

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262340 (11-Amino-3,3-dimethyl-12-p-tolyl-3,4,5,7,8,9,10,12...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262341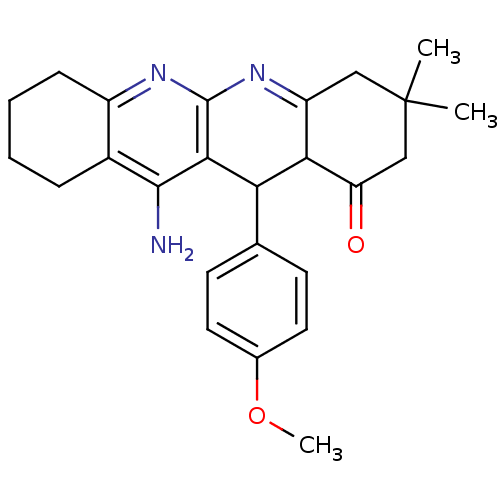 (11-Amino-12-(4-methoxy-phenyl)-3,3-dimethyl-3,4,5,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262282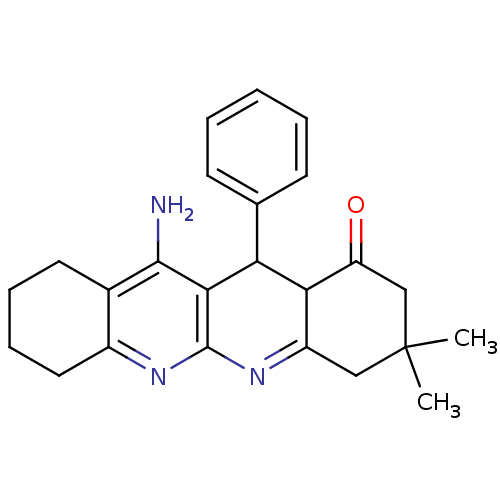 (11-Amino-3,3-dimethyl-12-phenyl-3,4,5,7,8,9,10,12-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 61.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262339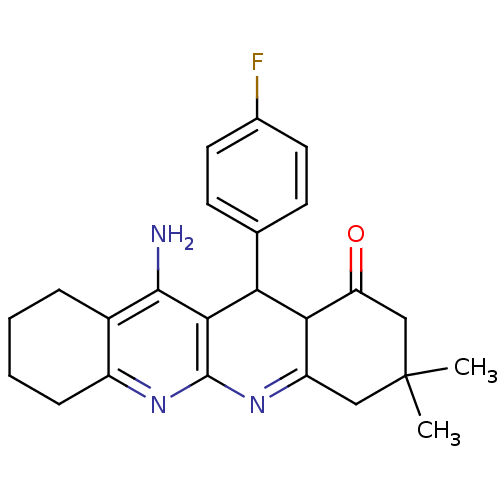 (11-Amino-12-(4-fluoro-phenyl)-3,3-dimethyl-3,4,5,7...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262342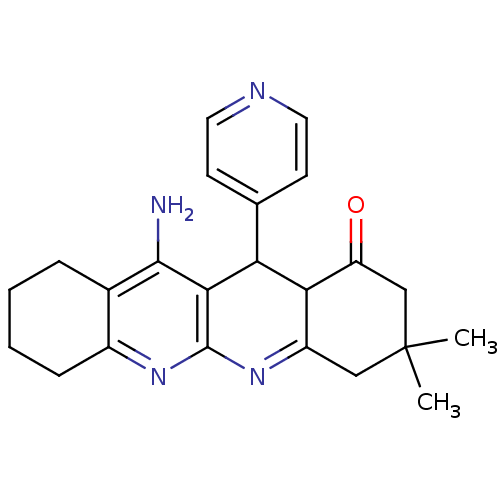 (11-Amino-3,3-dimethyl-12-pyridin-4-yl-3,4,5,7,8,9,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM31904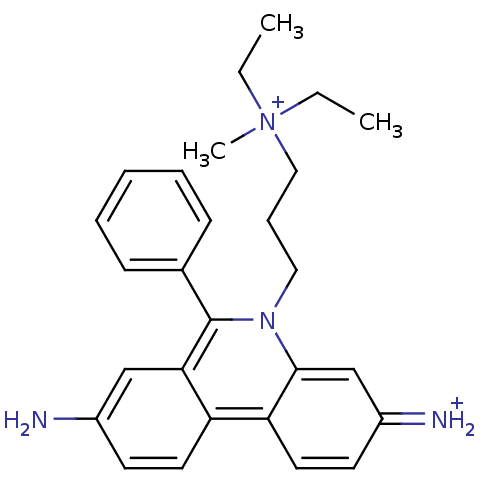 (CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.73 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31895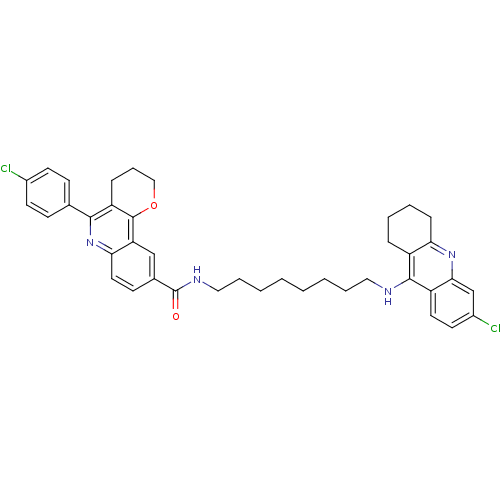 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 20....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.03 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.32 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31899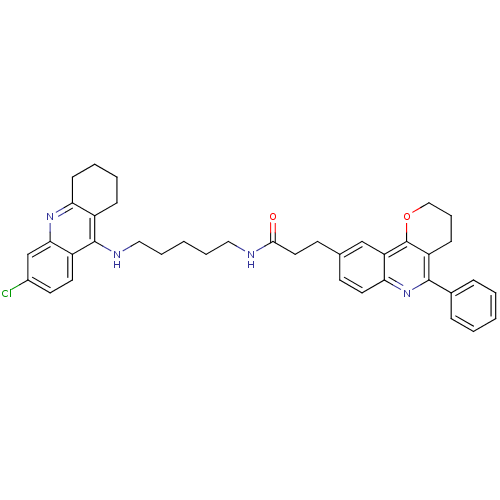 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 24....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.64 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31894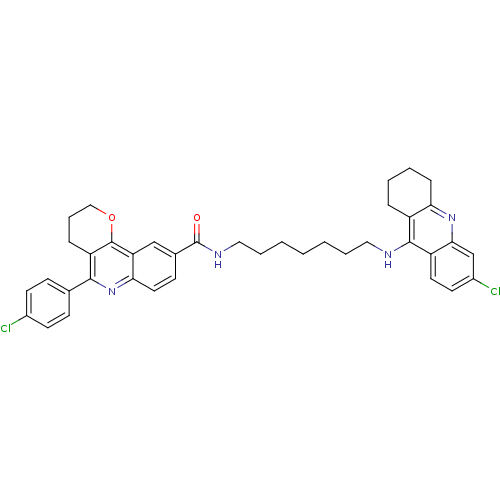 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 19....