 Found 68 hits with Last Name = 'hui' and Initial = 'yh'
Found 68 hits with Last Name = 'hui' and Initial = 'yh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243396
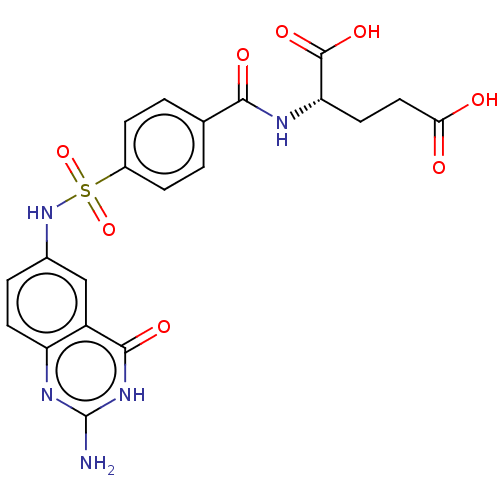
(CHEMBL1231520)Show SMILES Nc1nc2ccc(NS(=O)(=O)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H19N5O8S/c21-20-23-14-6-3-11(9-13(14)18(29)24-20)25-34(32,33)12-4-1-10(2-5-12)17(28)22-15(19(30)31)7-8-16(26)27/h1-6,9,15,25H,7-8H2,(H,22,28)(H,26,27)(H,30,31)(H3,21,23,24,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human AICARFT |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta-2B chain
(Bos taurus) | BDBM50005480

((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...)Show InChI InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta-2B chain
(Bos taurus) | BDBM50109343
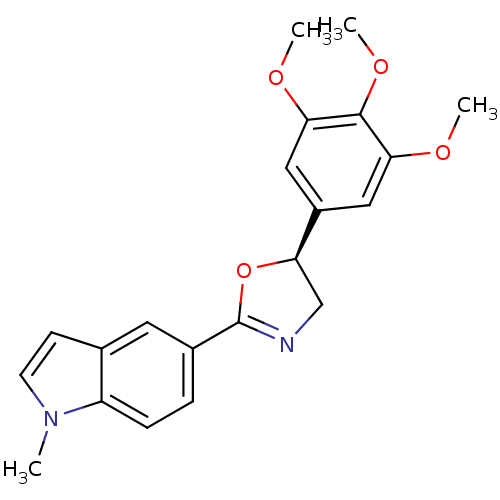
(1-Methyl-5-[(S)-5-(3,4,5-trimethoxy-phenyl)-4,5-di...)Show SMILES COc1cc(cc(OC)c1OC)[C@H]1CN=C(O1)c1ccc2n(C)ccc2c1 |c:15| Show InChI InChI=1S/C21H22N2O4/c1-23-8-7-13-9-14(5-6-16(13)23)21-22-12-19(27-21)15-10-17(24-2)20(26-4)18(11-15)25-3/h5-11,19H,12H2,1-4H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50014846
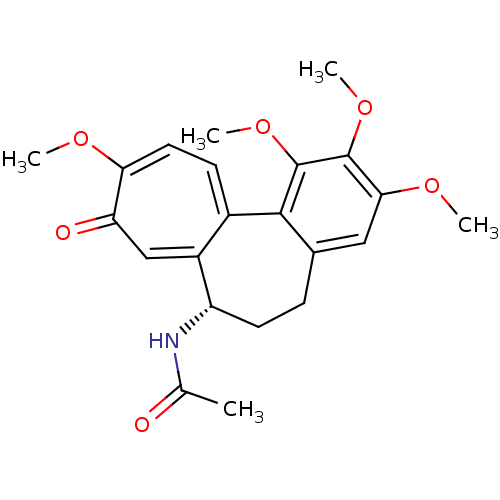
((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...)Show SMILES COc1cc2CC[C@H](NC(C)=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta-2B chain
(Bos taurus) | BDBM50109342
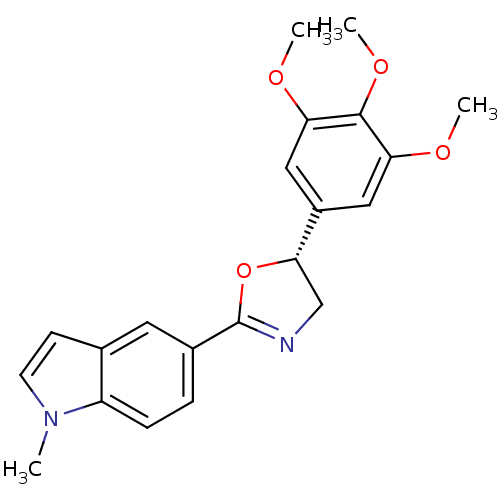
(1-Methyl-5-[(R)-5-(3,4,5-trimethoxy-phenyl)-4,5-di...)Show SMILES COc1cc(cc(OC)c1OC)[C@@H]1CN=C(O1)c1ccc2n(C)ccc2c1 |c:15| Show InChI InChI=1S/C21H22N2O4/c1-23-8-7-13-9-14(5-6-16(13)23)21-22-12-19(27-21)15-10-17(24-2)20(26-4)18(11-15)25-3/h5-11,19H,12H2,1-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50012278
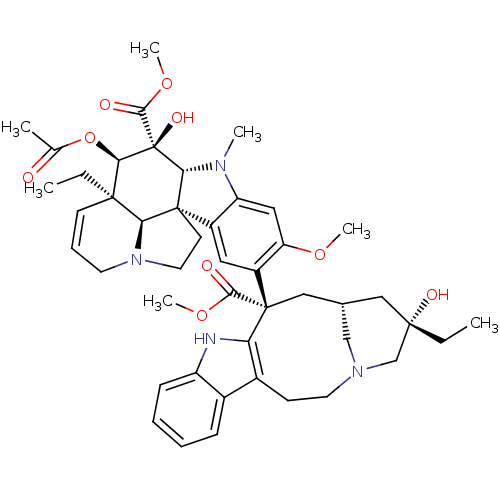
