 Found 360 hits with Last Name = 'humphreys' and Initial = 'd'
Found 360 hits with Last Name = 'humphreys' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118030
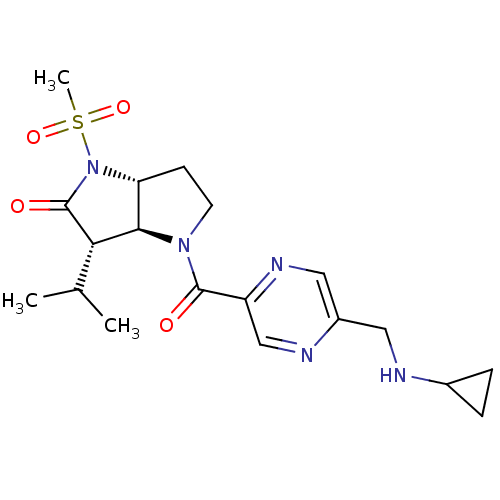
(4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...)Show SMILES CC(C)[C@H]1[C@H]2[C@@H](CCN2C(=O)c2cnc(CNC3CC3)cn2)N(C1=O)S(C)(=O)=O Show InChI InChI=1S/C19H27N5O4S/c1-11(2)16-17-15(24(19(16)26)29(3,27)28)6-7-23(17)18(25)14-10-21-13(9-22-14)8-20-12-4-5-12/h9-12,15-17,20H,4-8H2,1-3H3/t15-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) |
J Med Chem 45: 3878-90 (2002)
BindingDB Entry DOI: 10.7270/Q2HM596S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118028
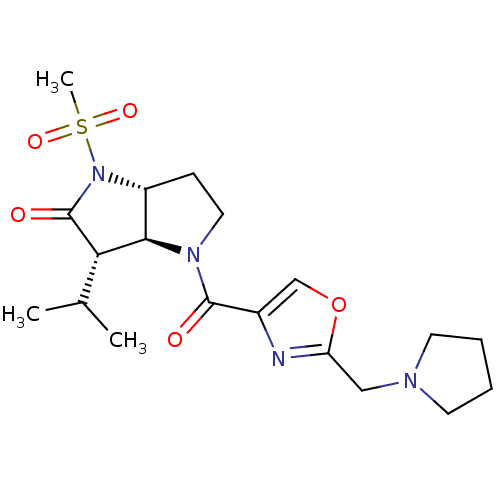
(3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...)Show SMILES CC(C)[C@H]1[C@H]2[C@@H](CCN2C(=O)c2coc(CN3CCCC3)n2)N(C1=O)S(C)(=O)=O Show InChI InChI=1S/C19H28N4O5S/c1-12(2)16-17-14(23(19(16)25)29(3,26)27)6-9-22(17)18(24)13-11-28-15(20-13)10-21-7-4-5-8-21/h11-12,14,16-17H,4-10H2,1-3H3/t14-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) |
J Med Chem 45: 3878-90 (2002)
BindingDB Entry DOI: 10.7270/Q2HM596S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118028

(3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...)Show SMILES CC(C)[C@H]1[C@H]2[C@@H](CCN2C(=O)c2coc(CN3CCCC3)n2)N(C1=O)S(C)(=O)=O Show InChI InChI=1S/C19H28N4O5S/c1-12(2)16-17-14(23(19(16)25)29(3,26)27)6-9-22(17)18(24)13-11-28-15(20-13)10-21-7-4-5-8-21/h11-12,14,16-17H,4-10H2,1-3H3/t14-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | 4.90 | 3.05E+4 | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay |
J Med Chem 45: 3878-90 (2002)
BindingDB Entry DOI: 10.7270/Q2HM596S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118029

