

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasmepsin I (Plasmodium falciparum) | BDBM8007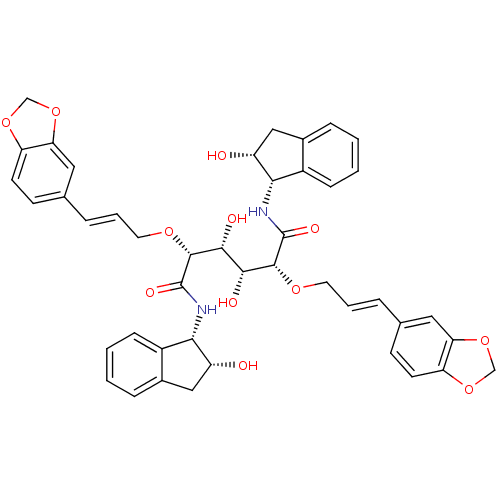 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(2H-1,3-benzodioxol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8005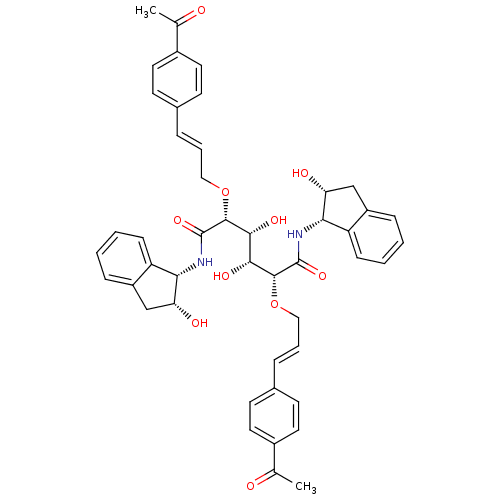 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1166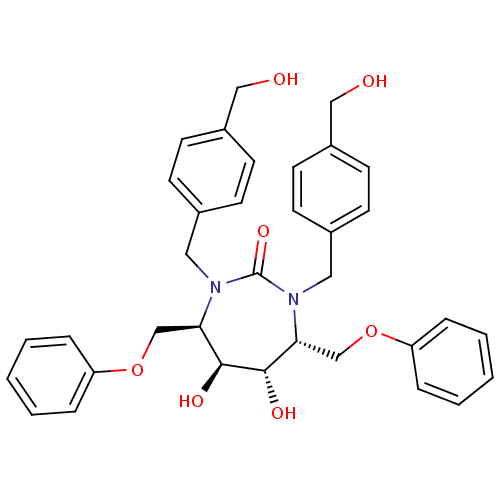 ((4R,5S,6S,7R)-1,3-Bis{[4-(hydroxymethyl)phenyl]met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-53.4 | 10 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM150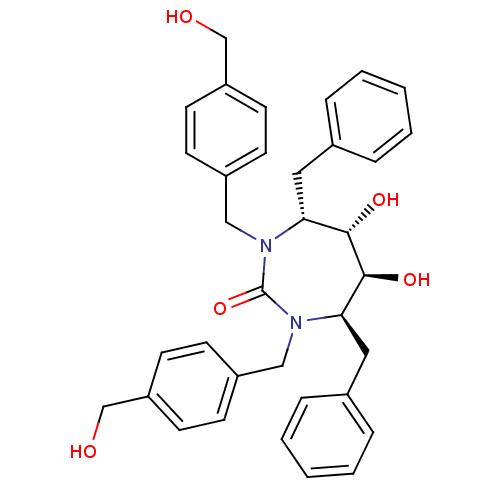 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <1 | <-53.4 | 15 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8008 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8002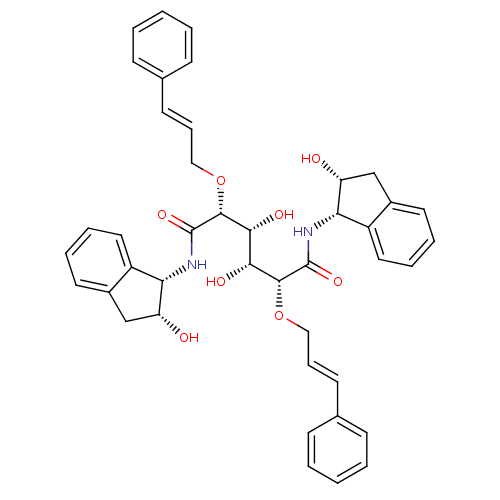 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8003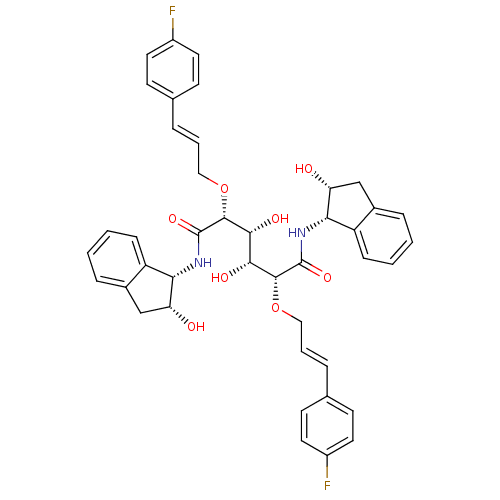 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-fluorophenyl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8015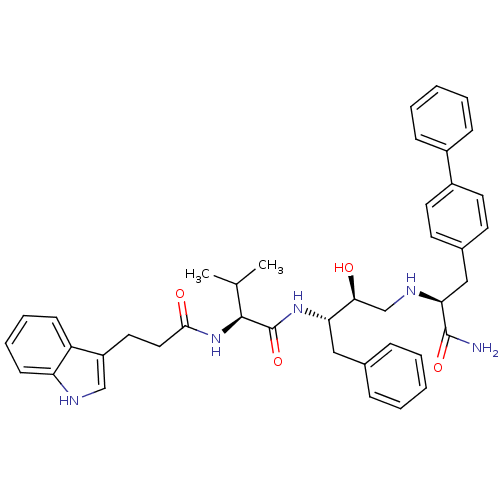 ((2S)-N-[(2S,3S)-4-{[(1S)-1-carbamoyl-2-(4-phenylph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM346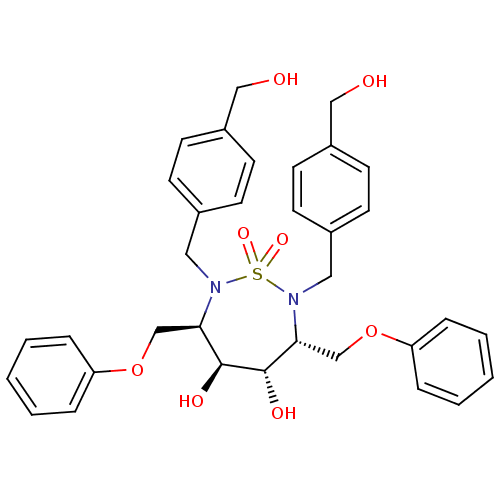 ((3R,4S,5S,6R)-4,5-dihydroxy-2,7-bis({[4-(hydroxyme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | -49.4 