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31895 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 20....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31900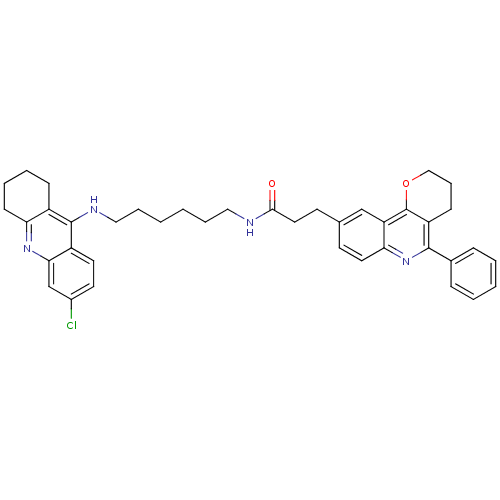 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 25....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31902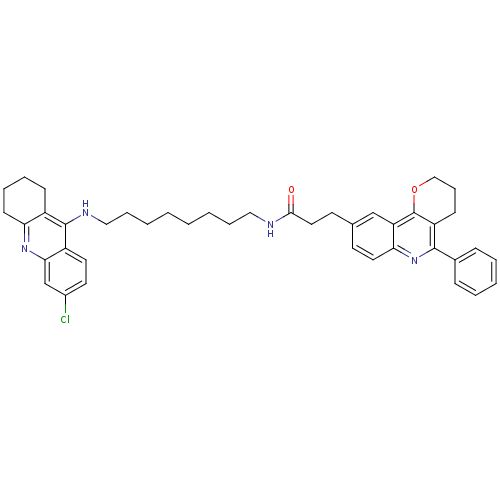 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 27....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31901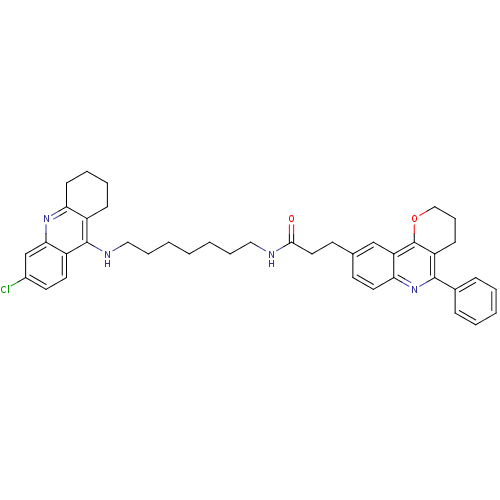 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 26....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31898 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 23....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.6 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31894 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 19....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31893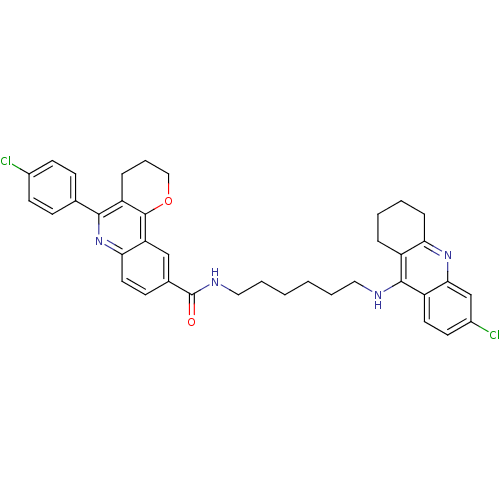 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 18....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.2 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31893 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 18....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31899 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 24....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23.9 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31896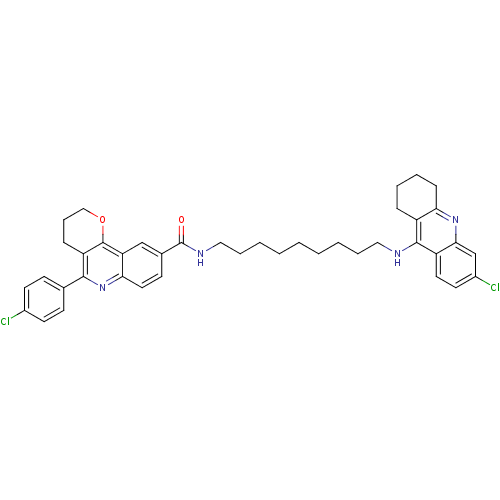 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 21....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31896 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 21....