(3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...)Show SMILES Cl.[H][C@@]12CCN(C(=O)\C=C\CN3CCCCC3)[C@@]1([H])[C@H](C(C)C)C(=O)N2S(C)(=O)=O |r| Show InChI InChI=1S/C19H31N3O4S/c1-14(2)17-18-15(22(19(17)24)27(3,25)26)9-13-21(18)16(23)8-7-12-20-10-5-4-6-11-20/h7-8,14-15,17-18H,4-6,9-13H2,1-3H3/b8-7+/t15-,17+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) |
J Med Chem 45: 3878-90 (2002)
BindingDB Entry DOI: 10.7270/Q2HM596S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118029

(3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...)Show SMILES Cl.[H][C@@]12CCN(C(=O)\C=C\CN3CCCCC3)[C@@]1([H])[C@H](C(C)C)C(=O)N2S(C)(=O)=O |r| Show InChI InChI=1S/C19H31N3O4S/c1-14(2)17-18-15(22(19(17)24)27(3,25)26)9-13-21(18)16(23)8-7-12-20-10-5-4-6-11-20/h7-8,14-15,17-18H,4-6,9-13H2,1-3H3/b8-7+/t15-,17+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | 0.00000207 | 6.63E+3 | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay |
J Med Chem 45: 3878-90 (2002)
BindingDB Entry DOI: 10.7270/Q2HM596S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118027
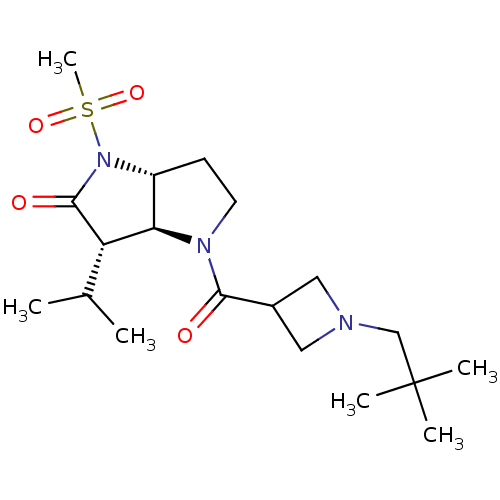
(4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...)Show SMILES CC(C)[C@H]1[C@H]2[C@@H](CCN2C(=O)C2CN(CC(C)(C)C)C2)N(C1=O)S(C)(=O)=O Show InChI InChI=1S/C19H33N3O4S/c1-12(2)15-16-14(22(18(15)24)27(6,25)26)7-8-21(16)17(23)13-9-20(10-13)11-19(3,4)5/h12-16H,7-11H2,1-6H3/t14-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) |
J Med Chem 45: 3878-90 (2002)
BindingDB Entry DOI: 10.7270/Q2HM596S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118027