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | J Med Chem 44: 155-69 (2001) Article DOI: 10.1021/jm001024j BindingDB Entry DOI: 10.7270/Q2JM27T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM342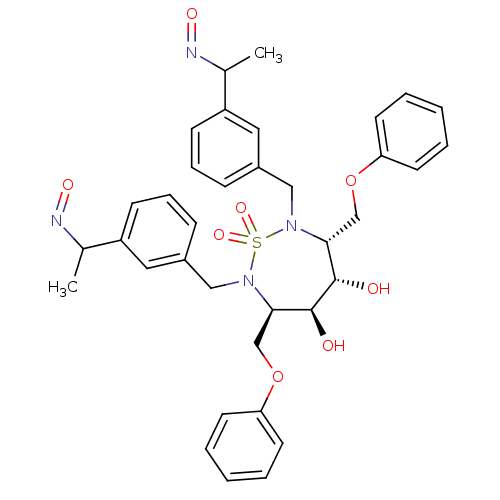 ((3R,4S,5S,6R)-4,5-dihydroxy-2,7-bis({3-[(1E)-1-(hy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | -49.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | J Med Chem 44: 155-69 (2001) Article DOI: 10.1021/jm001024j BindingDB Entry DOI: 10.7270/Q2JM27T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8011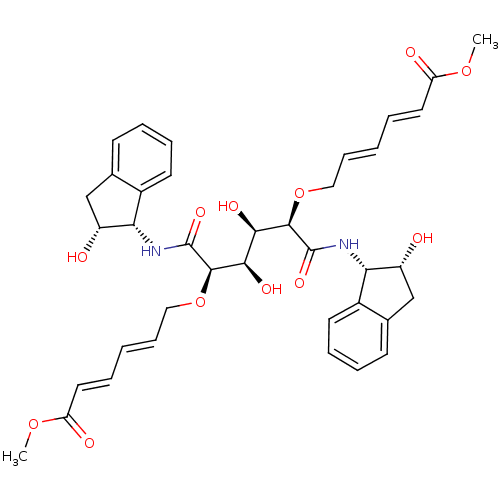 ((2R,3R,4R,5R)-N1,N6-Bis[(1S,2R)-2-hydroxy-1-indany...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8019 (2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8010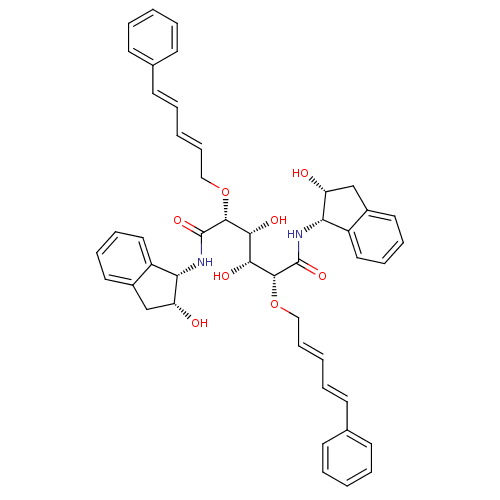 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8010 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8004 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8005 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8012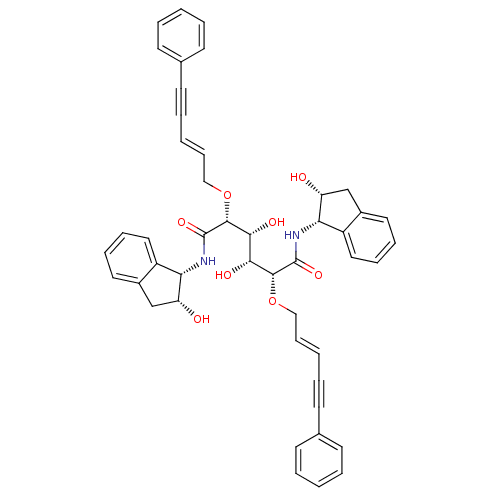 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM340 ((3R,4S,5S,6R)-3,6-Bis(phenoxymethyl)-4,5-dihydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 7.30 | -47.