med Chem Lett 19: 1718-21 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.086
BindingDB Entry DOI: 10.7270/Q2CJ8BTG |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27965
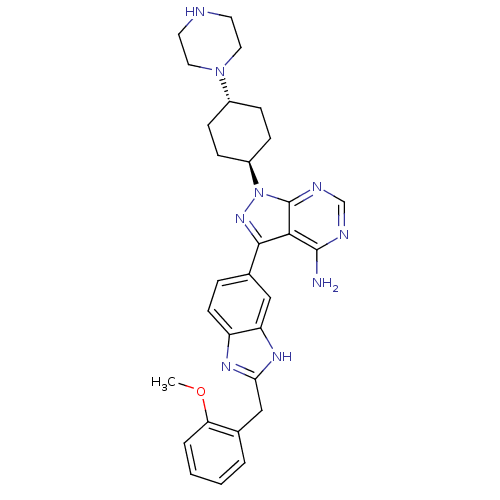
(3-{2-[(2-methoxyphenyl)methyl]-1H-1,3-benzodiazol-...)Show SMILES COc1ccccc1Cc1nc2ccc(cc2[nH]1)-c1nn([C@H]2CC[C@@H](CC2)N2CCNCC2)c2ncnc(N)c12 |r,wU:24.30,wD:21.23,(8.83,13.92,;7.49,13.15,;7.49,11.61,;8.83,10.84,;8.83,9.3,;7.49,8.53,;6.16,9.3,;6.16,10.84,;4.72,11.39,;3.53,10.41,;2.04,10.8,;1.21,9.5,;-.31,9.25,;-.85,7.81,;.12,6.62,;1.64,6.87,;2.19,8.31,;3.62,8.87,;-.35,5.15,;.55,3.91,;-.35,2.66,;.42,1.33,;-.41,.03,;.29,-1.34,;1.83,-1.41,;2.66,-.11,;1.96,1.26,;2.6,-2.74,;1.94,-4.13,;2.81,-5.4,;4.35,-5.28,;5.01,-3.89,;4.14,-2.62,;-1.82,3.14,;-3.15,2.37,;-4.48,3.14,;-4.48,4.68,;-3.15,5.45,;-3.15,6.99,;-1.82,4.68,)| Show InChI InChI=1S/C30H35N9O/c1-40-25-5-3-2-4-19(25)17-26-35-23-11-6-20(16-24(23)36-26)28-27-29(31)33-18-34-30(27)39(37-28)22-9-7-21(8-10-22)38-14-12-32-13-15-38/h2-6,11,16,18,21-22,32H,7-10,12-15,17H2,1H3,(H,35,36)(H2,31,33,34)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... |
Bioorg Med Chem Lett 19: 1718-21 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.086
BindingDB Entry DOI: 10.7270/Q2CJ8BTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50315888