(4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...)Show SMILES CC(C)[C@H]1[C@H]2[C@@H](CCN2C(=O)C2CN(CC(C)(C)C)C2)N(C1=O)S(C)(=O)=O Show InChI InChI=1S/C19H33N3O4S/c1-12(2)15-16-14(22(18(15)24)27(6,25)26)7-8-21(16)17(23)13-9-20(10-13)11-19(3,4)5/h12-16H,7-11H2,1-6H3/t14-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.73 | n/a | n/a | n/a | n/a | 0.0000109 | 6.31E+3 | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay |
J Med Chem 45: 3878-90 (2002)
BindingDB Entry DOI: 10.7270/Q2HM596S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50096484
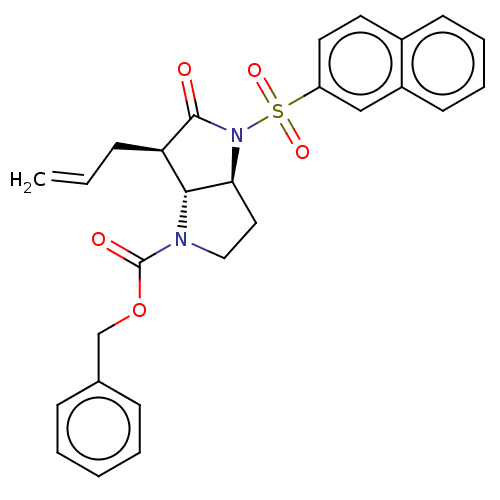
((4S,6R)-6-Allyl-4-(naphthalene-2-sulfonyl)-5-oxo-h...)Show SMILES [H][C@]12CCN(C(=O)OCc3ccccc3)[C@]1([H])[C@@H](CC=C)C(=O)N2S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C27H26N2O5S/c1-2-8-23-25-24(15-16-28(25)27(31)34-18-19-9-4-3-5-10-19)29(26(23)30)35(32,33)22-14-13-20-11-6-7-12-21(20)17-22/h2-7,9-14,17,23-25H,1,8,15-16,18H2/t23-,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human neutrophil elastase (HNE) |
Bioorg Med Chem Lett 11: 243-6 (2001)
BindingDB Entry DOI: 10.7270/Q2K074SH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50354851
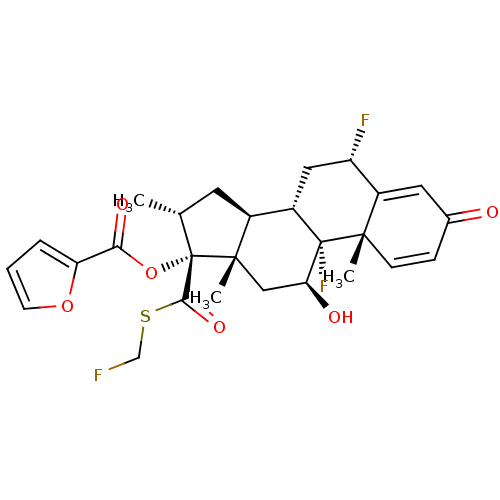
(FLUTICASONE FUROATE | Veramyst)Show SMILES C[C@@H]1C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(OC(=O)c1ccco1)C(=O)SCF |r,c:12,t:8| Show InChI InChI=1S/C27H29F3O6S/c1-14-9-16-17-11-19(29)18-10-15(31)6-7-24(18,2)26(17,30)21(32)12-25(16,3)27(14,23(34)37-13-28)36-22(33)20-5-4-8-35-20/h4-8,10,14,16-17,19,21,32H,9,11-13H2,1-3H3/t14-,16+,17+,19+,21+,24+,25+,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50066997
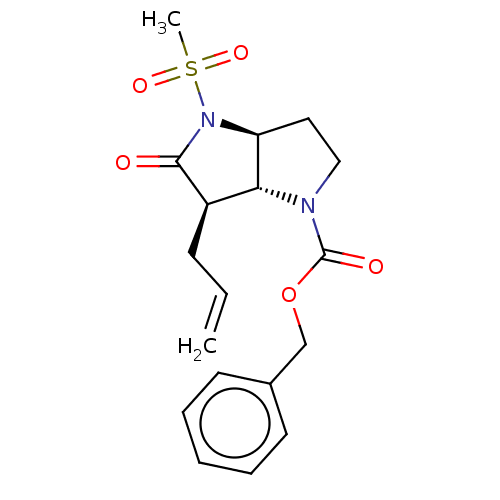
((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...)Show SMILES [H][C@]12CCN(C(=O)OCc3ccccc3)[C@]1([H])[C@@H](CC=C)C(=O)N2S(C)(=O)=O Show InChI InChI=1S/C18H22N2O5S/c1-3-7-14-16-15(20(17(14)21)26(2,23)24)10-11-19(16)18(22)25-12-13-8-5-4-6-9-13/h3-6,8-9,14-16H,1,7,10-12H2,2H3/t14-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human neutrophil elastase (HNE) |
Bioorg Med Chem Lett 11: 243-6 (2001)
BindingDB Entry DOI: 10.7270/Q2K074SH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414604