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | J Med Chem 44: 155-69 (2001) Article DOI: 10.1021/jm001024j BindingDB Entry DOI: 10.7270/Q2JM27T6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8006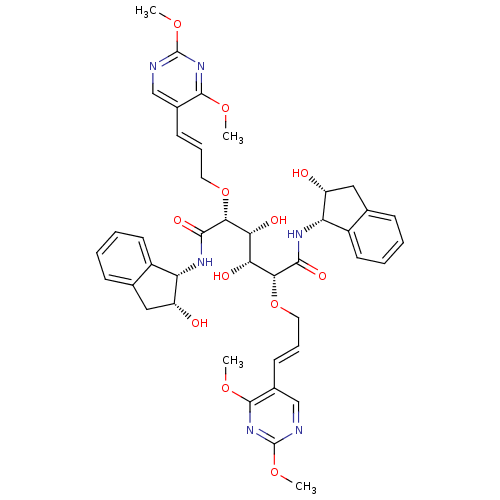 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(2,4-dimethoxypyrim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8011 ((2R,3R,4R,5R)-N1,N6-Bis[(1S,2R)-2-hydroxy-1-indany...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.90 | -45.5 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8015 ((2S)-N-[(2S,3S)-4-{[(1S)-1-carbamoyl-2-(4-phenylph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM335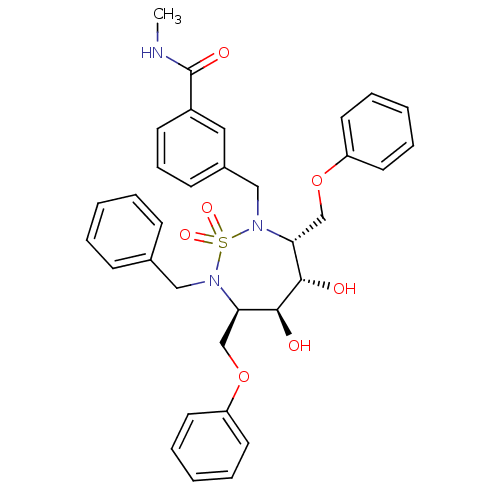 ((3R,4S,5S,6R)-3,6-Bis(phenoxymethyl)-4,5-dihydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 11 | -46.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | J Med Chem 44: 155-69 (2001) Article DOI: 10.1021/jm001024j BindingDB Entry DOI: 10.7270/Q2JM27T6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1157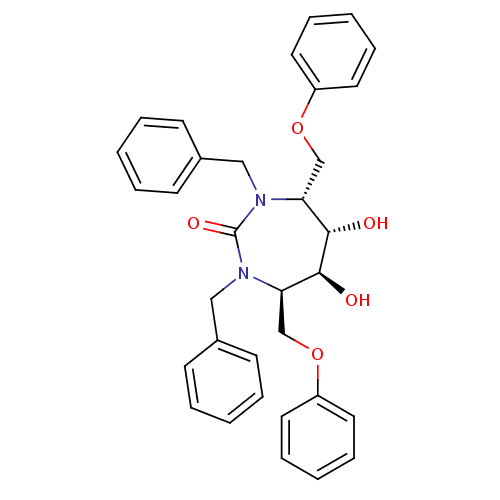 ((4R,5S,6S,7R)-1,3-Dibenzyl-4,7-bis(phenoxymethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 12.2 | -47.0 | 80 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8006 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(2,4-dimethoxypyrim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8007 