(CHEMBL1090360 | N-(3-(5-(2-(3-morpholinophenylamin...)Show SMILES O=C(Cc1ccccc1)Nc1cccc(c1)-c1nc2sccn2c1-c1ccnc(Nc2cccc(c2)N2CCOCC2)n1 Show InChI InChI=1S/C33H29N7O2S/c41-29(20-23-6-2-1-3-7-23)35-25-9-4-8-24(21-25)30-31(40-16-19-43-33(40)38-30)28-12-13-34-32(37-28)36-26-10-5-11-27(22-26)39-14-17-42-18-15-39/h1-13,16,19,21-22H,14-15,17-18,20H2,(H,35,41)(H,34,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lyn |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27966
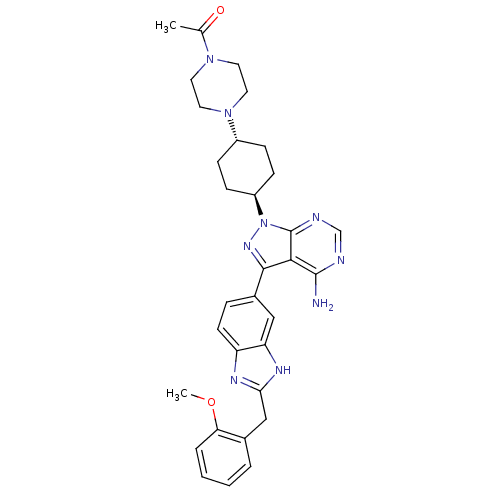
(1-{4-[4-(4-amino-3-{2-[(2-methoxyphenyl)methyl]-1H...)Show SMILES COc1ccccc1Cc1nc2ccc(cc2[nH]1)-c1nn([C@H]2CC[C@@H](CC2)N2CCN(CC2)C(C)=O)c2ncnc(N)c12 |r,wU:24.30,wD:21.23,(8.83,13.92,;7.49,13.15,;7.49,11.61,;8.83,10.84,;8.83,9.3,;7.49,8.53,;6.16,9.3,;6.16,10.84,;4.72,11.39,;3.53,10.41,;2.04,10.8,;1.21,9.5,;-.31,9.25,;-.85,7.81,;.12,6.62,;1.64,6.87,;2.19,8.31,;3.62,8.87,;-.35,5.15,;.55,3.91,;-.35,2.66,;.42,1.33,;-.41,.03,;.29,-1.34,;1.83,-1.41,;2.66,-.11,;1.96,1.26,;2.6,-2.74,;1.94,-4.13,;2.81,-5.4,;4.35,-5.28,;5.01,-3.89,;4.14,-2.62,;5.22,-6.55,;4.55,-7.94,;6.75,-6.43,;-1.82,3.14,;-3.15,2.37,;-4.48,3.14,;-4.48,4.68,;-3.15,5.45,;-3.15,6.99,;-1.82,4.68,)| Show InChI InChI=1S/C32H37N9O2/c1-20(42)39-13-15-40(16-14-39)23-8-10-24(11-9-23)41-32-29(31(33)34-19-35-32)30(38-41)22-7-12-25-26(17-22)37-28(36-25)18-21-5-3-4-6-27(21)43-2/h3-7,12,17,19,23-24H,8-11,13-16,18H2,1-2H3,(H,36,37)(H2,33,34,35)/t23-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... |
Bioorg Med Chem Lett 19: 1718-21 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.086
BindingDB Entry DOI: 10.7270/Q2CJ8BTG |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315886

(2-phenyl-N-(3-(5-(2-(1,2,3,4-tetrahydroisoquinolin...)Show SMILES O=C(Cc1ccccc1)Nc1cccc(c1)-c1nc2sccn2c1-c1ccnc(Nc2ccc3CNCCc3c2)n1 Show InChI InChI=1S/C32H27N7OS/c40-28(17-21-5-2-1-3-6-21)35-25-8-4-7-23(19-25)29-30(39-15-16-41-32(39)38-29)27-12-14-34-31(37-27)36-26-10-9-24-20-33-13-11-22(24)18-26/h1-10,12,14-16,18-19,33H,11,13,17,20H2,(H,35,40)(H,34,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IGF1R expressed in Sf21 cells by time resolved fluorescence assay |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50315895

(CHEMBL1090357 | N-(3-(5-(2-(4-(2-(dimethylamino)et...)Show SMILES CN(C)CCc1ccc(Nc2nccc(n2)-c2c(nc3sccn23)-c2cccc(NC(=O)c3c(F)cccc3F)c2)cc1 Show InChI InChI=1S/C32H27F2N7OS/c1-40(2)16-14-20-9-11-22(12-10-20)37-31-35-15-13-26(38-31)29-28(39-32-41(29)17-18-43-32)21-5-3-6-23(19-21)36-30(42)27-24(33)7-4-8-25(27)34/h3-13,15,17-19H,14,16H2,1-2H3,(H,36,42)(H,35,37,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 intracellular phosphorylation in human N87 cells by ELISA |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM28514

((3R,5S)-5-{2-[4-({3-chloro-4-[(3-fluorophenyl)meth...)Show SMILES CN(C)C(=O)O[C@H]1CN[C@@H](C1)C#Cc1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 |r| Show InChI InChI=1S/C28H25ClFN5O3S/c1-35(2)28(36)38-21-11-19(31-14-21)6-8-22-13-24-26(39-22)27(33-16-32-24)34-20-7-9-25(23(29)12-20)37-15-17-4-3-5-18(30)10-17/h3-5,7,9-10,12-13,16,19,21,31H,11,14-15H2,1-2H3,(H,32,33,34)/t19-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK
| Assay Description
Assays were performed in 96-well polystyrene round-bottomed plates in reaction buffer containing biotinylated substrate and purified kinase in the pr... |
Bioorg Med Chem Lett 19: 21-6 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.023
BindingDB Entry DOI: 10.7270/Q2NV9GKZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM28514