((2ALPHA,2''BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLAS...)Show SMILES CC[C@]1(O)C[C@@H]2CN(C1)CCc1c([nH]c3ccccc13)[C@@](C2)(C(=O)OC)c1cc2c(cc1OC)N(C)[C@@H]1[C@]22CCN3CC=C[C@](CC)([C@@H]23)[C@@H](OC(C)=O)[C@]1(O)C(=O)OC |r,c:48| Show InChI InChI=1S/C46H58N4O9/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3/t28-,37-,38+,39+,42-,43+,44+,45-,46-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243463
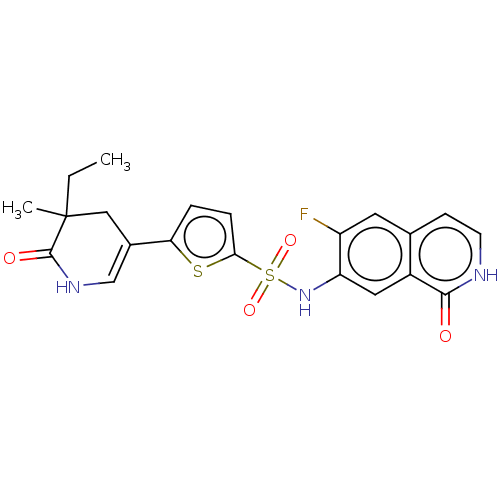
(CHEMBL4100363)Show SMILES CCC1(C)CC(=CNC1=O)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |c:5| Show InChI InChI=1S/C21H20FN3O4S2/c1-3-21(2)10-13(11-24-20(21)27)17-4-5-18(30-17)31(28,29)25-16-9-14-12(8-15(16)22)6-7-23-19(14)26/h4-9,11,25H,3,10H2,1-2H3,(H,23,26)(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243462
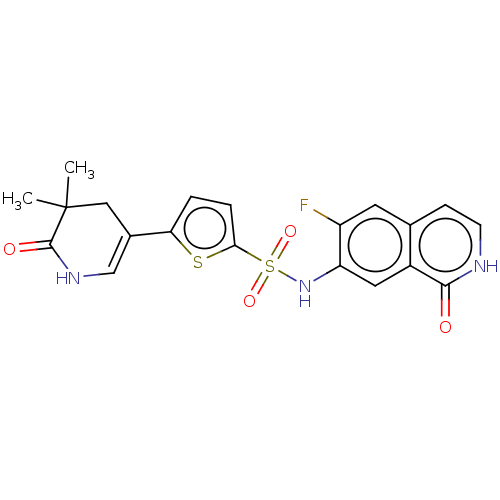
(CHEMBL4083899)Show SMILES CC1(C)CC(=CNC1=O)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |c:4| Show InChI InChI=1S/C20H18FN3O4S2/c1-20(2)9-12(10-23-19(20)26)16-3-4-17(29-16)30(27,28)24-15-8-13-11(7-14(15)21)5-6-22-18(13)25/h3-8,10,24H,9H2,1-2H3,(H,22,25)(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243461
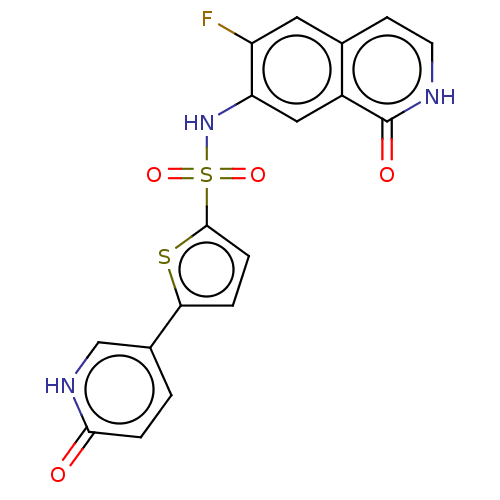
(CHEMBL4075503)Show SMILES Fc1cc2cc[nH]c(=O)c2cc1NS(=O)(=O)c1ccc(s1)-c1ccc(=O)[nH]c1 Show InChI InChI=1S/C18H12FN3O4S2/c19-13-7-10-5-6-20-18(24)12(10)8-14(13)22-28(25,26)17-4-2-15(27-17)11-1-3-16(23)21-9-11/h1-9,22H,(H,20,24)(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243486
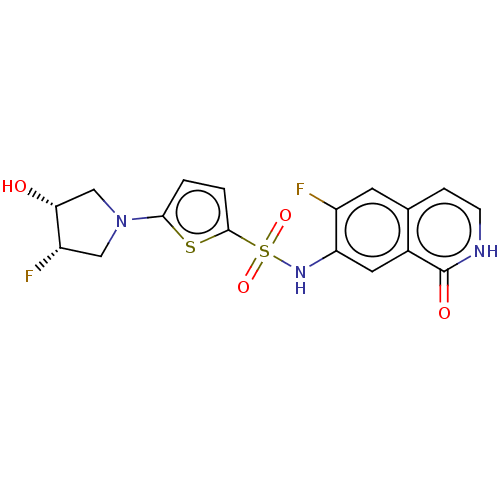
(CHEMBL4081385)Show SMILES O[C@@H]1CN(C[C@@H]1F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H15F2N3O4S2/c18-11-5-9-3-4-20-17(24)10(9)6-13(11)21-28(25,26)16-2-1-15(27-16)22-7-12(19)14(23)8-22/h1-6,12,14,21,23H,7-8H2,(H,20,24)/t12-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243487