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(2H-1,3-benzodioxol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1164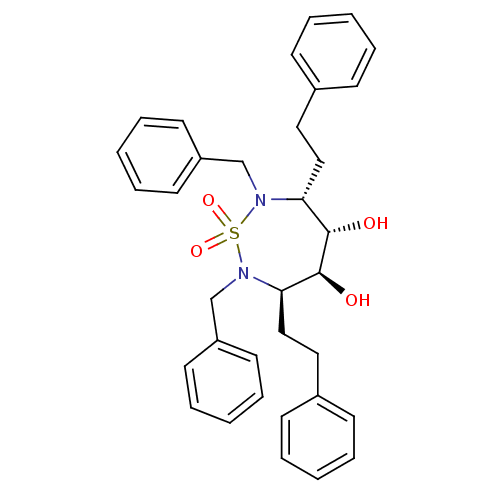 ((3R,4S,5S,6R)-2,7-Dibenzyl-3,6-bis(2-phenylethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14.7 | -46.5 | 200 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8010 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8008 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1163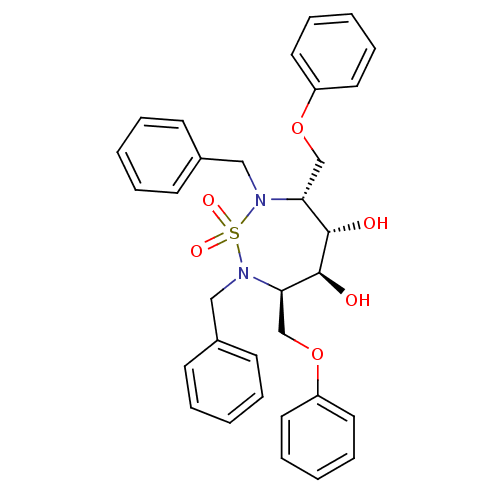 ((3R,4S,5S,6R)-2,7-Dibenzyl-3,6-bis(phenoxymethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 19.1 | -45.8 | 200 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Uppsala University | Assay Description The IC50 value is the inhibitor concentration that results in 50% of HIV-1 protease activity measured by a spectrophotometric assay using a chromopho... | J Med Chem 40: 885-97 (1997) Article DOI: 10.1021/jm960728j BindingDB Entry DOI: 10.7270/Q24B2ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1163 ((3R,4S,5S,6R)-2,7-Dibenzyl-3,6-bis(phenoxymethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 23 | -44.