((3R,5S)-5-{2-[4-({3-chloro-4-[(3-fluorophenyl)meth...)Show SMILES CN(C)C(=O)O[C@H]1CN[C@@H](C1)C#Cc1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 |r| Show InChI InChI=1S/C28H25ClFN5O3S/c1-35(2)28(36)38-21-11-19(31-14-21)6-8-22-13-24-26(39-22)27(33-16-32-24)34-20-7-9-25(23(29)12-20)37-15-17-4-3-5-18(30)10-17/h3-5,7,9-10,12-13,16,19,21,31H,11,14-15H2,1-2H3,(H,32,33,34)/t19-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR kinase |
Bioorg Med Chem Lett 18: 5738-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.090
BindingDB Entry DOI: 10.7270/Q2H70FTT |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50315888

(CHEMBL1090360 | N-(3-(5-(2-(3-morpholinophenylamin...)Show SMILES O=C(Cc1ccccc1)Nc1cccc(c1)-c1nc2sccn2c1-c1ccnc(Nc2cccc(c2)N2CCOCC2)n1 Show InChI InChI=1S/C33H29N7O2S/c41-29(20-23-6-2-1-3-7-23)35-25-9-4-8-24(21-25)30-31(40-16-19-43-33(40)38-30)28-12-13-34-32(37-28)36-26-10-5-11-27(22-26)39-14-17-42-18-15-39/h1-13,16,19,21-22H,14-15,17-18,20H2,(H,35,41)(H,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50327951
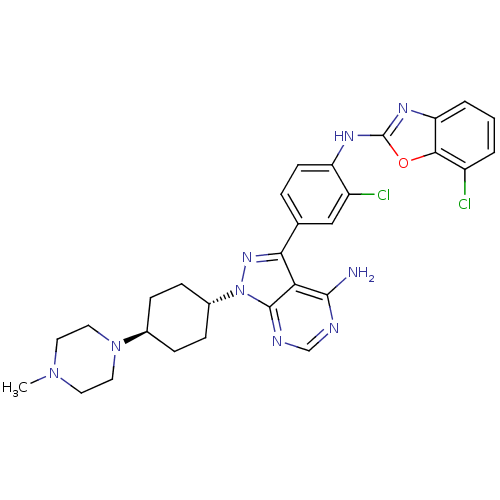
(CHEMBL1256433 | trans-N-(4-(4-amino-1-(4-(4-methyl...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)c(Cl)c2)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(10.51,-36.15,;10.04,-34.69,;8.54,-34.35,;8.07,-32.89,;9.1,-31.75,;10.62,-32.07,;11.08,-33.55,;8.63,-30.29,;9.67,-29.15,;9.2,-27.67,;7.69,-27.36,;6.66,-28.5,;7.13,-29.96,;7.21,-25.89,;8.12,-24.66,;7.21,-23.41,;7.98,-22.07,;9.52,-22.07,;10.29,-20.74,;9.52,-19.41,;10.28,-18.07,;11.82,-18.06,;12.72,-16.81,;14.19,-17.28,;15.51,-16.51,;16.84,-17.26,;16.84,-18.8,;15.52,-19.57,;15.52,-21.11,;14.19,-18.81,;12.73,-19.3,;7.97,-19.41,;7.19,-18.09,;7.21,-20.75,;5.75,-23.88,;4.42,-23.11,;4.42,-21.57,;3.09,-23.89,;3.08,-25.42,;4.43,-26.19,;5.75,-25.42,)| Show InChI InChI=1S/C29H31Cl2N9O/c1-38-11-13-39(14-12-38)18-6-8-19(9-7-18)40-28-24(27(32)33-16-34-28)25(37-40)17-5-10-22(21(31)15-17)35-29-36-23-4-2-3-20(30)26(23)41-29/h2-5,10,15-16,18-19H,6-9,11-14H2,1H3,(H,35,36)(H2,32,33,34)/t18-,19- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 20: 6067-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.052
BindingDB Entry DOI: 10.7270/Q29S1R7Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM28502
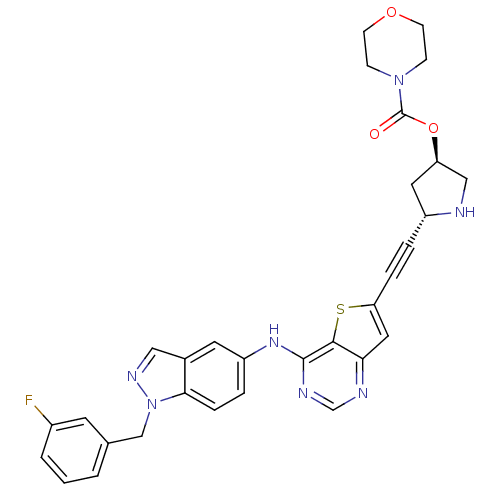
((3R,5S)-5-{2-[4-({1-[(3-fluorophenyl)methyl]-1H-in...)Show SMILES Fc1cccc(Cn2ncc3cc(Nc4ncnc5cc(sc45)C#C[C@@H]4C[C@H](CN4)OC(=O)N4CCOCC4)ccc23)c1 |r| Show InChI InChI=1S/C31H28FN7O3S/c32-22-3-1-2-20(12-22)18-39-28-7-5-24(13-21(28)16-36-39)37-30-29-27(34-19-35-30)15-26(43-29)6-4-23-14-25(17-33-23)42-31(40)38-8-10-41-11-9-38/h1-3,5,7,12-13,15-16,19,23,25,33H,8-11,14,17-18H2,(H,34,35,37)/t23-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
Assays were performed in 96-well polystyrene round-bottomed plates in reaction buffer containing biotinylated substrate and purified kinase in the pr... |
Bioorg Med Chem Lett 19: 1332-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.080
BindingDB Entry DOI: 10.7270/Q2SN078S |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM28490