(CHEMBL4091668)Show SMILES O[C@@H]1CN(CC1(F)F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H14F3N3O4S2/c18-11-5-9-3-4-21-16(25)10(9)6-12(11)22-29(26,27)15-2-1-14(28-15)23-7-13(24)17(19,20)8-23/h1-6,13,22,24H,7-8H2,(H,21,25)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243441
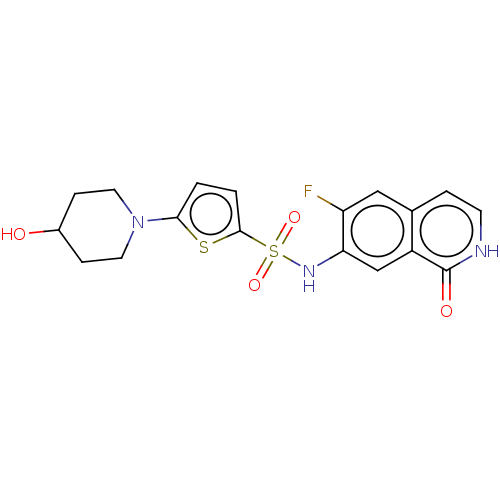
(CHEMBL4076500)Show SMILES OC1CCN(CC1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F Show InChI InChI=1S/C18H18FN3O4S2/c19-14-9-11-3-6-20-18(24)13(11)10-15(14)21-28(25,26)17-2-1-16(27-17)22-7-4-12(23)5-8-22/h1-3,6,9-10,12,21,23H,4-5,7-8H2,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243443
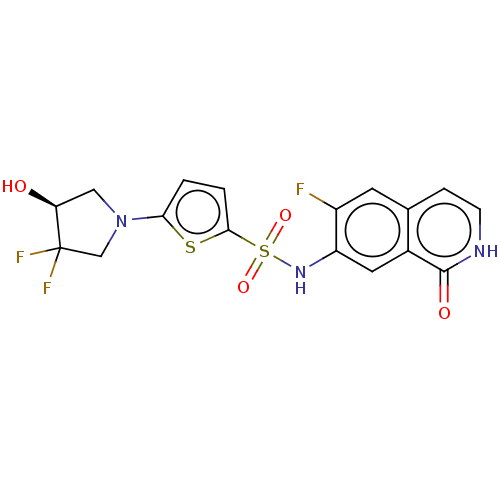
(CHEMBL4070790)Show SMILES O[C@H]1CN(CC1(F)F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H14F3N3O4S2/c18-11-5-9-3-4-21-16(25)10(9)6-12(11)22-29(26,27)15-2-1-14(28-15)23-7-13(24)17(19,20)8-23/h1-6,13,22,24H,7-8H2,(H,21,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243485
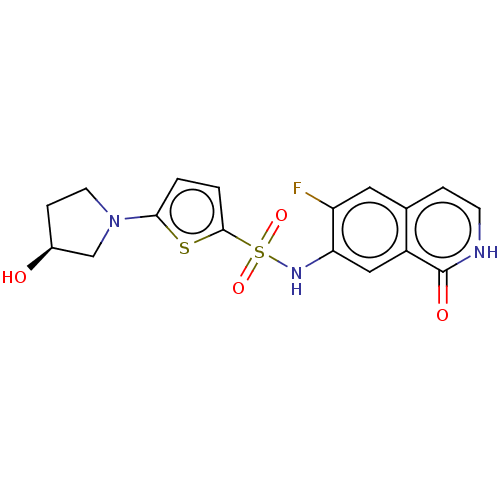
(CHEMBL4074469)Show SMILES O[C@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243415
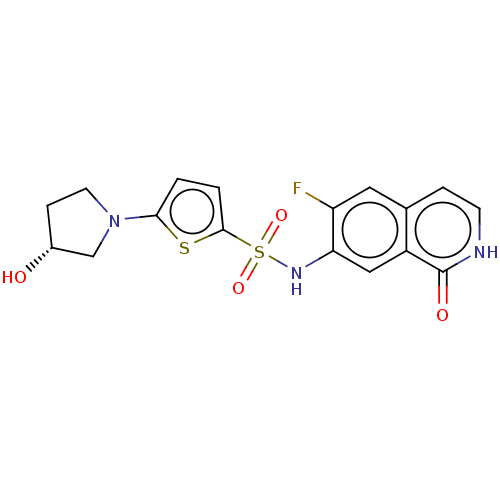
(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243434
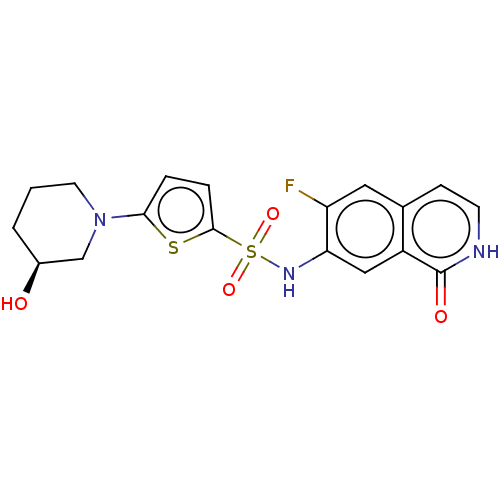
(CHEMBL4079085)Show SMILES O[C@H]1CCCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C18H18FN3O4S2/c19-14-8-11-5-6-20-18(24)13(11)9-15(14)21-28(25,26)17-4-3-16(27-17)22-7-1-2-12(23)10-22/h3-6,8-9,12,21,23H,1-2,7,10H2,(H,20,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243442
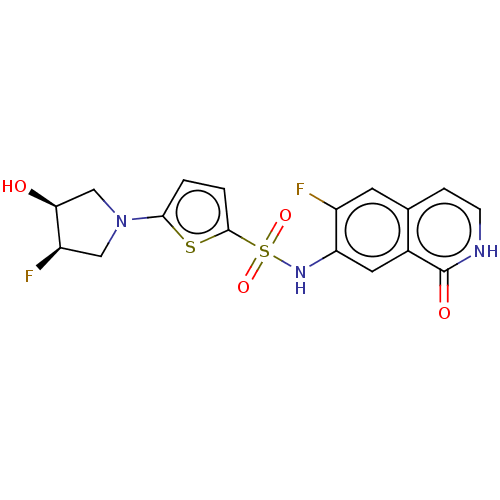
(CHEMBL4099409)Show SMILES O[C@H]1CN(C[C@H]1F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H15F2N3O4S2/c18-11-5-9-3-4-20-17(24)10(9)6-13(11)21-28(25,26)16-2-1-15(27-16)22-7-12(19)14(23)8-22/h1-6,12,14,21,23H,7-8H2,(H,20,24)/t12-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243433
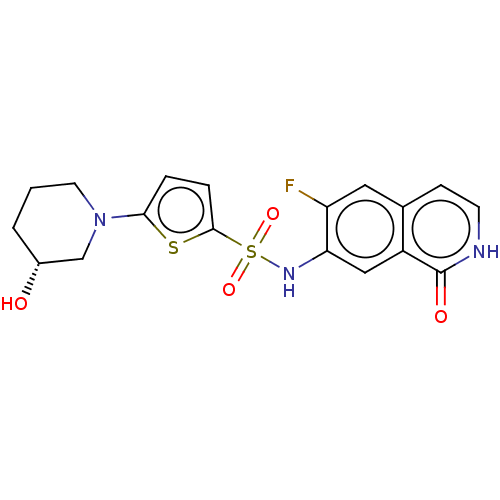