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | J Med Chem 44: 155-69 (2001) Article DOI: 10.1021/jm001024j BindingDB Entry DOI: 10.7270/Q2JM27T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8009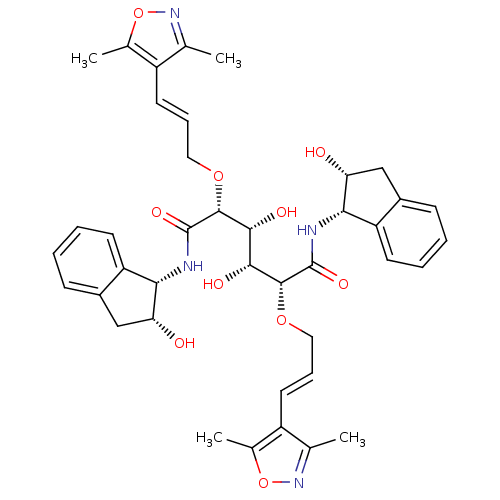 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(3,5-dimethyl-1,2-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM358 ((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N,N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8001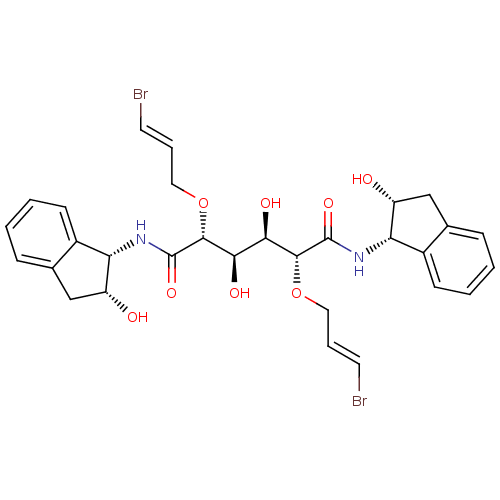 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-bromoprop-2-en-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8002 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | -42.6 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM8015 ((2S)-N-[(2S,3S)-4-{[(1S)-1-carbamoyl-2-(4-phenylph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8014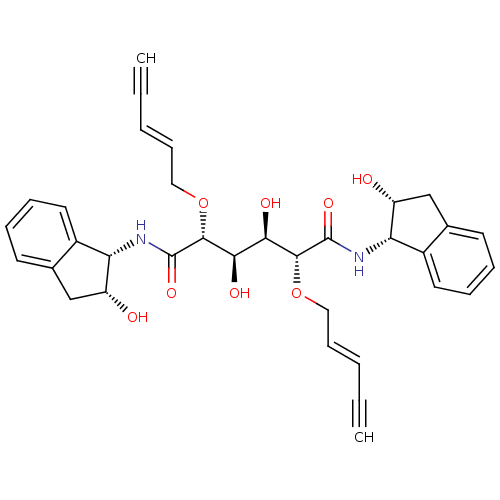 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8013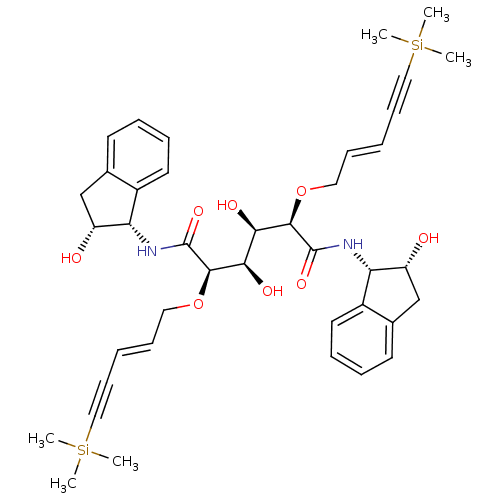 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8014 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM326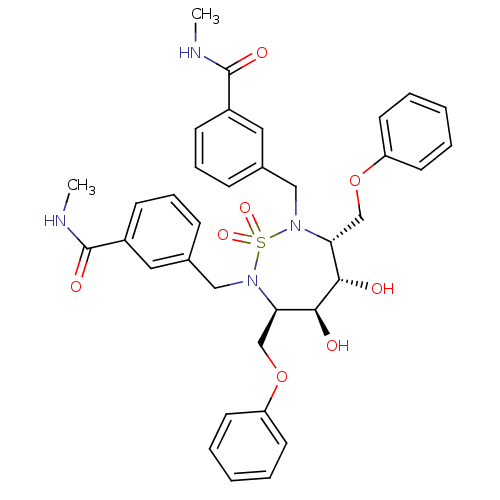 ((3R,4S,5S,6R)-2,7-Bis[3-(N-methylcarbamoyl)benzyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | -43.