((3R,5S)-5-[2-(4-{[3-chloro-4-(pyridin-2-ylmethoxy)...)Show SMILES Clc1cc(Nc2ncnc3cc(sc23)C#C[C@@H]2C[C@H](CN2)OC(=O)N2CCOCC2)ccc1OCc1ccccn1 |r| Show InChI InChI=1S/C29H27ClN6O4S/c30-24-14-20(5-7-26(24)39-17-21-3-1-2-8-31-21)35-28-27-25(33-18-34-28)15-23(41-27)6-4-19-13-22(16-32-19)40-29(37)36-9-11-38-12-10-36/h1-3,5,7-8,14-15,18-19,22,32H,9-13,16-17H2,(H,33,34,35)/t19-,22-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Assays were performed in 96-well polystyrene round-bottomed plates in reaction buffer containing biotinylated substrate and purified kinase in the pr... |
Bioorg Med Chem Lett 19: 1332-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.080
BindingDB Entry DOI: 10.7270/Q2SN078S |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM28502

((3R,5S)-5-{2-[4-({1-[(3-fluorophenyl)methyl]-1H-in...)Show SMILES Fc1cccc(Cn2ncc3cc(Nc4ncnc5cc(sc45)C#C[C@@H]4C[C@H](CN4)OC(=O)N4CCOCC4)ccc23)c1 |r| Show InChI InChI=1S/C31H28FN7O3S/c32-22-3-1-2-20(12-22)18-39-28-7-5-24(13-21(28)16-36-39)37-30-29-27(34-19-35-30)15-26(43-29)6-4-23-14-25(17-33-23)42-31(40)38-8-10-41-11-9-38/h1-3,5,7,12-13,15-16,19,23,25,33H,8-11,14,17-18H2,(H,34,35,37)/t23-,25-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Assays were performed in 96-well polystyrene round-bottomed plates in reaction buffer containing biotinylated substrate and purified kinase in the pr... |
Bioorg Med Chem Lett 19: 1332-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.080
BindingDB Entry DOI: 10.7270/Q2SN078S |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [671-1210,L858R]
(Homo sapiens (Human)) | BDBM27965