(CHEMBL4101204)Show SMILES O[C@@H]1CCCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C18H18FN3O4S2/c19-14-8-11-5-6-20-18(24)13(11)9-15(14)21-28(25,26)17-4-3-16(27-17)22-7-1-2-12(23)10-22/h3-6,8-9,12,21,23H,1-2,7,10H2,(H,20,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243477
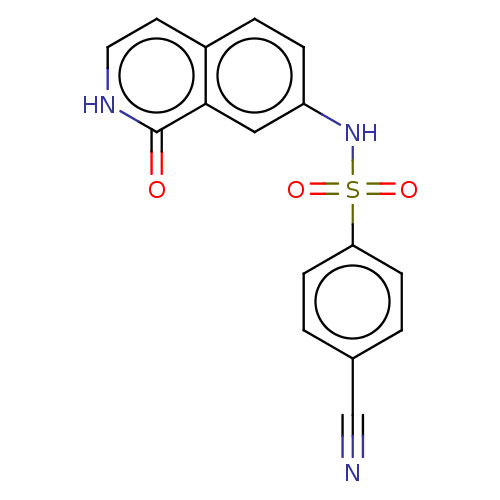
(CHEMBL4092503)Show SMILES O=c1[nH]ccc2ccc(NS(=O)(=O)c3ccc(cc3)C#N)cc12 Show InChI InChI=1S/C16H11N3O3S/c17-10-11-1-5-14(6-2-11)23(21,22)19-13-4-3-12-7-8-18-16(20)15(12)9-13/h1-9,19H,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 608 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111568
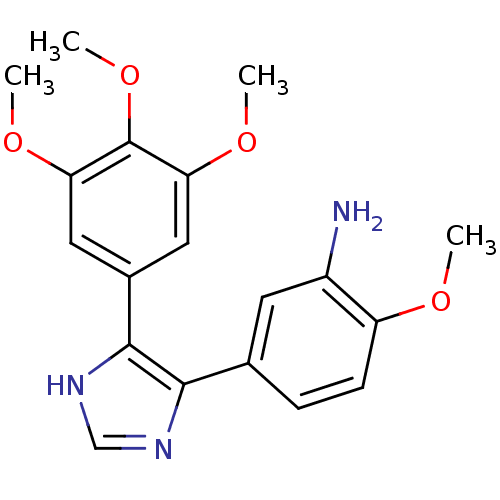
(2-Methoxy-5-[5-(3,4,5-trimethoxy-phenyl)-3H-imidaz...)Show SMILES COc1ccc(cc1N)-c1nc[nH]c1-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C19H21N3O4/c1-23-14-6-5-11(7-13(14)20)17-18(22-10-21-17)12-8-15(24-2)19(26-4)16(9-12)25-3/h5-10H,20H2,1-4H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243414
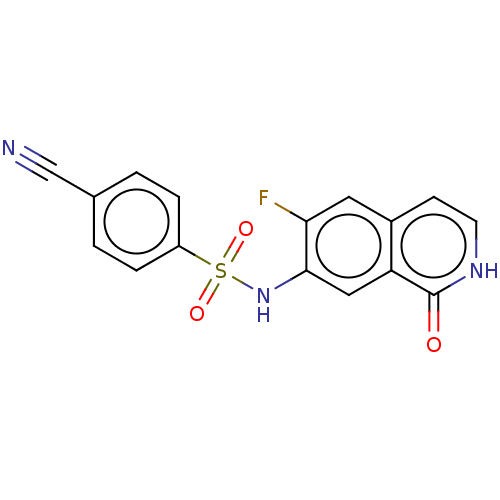
(CHEMBL4084757)Show SMILES Fc1cc2cc[nH]c(=O)c2cc1NS(=O)(=O)c1ccc(cc1)C#N Show InChI InChI=1S/C16H10FN3O3S/c17-14-7-11-5-6-19-16(21)13(11)8-15(14)20-24(22,23)12-3-1-10(9-18)2-4-12/h1-8,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111579
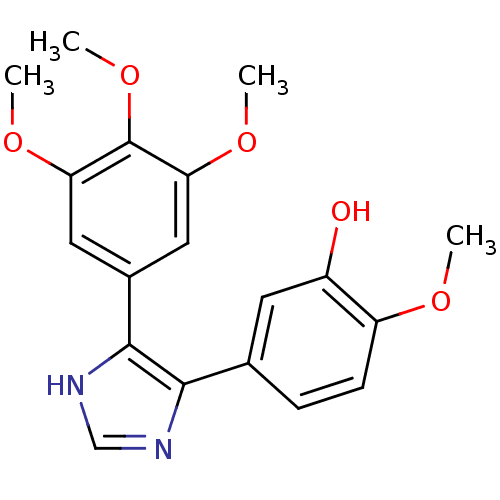
(2-Methoxy-5-[5-(3,4,5-trimethoxy-phenyl)-3H-imidaz...)Show SMILES COc1ccc(cc1O)-c1nc[nH]c1-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C19H20N2O5/c1-23-14-6-5-11(7-13(14)22)17-18(21-10-20-17)12-8-15(24-2)19(26-4)16(9-12)25-3/h5-10,22H,1-4H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111574

(1-Methyl-5-[4-(3,4,5-trimethoxy-phenyl)-oxazol-5-y...)Show SMILES COc1cc(cc(OC)c1OC)-c1ncoc1-c1ccc2n(C)ccc2c1 Show InChI InChI=1S/C21H20N2O4/c1-23-8-7-13-9-14(5-6-16(13)23)20-19(22-12-27-20)15-10-17(24-2)21(26-4)18(11-15)25-3/h5-12H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111554
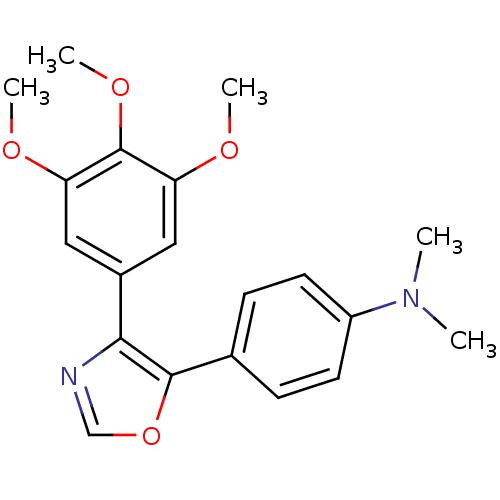