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | J Med Chem 44: 155-69 (2001) Article DOI: 10.1021/jm001024j BindingDB Entry DOI: 10.7270/Q2JM27T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8012 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM344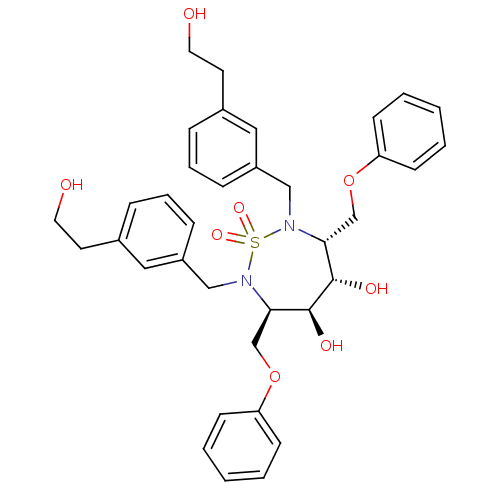 ((3R,4S,5S,6R)-4,5-dihydroxy-2,7-bis({[3-(2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | -42.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | J Med Chem 44: 155-69 (2001) Article DOI: 10.1021/jm001024j BindingDB Entry DOI: 10.7270/Q2JM27T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8001 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-bromoprop-2-en-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | -41.4 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8018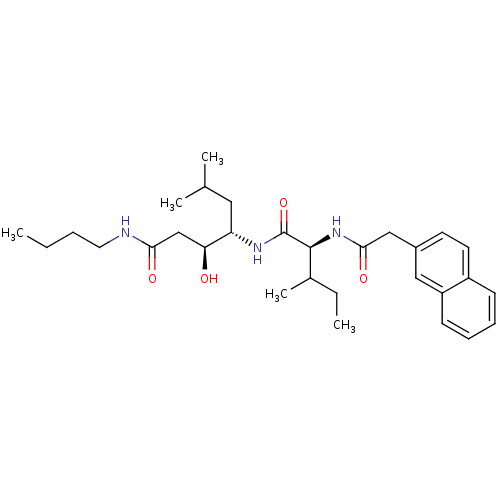 ((3S,4S)-N-butyl-3-hydroxy-6-methyl-4-[(2S,3S)-3-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8003 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-fluorophenyl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 59 | -40.8 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM343 ((3R,4S,5S,6R)-2,7-bis[(3-acetylphenyl)methyl]-4,5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | -42.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | J Med Chem 44: 155-69 (2001) Article DOI: 10.1021/jm001024j BindingDB Entry DOI: 10.7270/Q2JM27T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM333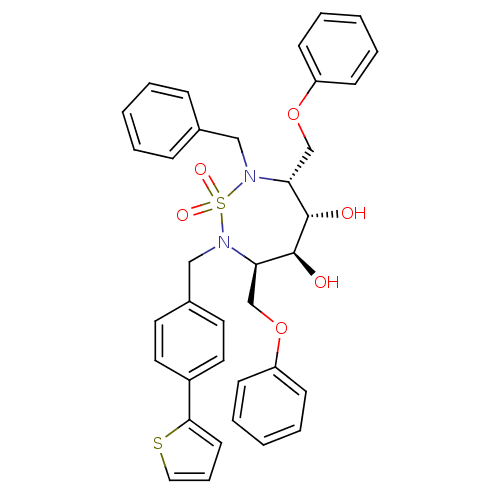 ((3R,4S,5S,6R)-2-benzyl-4,5-dihydroxy-3,6-bis(pheno...