(CHEMBL551816)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H22Cl2F4N4O2/c1-2-35(24(37)23-19(27)5-3-6-20(23)28)15-25(38,26(30,31)32)14-33-21-7-4-8-22-18(21)13-34-36(22)17-11-9-16(29)10-12-17/h3-13,33,38H,2,14-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414609

(CHEMBL550730)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1F Show InChI InChI=1S/C26H23F5N4O2/c1-2-34(24(36)19-6-3-4-7-21(19)28)16-25(37,26(29,30)31)15-32-22-8-5-9-23-20(22)14-33-35(23)18-12-10-17(27)11-13-18/h3-14,32,37H,2,15-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414610
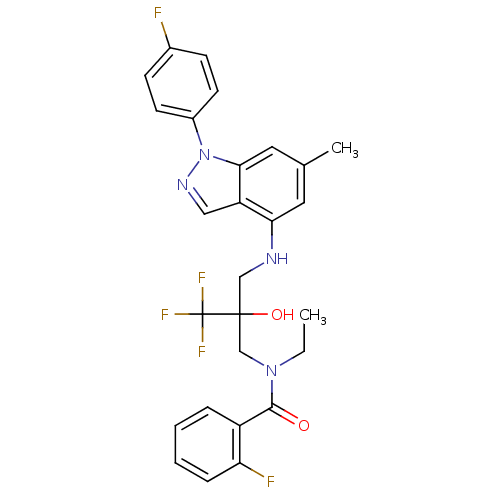
(CHEMBL564160)Show SMILES CCN(CC(O)(CNc1cc(C)cc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1F Show InChI InChI=1S/C27H25F5N4O2/c1-3-35(25(37)20-6-4-5-7-22(20)29)16-26(38,27(30,31)32)15-33-23-12-17(2)13-24-21(23)14-34-36(24)19-10-8-18(28)9-11-19/h4-14,33,38H,3,15-16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414607
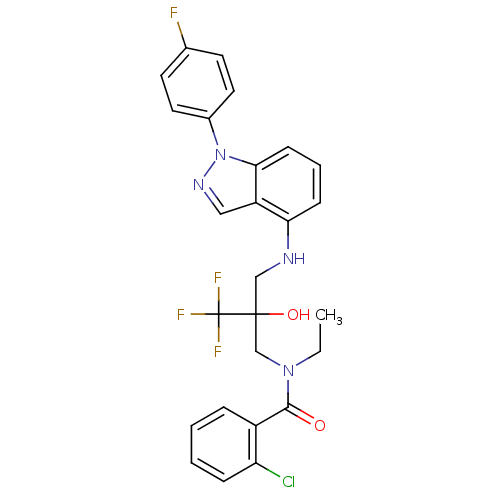
(CHEMBL563812)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1Cl Show InChI InChI=1S/C26H23ClF4N4O2/c1-2-34(24(36)19-6-3-4-7-21(19)27)16-25(37,26(29,30)31)15-32-22-8-5-9-23-20(22)14-33-35(23)18-12-10-17(28)11-13-18/h3-14,32,37H,2,15-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414611
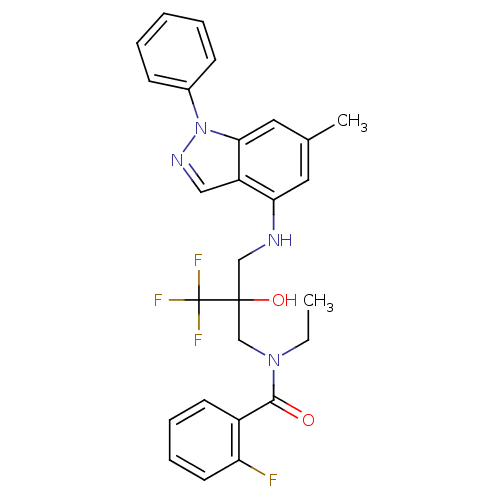
(CHEMBL560797)Show SMILES CCN(CC(O)(CNc1cc(C)cc2n(ncc12)-c1ccccc1)C(F)(F)F)C(=O)c1ccccc1F Show InChI InChI=1S/C27H26F4N4O2/c1-3-34(25(36)20-11-7-8-12-22(20)28)17-26(37,27(29,30)31)16-32-23-13-18(2)14-24-21(23)15-33-35(24)19-9-5-4-6-10-19/h4-15,32,37H,3,16-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414608