(CHEMBL296066 | Dimethyl-{4-[4-(3,4,5-trimethoxy-ph...)Show InChI InChI=1S/C20H22N2O4/c1-22(2)15-8-6-13(7-9-15)19-18(21-12-26-19)14-10-16(23-3)20(25-5)17(11-14)24-4/h6-12H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111558

(2-Methoxy-5-[4-(3,4,5-trimethoxy-phenyl)-oxazol-5-...)Show InChI InChI=1S/C19H20N2O5/c1-22-14-6-5-11(7-13(14)20)18-17(21-10-26-18)12-8-15(23-2)19(25-4)16(9-12)24-3/h5-10H,20H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111567

(2-Methoxy-5-[4-(3,4,5-trimethoxy-phenyl)-oxazol-5-...)Show InChI InChI=1S/C19H19NO6/c1-22-14-6-5-11(7-13(14)21)18-17(20-10-26-18)12-8-15(23-2)19(25-4)16(9-12)24-3/h5-10,21H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243396

(CHEMBL1231520)Show SMILES Nc1nc2ccc(NS(=O)(=O)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H19N5O8S/c21-20-23-14-6-3-11(9-13(14)18(29)24-20)25-34(32,33)12-4-1-10(2-5-12)17(28)22-15(19(30)31)7-8-16(26)27/h1-6,9,15,25H,7-8H2,(H,22,28)(H,26,27)(H,30,31)(H3,21,23,24,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111566

(1-Methyl-6-[5-(3,4,5-trimethoxy-phenyl)-3H-imidazo...)Show SMILES COc1cc(cc(OC)c1OC)-c1[nH]cnc1-c1ccc2ccn(C)c2c1 Show InChI InChI=1S/C21H21N3O3/c1-24-8-7-13-5-6-14(9-16(13)24)19-20(23-12-22-19)15-10-17(25-2)21(27-4)18(11-15)26-3/h5-12H,1-4H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50005480

((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...)Show InChI InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111555

(1-Methyl-5-[3-(3,4,5-trimethoxy-phenyl)-3H-imidazo...)Show SMILES COc1cc(cc(OC)c1OC)-n1cncc1-c1ccc2n(C)ccc2c1 Show InChI InChI=1S/C21H21N3O3/c1-23-8-7-15-9-14(5-6-17(15)23)18-12-22-13-24(18)16-10-19(25-2)21(27-4)20(11-16)26-3/h5-13H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111569
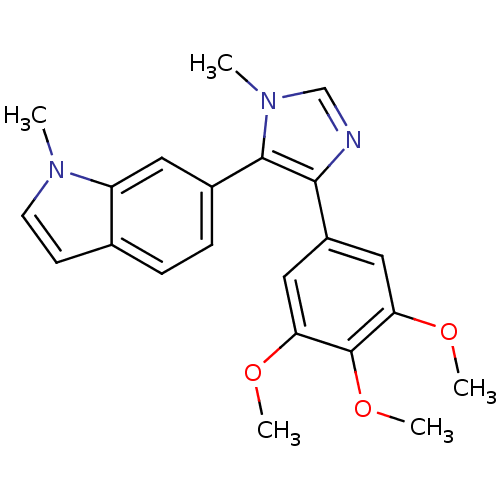