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 63 | -41.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | J Med Chem 44: 155-69 (2001) Article DOI: 10.1021/jm001024j BindingDB Entry DOI: 10.7270/Q2JM27T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM8019 (2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8028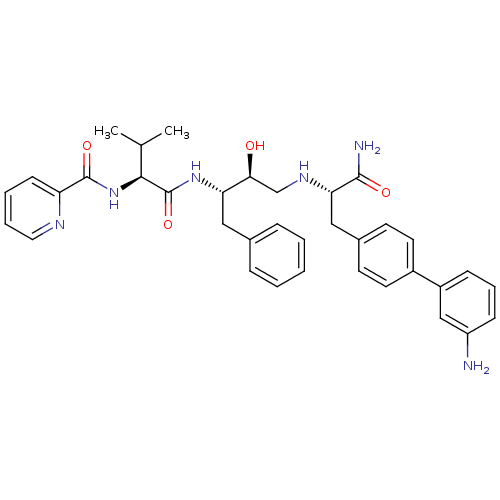 ((2S)-N-[(2S,3S)-4-{[(1S)-2-[4-(3-aminophenyl)pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 46: 734-46 (2003) Article DOI: 10.1021/jm020951i BindingDB Entry DOI: 10.7270/Q2N29V56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8026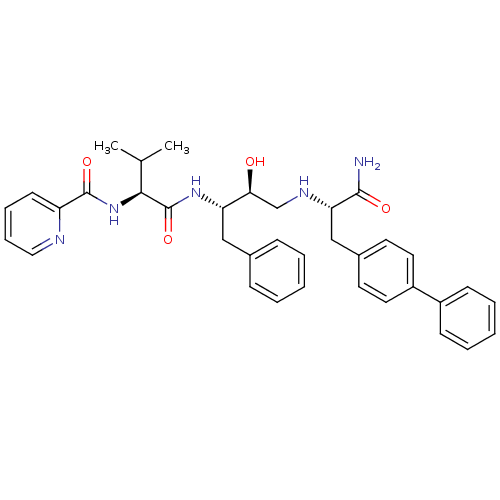 ((2S)-N-[(2S,3S)-4-{[(1S)-1-carbamoyl-2-(4-phenylph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 46: 734-46 (2003) Article DOI: 10.1021/jm020951i BindingDB Entry DOI: 10.7270/Q2N29V56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM347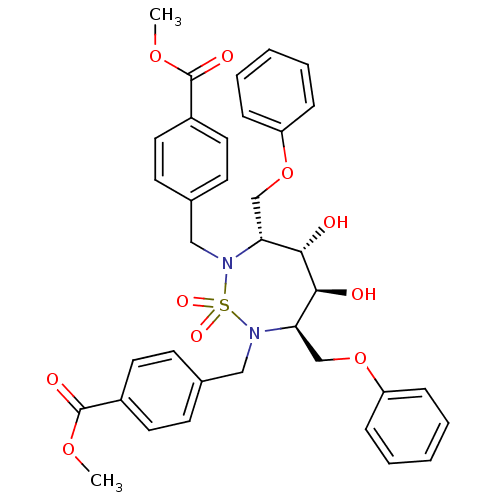 (CHEMBL133573 | Cyclic Sulfamide deriv. 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | -41.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | J Med Chem 44: 155-69 (2001) Article DOI: 10.1021/jm001024j BindingDB Entry DOI: 10.7270/Q2JM27T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 151 total ) | Next | Last >> |