(3-{2-[(2-methoxyphenyl)methyl]-1H-1,3-benzodiazol-...)Show SMILES COc1ccccc1Cc1nc2ccc(cc2[nH]1)-c1nn([C@H]2CC[C@@H](CC2)N2CCNCC2)c2ncnc(N)c12 |r,wU:24.30,wD:21.23,(8.83,13.92,;7.49,13.15,;7.49,11.61,;8.83,10.84,;8.83,9.3,;7.49,8.53,;6.16,9.3,;6.16,10.84,;4.72,11.39,;3.53,10.41,;2.04,10.8,;1.21,9.5,;-.31,9.25,;-.85,7.81,;.12,6.62,;1.64,6.87,;2.19,8.31,;3.62,8.87,;-.35,5.15,;.55,3.91,;-.35,2.66,;.42,1.33,;-.41,.03,;.29,-1.34,;1.83,-1.41,;2.66,-.11,;1.96,1.26,;2.6,-2.74,;1.94,-4.13,;2.81,-5.4,;4.35,-5.28,;5.01,-3.89,;4.14,-2.62,;-1.82,3.14,;-3.15,2.37,;-4.48,3.14,;-4.48,4.68,;-3.15,5.45,;-3.15,6.99,;-1.82,4.68,)| Show InChI InChI=1S/C30H35N9O/c1-40-25-5-3-2-4-19(25)17-26-35-23-11-6-20(16-24(23)36-26)28-27-29(31)33-18-34-30(27)39(37-28)22-9-7-21(8-10-22)38-14-12-32-13-15-38/h2-6,11,16,18,21-22,32H,7-10,12-15,17H2,1H3,(H,35,36)(H2,31,33,34)/t21-,22- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Abbott Laboratories
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... |
Bioorg Med Chem Lett 19: 1718-21 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.086
BindingDB Entry DOI: 10.7270/Q2CJ8BTG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [671-1210,L858R]
(Homo sapiens (Human)) | BDBM27967
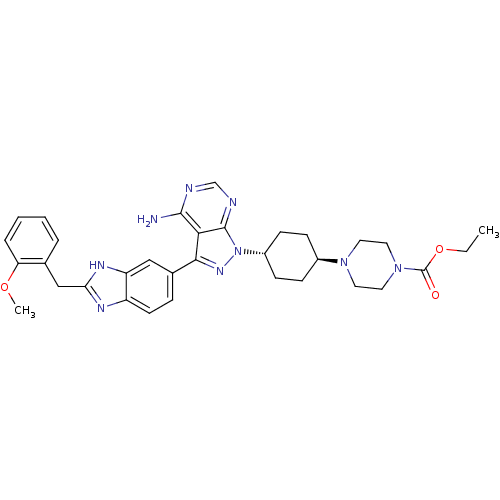
(ethyl 4-[4-(4-amino-3-{2-[(2-methoxyphenyl)methyl]...)Show SMILES CCOC(=O)N1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc3nc(Cc4ccccc4OC)[nH]c3c2)c2c(N)ncnc12 |r,wU:11.11,wD:14.18,(3.22,-10.25,;4.55,-9.48,;4.55,-7.94,;5.22,-6.55,;6.75,-6.43,;4.35,-5.28,;2.81,-5.4,;1.94,-4.13,;2.6,-2.74,;4.14,-2.62,;5.01,-3.89,;1.83,-1.41,;.29,-1.34,;-.41,.03,;.42,1.33,;1.96,1.26,;2.66,-.11,;-.35,2.66,;.55,3.91,;-.35,5.15,;.12,6.62,;-.85,7.81,;-.31,9.25,;1.21,9.5,;2.04,10.8,;3.53,10.41,;4.72,11.39,;6.16,10.84,;6.16,9.3,;7.49,8.53,;8.83,9.3,;8.83,10.84,;7.49,11.61,;7.49,13.15,;8.83,13.92,;3.62,8.87,;2.19,8.31,;1.64,6.87,;-1.82,4.68,;-3.15,5.45,;-3.15,6.99,;-4.48,4.68,;-4.48,3.14,;-3.15,2.37,;-1.82,3.14,)| Show InChI InChI=1S/C33H39N9O3/c1-3-45-33(43)41-16-14-40(15-17-41)23-9-11-24(12-10-23)42-32-29(31(34)35-20-36-32)30(39-42)22-8-13-25-26(18-22)38-28(37-25)19-21-6-4-5-7-27(21)44-2/h4-8,13,18,20,23-24H,3,9-12,14-17,19H2,1-2H3,(H,37,38)(H2,34,35,36)/t23-,24- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Abbott Laboratories
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... |
Bioorg Med Chem Lett 19: 1718-21 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.086
BindingDB Entry DOI: 10.7270/Q2CJ8BTG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM28513

((3R,5S)-5-{2-[4-({3-chloro-4-[(3-fluorophenyl)meth...)Show SMILES CCNC(=O)O[C@H]1CN[C@@H](C1)C#Cc1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 |r| Show InChI InChI=1S/C28H25ClFN5O3S/c1-2-31-28(36)38-21-11-19(32-14-21)6-8-22-13-24-26(39-22)27(34-16-33-24)35-20-7-9-25(23(29)12-20)37-15-17-4-3-5-18(30)10-17/h3-5,7,9-10,12-13,16,19,21,32H,2,11,14-15H2,1H3,(H,31,36)(H,33,34,35)/t19-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK
| Assay Description
Assays were performed in 96-well polystyrene round-bottomed plates in reaction buffer containing biotinylated substrate and purified kinase in the pr... |
Bioorg Med Chem Lett 19: 21-6 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.023
BindingDB Entry DOI: 10.7270/Q2NV9GKZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data