(1-Methyl-6-[3-methyl-5-(3,4,5-trimethoxy-phenyl)-3...)Show SMILES COc1cc(cc(OC)c1OC)-c1ncn(C)c1-c1ccc2ccn(C)c2c1 Show InChI InChI=1S/C22H23N3O3/c1-24-9-8-14-6-7-15(10-17(14)24)21-20(23-13-25(21)2)16-11-18(26-3)22(28-5)19(12-16)27-4/h6-13H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111580
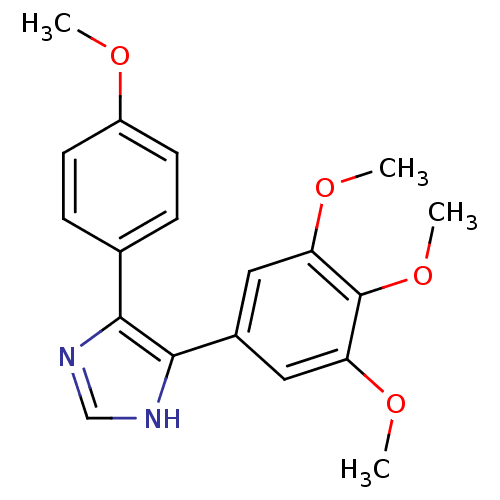
(5-(4-Methoxy-phenyl)-4-(3,4,5-trimethoxy-phenyl)-1...)Show SMILES COc1ccc(cc1)-c1nc[nH]c1-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C19H20N2O4/c1-22-14-7-5-12(6-8-14)17-18(21-11-20-17)13-9-15(23-2)19(25-4)16(10-13)24-3/h5-11H,1-4H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50109351
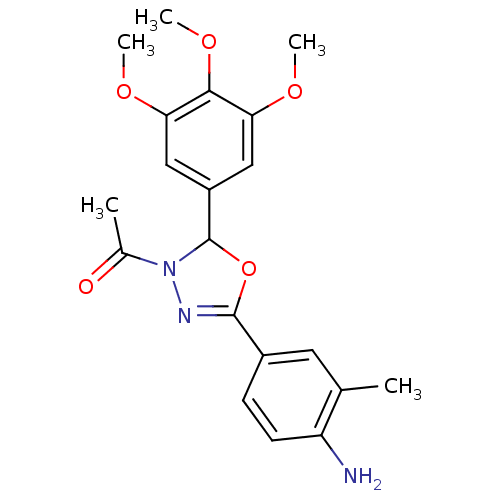
(1-[5-(4-Amino-3-methyl-phenyl)-2-(3,4,5-trimethoxy...)Show SMILES COc1cc(cc(OC)c1OC)C1OC(=NN1C(C)=O)c1ccc(N)c(C)c1 |c:15| Show InChI InChI=1S/C20H23N3O5/c1-11-8-13(6-7-15(11)21)19-22-23(12(2)24)20(28-19)14-9-16(25-3)18(27-5)17(10-14)26-4/h6-10,20H,21H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Activity towards binding at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111570
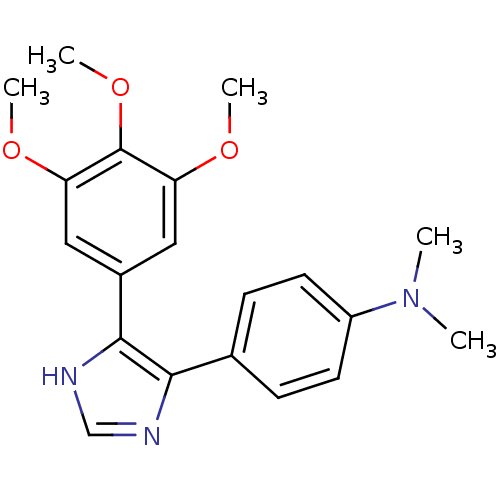
(CHEMBL296406 | Dimethyl-{4-[5-(3,4,5-trimethoxy-ph...)Show SMILES COc1cc(cc(OC)c1OC)-c1[nH]cnc1-c1ccc(cc1)N(C)C Show InChI InChI=1S/C20H23N3O3/c1-23(2)15-8-6-13(7-9-15)18-19(22-12-21-18)14-10-16(24-3)20(26-5)17(11-14)25-4/h6-12H,1-5H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111578

(2-Methoxy-5-[3-(3,4,5-trimethoxy-phenyl)-3H-imidaz...)Show InChI InChI=1S/C19H20N2O5/c1-23-16-6-5-12(7-15(16)22)14-10-20-11-21(14)13-8-17(24-2)19(26-4)18(9-13)25-3/h5-11,22H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111562
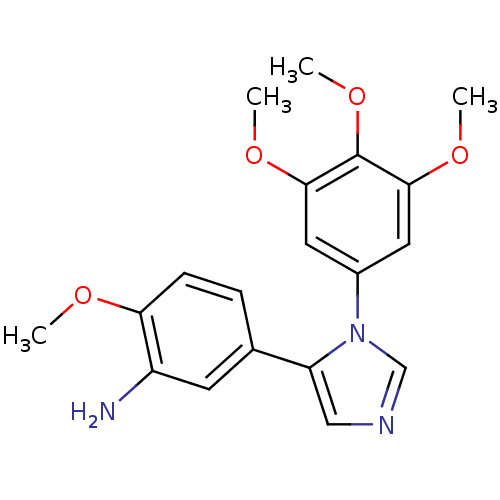
(2-Methoxy-5-[3-(3,4,5-trimethoxy-phenyl)-3H-imidaz...)Show InChI InChI=1S/C19H21N3O4/c1-23-16-6-5-12(7-14(16)20)15-10-21-11-22(15)13-8-17(24-2)19(26-4)18(9-13)25-3/h5-11H,20H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243413
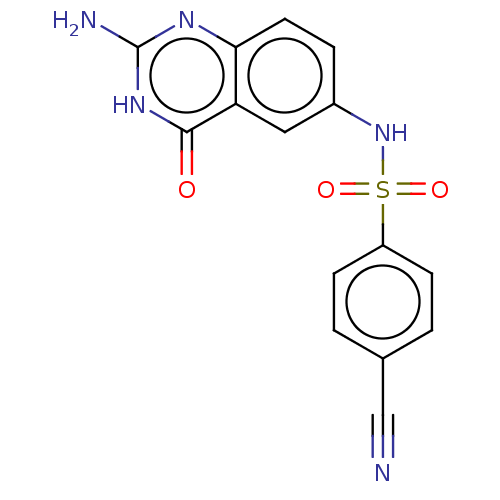