(CHEMBL550933)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1C Show InChI InChI=1S/C27H26F4N4O2/c1-3-34(25(36)21-8-5-4-7-18(21)2)17-26(37,27(29,30)31)16-32-23-9-6-10-24-22(23)15-33-35(24)20-13-11-19(28)12-14-20/h4-15,32,37H,3,16-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414612
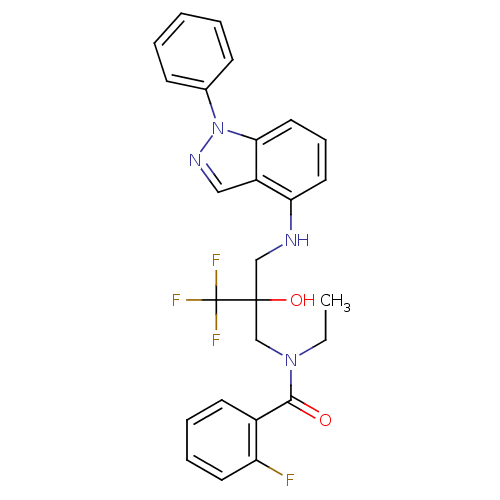
(CHEMBL559115)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccccc1)C(F)(F)F)C(=O)c1ccccc1F Show InChI InChI=1S/C26H24F4N4O2/c1-2-33(24(35)19-11-6-7-12-21(19)27)17-25(36,26(28,29)30)16-31-22-13-8-14-23-20(22)15-32-34(23)18-9-4-3-5-10-18/h3-15,31,36H,2,16-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414605
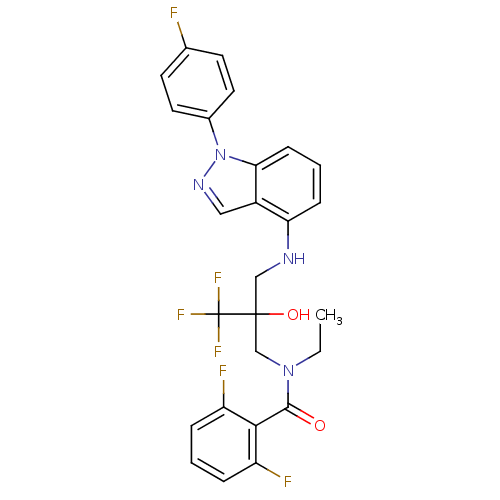
(CHEMBL556231)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1c(F)cccc1F Show InChI InChI=1S/C26H22F6N4O2/c1-2-35(24(37)23-19(28)5-3-6-20(23)29)15-25(38,26(30,31)32)14-33-21-7-4-8-22-18(21)13-34-36(22)17-11-9-16(27)10-12-17/h3-13,33,38H,2,14-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414613
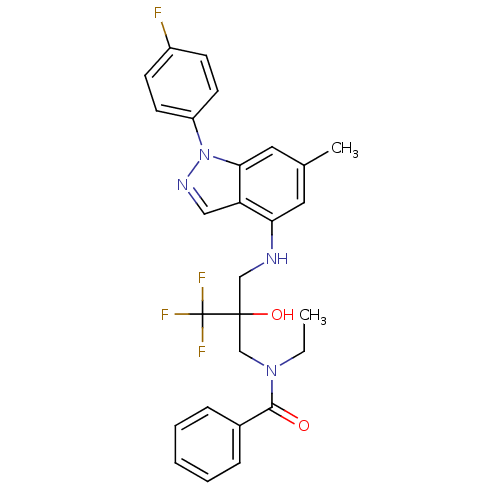
(CHEMBL561475)Show SMILES CCN(CC(O)(CNc1cc(C)cc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1 Show InChI InChI=1S/C27H26F4N4O2/c1-3-34(25(36)19-7-5-4-6-8-19)17-26(37,27(29,30)31)16-32-23-13-18(2)14-24-22(23)15-33-35(24)21-11-9-20(28)10-12-21/h4-15,32,37H,3,16-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414615