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.9 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31901 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 26....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29.9 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31900 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 25....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30.8 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31902 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 27....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34.1 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.2 | 22 |
CSIC | Assay Description The inhibitory activity of the compounds towards BuChE was determined following the method of Ellman using BuChE from human serum and butyrylthiochol... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of human serum BuChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM29401 (tacrine-dihydropyridine hybrid (tacripyrine), 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.2 | 22 |
CSIC | Assay Description The inhibitory activity of the compounds towards hAChE was determined following the method of Ellman using AChE from human serum and acetylthiocholin... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29400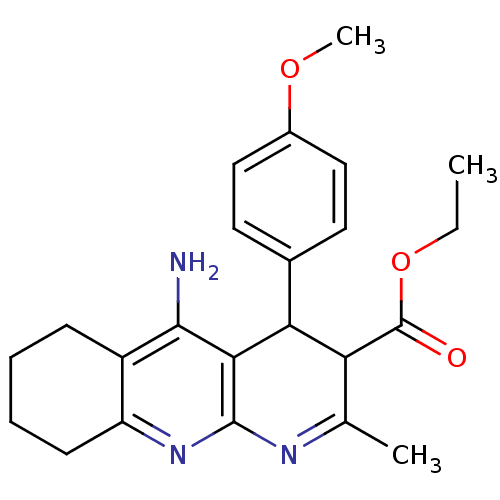 (CHEMBL374184 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by spectrophotometry | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29400 (CHEMBL374184 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29402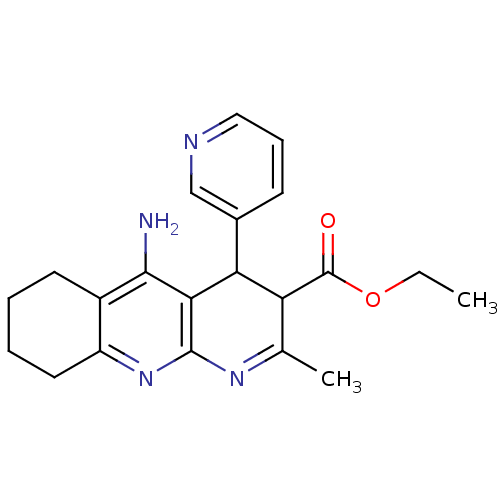 (tacrine-dihydropyridine hybrid (tacripyrine), 13) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31898 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 23....