(CHEMBL4077027)Show SMILES Nc1nc2ccc(NS(=O)(=O)c3ccc(cc3)C#N)cc2c(=O)[nH]1 Show InChI InChI=1S/C15H11N5O3S/c16-8-9-1-4-11(5-2-9)24(22,23)20-10-3-6-13-12(7-10)14(21)19-15(17)18-13/h1-7,20H,(H3,17,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111556

(CHEMBL297236 | Dimethyl-{4-[3-(3,4,5-trimethoxy-ph...)Show InChI InChI=1S/C20H23N3O3/c1-22(2)15-8-6-14(7-9-15)17-12-21-13-23(17)16-10-18(24-3)20(26-5)19(11-16)25-4/h6-13H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111559
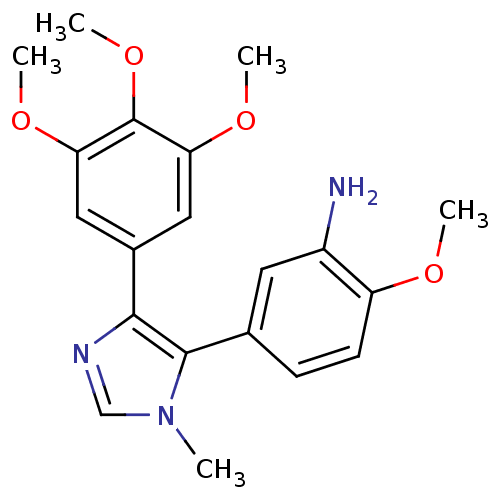
(2-Methoxy-5-[3-methyl-5-(3,4,5-trimethoxy-phenyl)-...)Show SMILES COc1ccc(cc1N)-c1c(ncn1C)-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C20H23N3O4/c1-23-11-22-18(19(23)12-6-7-15(24-2)14(21)8-12)13-9-16(25-3)20(27-5)17(10-13)26-4/h6-11H,21H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243508
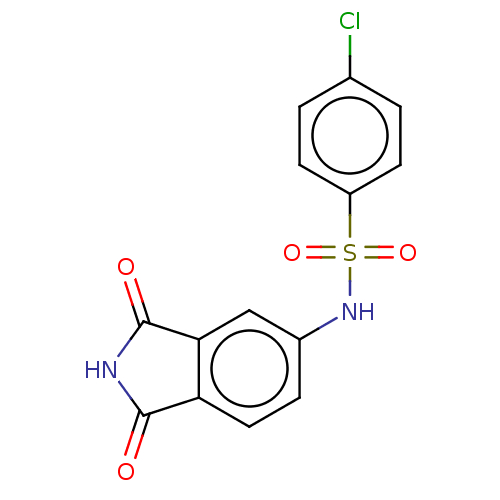
(CHEMBL4101760)Show SMILES Clc1ccc(cc1)S(=O)(=O)Nc1ccc2C(=O)NC(=O)c2c1 Show InChI InChI=1S/C14H9ClN2O4S/c15-8-1-4-10(5-2-8)22(20,21)17-9-3-6-11-12(7-9)14(19)16-13(11)18/h1-7,17H,(H,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111561

(CHEMBL47112 | Dimethyl-{4-[1-(3,4,5-trimethoxy-phe...)Show InChI InChI=1S/C20H23N3O3/c1-22(2)15-8-6-14(7-9-15)20-21-10-11-23(20)16-12-17(24-3)19(26-5)18(13-16)25-4/h6-13H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111582
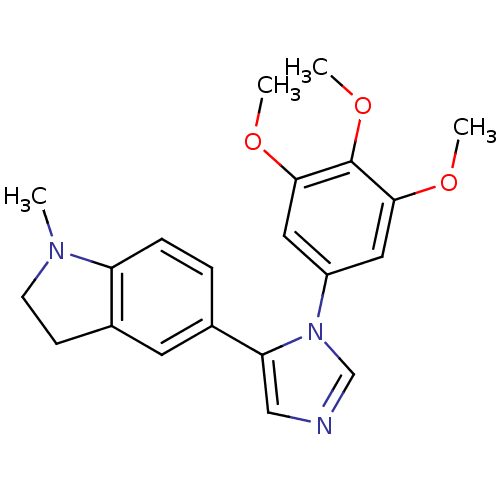
(1-Methyl-5-[3-(3,4,5-trimethoxy-phenyl)-3H-imidazo...)Show SMILES COc1cc(cc(OC)c1OC)-n1cncc1-c1ccc2N(C)CCc2c1 Show InChI InChI=1S/C21H23N3O3/c1-23-8-7-15-9-14(5-6-17(15)23)18-12-22-13-24(18)16-10-19(25-2)21(27-4)20(11-16)26-3/h5-6,9-13H,7-8H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50111565
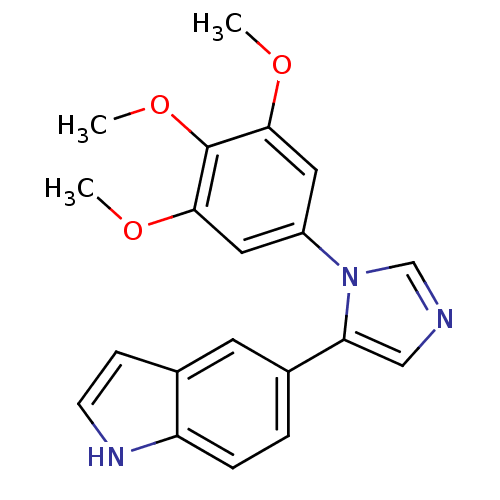
(5-[3-(3,4,5-Trimethoxy-phenyl)-3H-imidazol-4-yl]-1...)Show SMILES COc1cc(cc(OC)c1OC)-n1cncc1-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C20H19N3O3/c1-24-18-9-15(10-19(25-2)20(18)26-3)23-12-21-11-17(23)14-4-5-16-13(8-14)6-7-22-16/h4-12,22H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Concentration needed to inhibit tubulin polymerization by 50% |