(CHEMBL559942)Show SMILES CCN(CC(O)(CNc1cc(C)cc2n(ncc12)-c1ccccc1)C(F)(F)F)C(=O)c1ccccc1 Show InChI InChI=1S/C27H27F3N4O2/c1-3-33(25(35)20-10-6-4-7-11-20)18-26(36,27(28,29)30)17-31-23-14-19(2)15-24-22(23)16-32-34(24)21-12-8-5-9-13-21/h4-16,31,36H,3,17-18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50066999

((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...)Show SMILES CCC[C@@H](NC(=O)N1[C@@H](Oc2ccc(cc2)C(=O)N2CCN(C)CC2)C(CC)(CC)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C31H40N4O6/c1-5-8-24(22-11-14-25-26(19-22)40-20-39-25)32-30(38)35-28(37)31(6-2,7-3)29(35)41-23-12-9-21(10-13-23)27(36)34-17-15-33(4)16-18-34/h9-14,19,24,29H,5-8,15-18,20H2,1-4H3,(H,32,38)/t24-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 |
Bioorg Med Chem Lett 11: 243-6 (2001)
BindingDB Entry DOI: 10.7270/Q2K074SH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414606
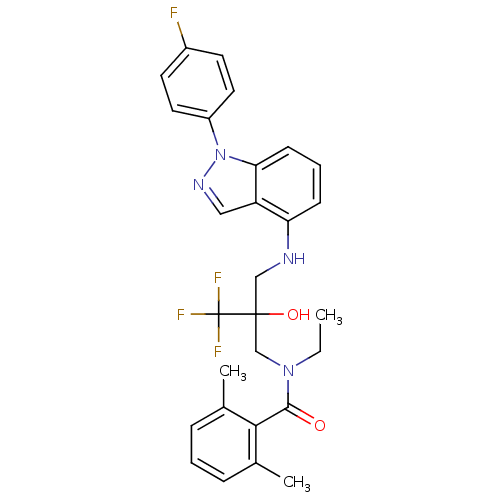
(CHEMBL550258)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C28H28F4N4O2/c1-4-35(26(37)25-18(2)7-5-8-19(25)3)17-27(38,28(30,31)32)16-33-23-9-6-10-24-22(23)15-34-36(24)21-13-11-20(29)12-14-21/h5-15,33,38H,4,16-17H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50096486
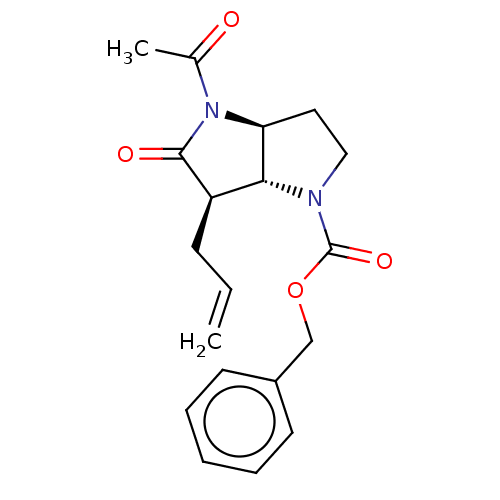
(CHEMBL2367646 | benzyl (3aS,6aR)-4-acetyl-6-allyl-...)Show SMILES [H][C@]12CCN(C(=O)OCc3ccccc3)[C@]1([H])[C@@H](CC=C)C(=O)N2C(C)=O Show InChI InChI=1S/C19H22N2O4/c1-3-7-15-17-16(21(13(2)22)18(15)23)10-11-20(17)19(24)25-12-14-8-5-4-6-9-14/h3-6,8-9,15-17H,1,7,10-12H2,2H3/t15-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human neutrophil elastase (HNE) |
Bioorg Med Chem Lett 11: 243-6 (2001)
BindingDB Entry DOI: 10.7270/Q2K074SH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414618