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48.1 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31897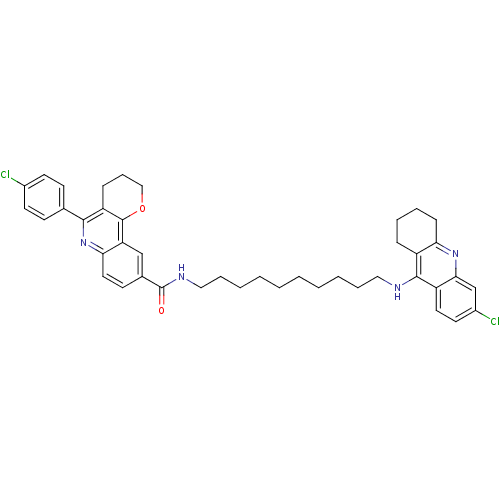 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 22....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29391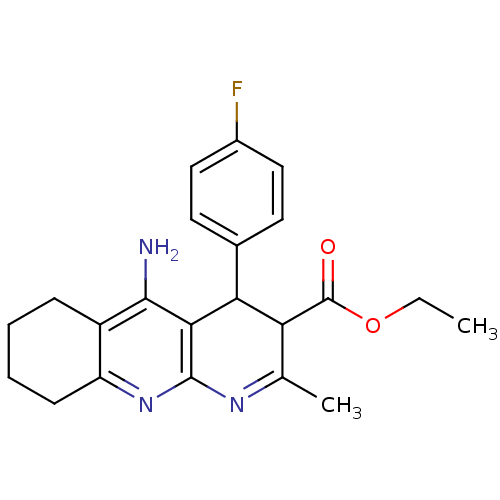 (CHEMBL219172 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29391 (CHEMBL219172 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by spectrophotometry | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM29399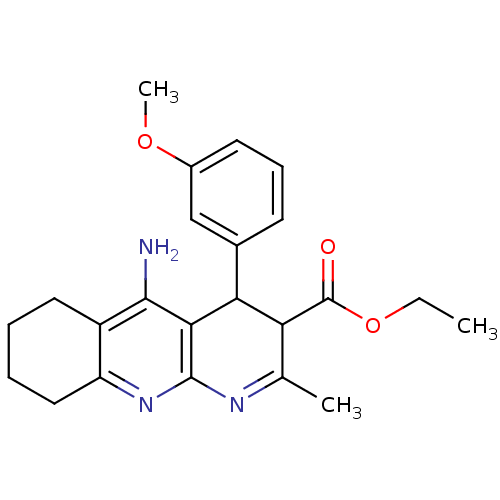 (CHEMBL218940 | tacrine-dihydropyridine hybrid (tac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.2 | 22 |
CSIC | Assay Description The inhibitory activity of the compounds towards hAChE was determined following the method of Ellman using AChE from human serum and acetylthiocholin... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29399 (CHEMBL218940 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM29397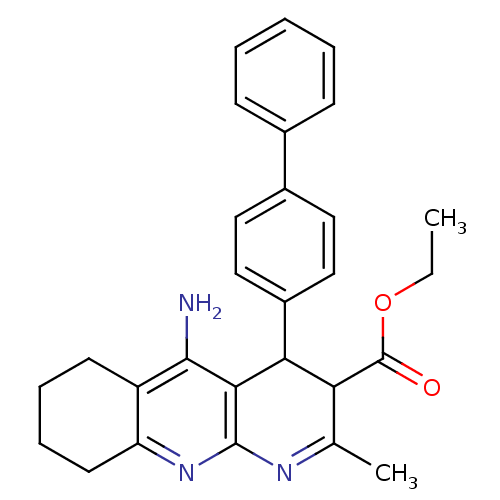 (tacrine-dihydropyridine hybrid (tacripyrine), 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 7.2 | 22 |
CSIC | Assay Description The inhibitory activity of the compounds towards hAChE was determined following the method of Ellman using AChE from human serum and acetylthiocholin... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29390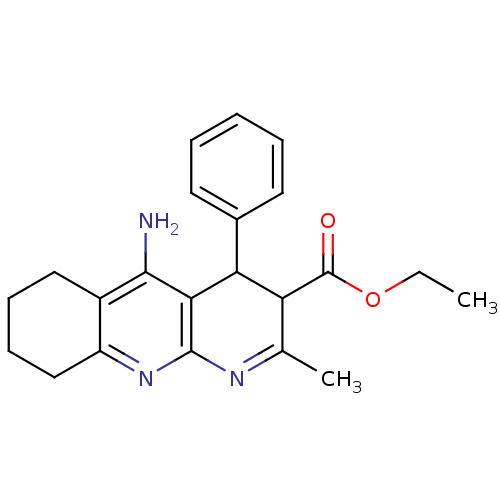 (CHEMBL218939 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29390 (CHEMBL218939 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by spectrophotometry | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29397 (tacrine-dihydropyridine hybrid (tacripyrine), 8) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29396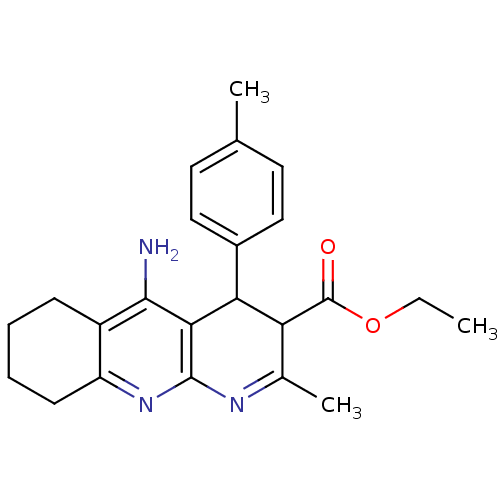 (CHEMBL219406 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29396 (CHEMBL219406 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by spectrophotometry | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31897 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 22....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93.7 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29401 (tacrine-dihydropyridine hybrid (tacripyrine), 12) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM29400 (CHEMBL374184 | tacrine-dihydropyridine hybrid (tac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | 7.2 | 22 |
CSIC | Assay Description The inhibitory activity of the compounds towards hAChE was determined following the method of Ellman using AChE from human serum and acetylthiocholin... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM29400 (CHEMBL374184 | tacrine-dihydropyridine hybrid (tac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29394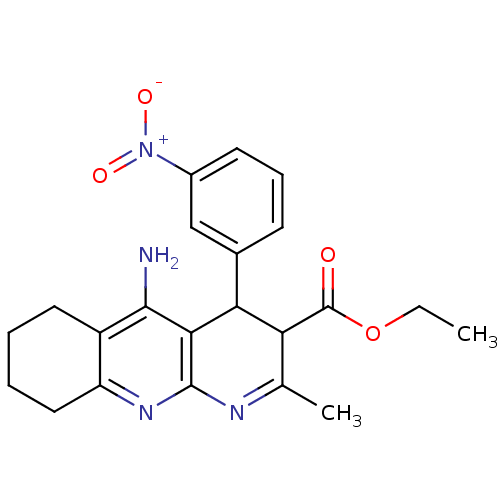 (CHEMBL220294 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM29390 (CHEMBL218939 | tacrine-dihydropyridine hybrid (tac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 134 total ) | Next | Last >> |