J Med Chem 45: 1697-711 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GKQ |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243397
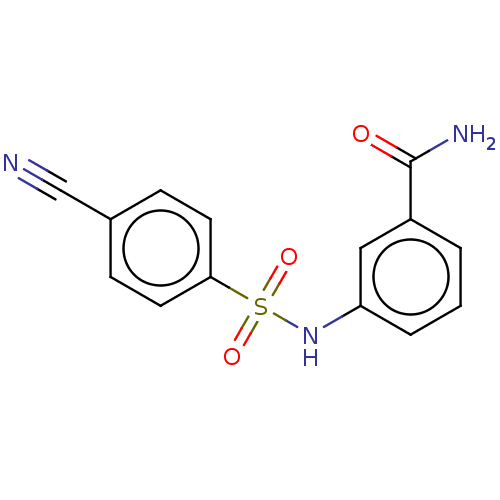
(CHEMBL4100255)Show InChI InChI=1S/C14H11N3O3S/c15-9-10-4-6-13(7-5-10)21(19,20)17-12-3-1-2-11(8-12)14(16)18/h1-8,17H,(H2,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243398
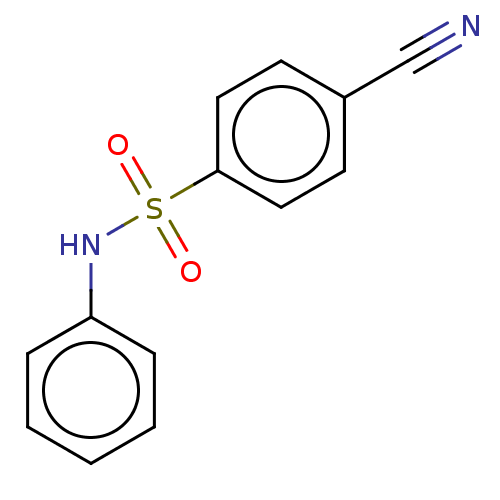
(CHEMBL4082237)Show InChI InChI=1S/C13H10N2O2S/c14-10-11-6-8-13(9-7-11)18(16,17)15-12-4-2-1-3-5-12/h1-9,15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MTHFD2 (unknown origin) using NAD/5,10-methylene THF as substrate after 30 mins by HPLC method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase 2, mitochondrial
(Homo sapiens) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MTHFD2L (unknown origin) using NAD/5,10-methylene THF as substrate after 2 hrs by HPLC method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
C-1-tetrahydrofolate synthase, cytoplasmic
(Homo sapiens (Human)) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MTHFD1 (unknown origin) using NADP/5,10-methylene THF as substrate after 30 mins by HPLC method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Serine hydroxymethyltransferase, cytosolic
(Homo sapiens (Human)) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SHMT1 (unknown origin) using serine/THF as substrate after 50 mins by HPLC method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15B
(Rattus norvegicus) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase (unknown origin) using dUMP/5,10-methylene THF as substrate after 1 hr by mass spectrometric method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data