(CHEMBL563595)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccccc1)C(F)(F)F)C(=O)c1ccccc1 Show InChI InChI=1S/C26H25F3N4O2/c1-2-32(24(34)19-10-5-3-6-11-19)18-25(35,26(27,28)29)17-30-22-14-9-15-23-21(22)16-31-33(23)20-12-7-4-8-13-20/h3-16,30,35H,2,17-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414614
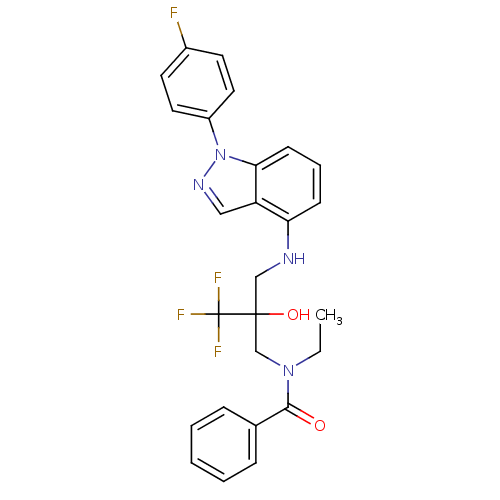
(CHEMBL561276)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1 Show InChI InChI=1S/C26H24F4N4O2/c1-2-33(24(35)18-7-4-3-5-8-18)17-25(36,26(28,29)30)16-31-22-9-6-10-23-21(22)15-32-34(23)20-13-11-19(27)12-14-20/h3-15,31,36H,2,16-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50096488
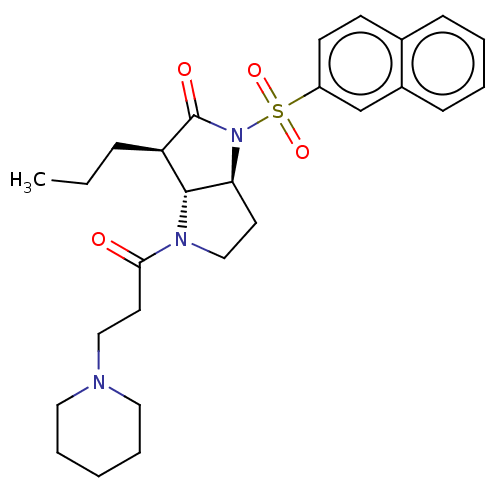
((1S,3R)-1-(Naphthalene-2-sulfonyl)-4-(3-piperidin-...)Show SMILES Cl.[H][C@]12CCN(C(=O)CCN3CCCCC3)[C@]1([H])[C@@H](CCC)C(=O)N2S(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C27H35N3O4S.ClH/c1-2-8-23-26-24(13-18-29(26)25(31)14-17-28-15-6-3-7-16-28)30(27(23)32)35(33,34)22-12-11-20-9-4-5-10-21(20)19-22;/h4-5,9-12,19,23-24,26H,2-3,6-8,13-18H2,1H3;1H/t23-,24+,26-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D4-like receptor labelled with [3H]YM-09151-2 in retina |
Bioorg Med Chem Lett 11: 243-6 (2001)
BindingDB Entry DOI: 10.7270/Q2K074SH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 17: 4737-45 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.066
BindingDB Entry DOI: 10.7270/Q2KD206G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50096488

((1S,3R)-1-(Naphthalene-2-sulfonyl)-4-(3-piperidin-...)Show SMILES Cl.[H][C@]12CCN(C(=O)CCN3CCCCC3)[C@]1([H])[C@@H](CCC)C(=O)N2S(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C27H35N3O4S.ClH/c1-2-8-23-26-24(13-18-29(26)25(31)14-17-28-15-6-3-7-16-28)30(27(23)32)35(33,34)22-12-11-20-9-4-5-10-21(20)19-22;/h4-5,9-12,19,23-24,26H,2-3,6-8,13-18H2,1H3;1H/t23-,24+,26-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human neutrophil elastase (HNE) |
Bioorg Med Chem Lett 11: 243-6 (2001)
BindingDB Entry DOI: 10.7270/Q2K074SH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
J Med Chem 50: 6519-34 (2007)
Article DOI: 10.1021/jm070778w
BindingDB Entry DOI: 10.7270/Q21R6RRR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411047
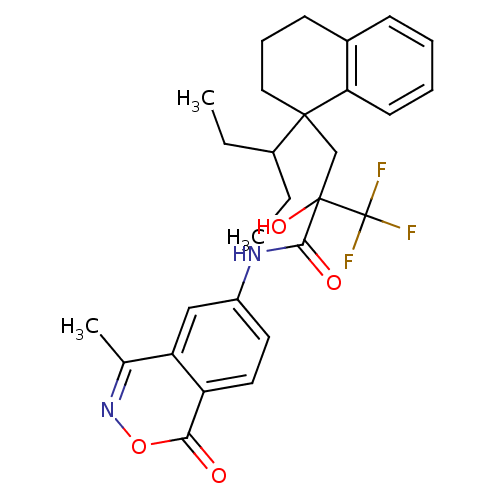
(CHEMBL211441)Show SMILES CCC(CC)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C28H31F3N2O4/c1-4-19(5-2)26(14-8-10-18-9-6-7-11-23(18)26)16-27(36,28(29,30)31)25(35)32-20-12-13-21-22(15-20)17(3)33-37-24(21)34/h6-7,9,11-13,15,19,36H,4-5,8,10,14,16H2,1-3H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
J Med Chem 50: 6519-34 (2007)
Article DOI: 10.1021/jm070778w
BindingDB Entry DOI: 10.7270/Q21R6RRR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411047

(CHEMBL211441)Show SMILES CCC(CC)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C28H31F3N2O4/c1-4-19(5-2)26(14-8-10-18-9-6-7-11-23(18)26)16-27(36,28(29,30)31)25(35)32-20-12-13-21-22(15-20)17(3)33-37-24(21)34/h6-7,9,11-13,15,19,36H,4-5,8,10,14,16H2,1-3H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414623

(CHEMBL394168)Show SMILES CCC1(CC(O)(CNC(=O)c2cnn(c2N)-c2ccccc2)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H29F3N4O2/c1-2-24(14-8-10-18-9-6-7-13-21(18)24)16-25(35,26(27,28)29)17-31-23(34)20-15-32-33(22(20)30)19-11-4-3-5-12-19/h3-7,9,11-13,15,35H,2,8,10,14,16-17,30H2,1H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411480

(CHEMBL398572)Show SMILES CCC1(CC(O)(Cc2ccnc3ccccc23)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C25H26F3NO/c1-2-23(14-7-9-18-8-3-5-11-21(18)23)17-24(30,25(26,27)28)16-19-13-15-29-22-12-6-4-10-20(19)22/h3-6,8,10-13,15,30H,2,7,9,14,16-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
J Med Chem 50: 6519-34 (2007)
Article DOI: 10.1021/jm070778w
BindingDB Entry DOI: 10.7270/Q21R6RRR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414623

(CHEMBL394168)Show SMILES CCC1(CC(O)(CNC(=O)c2cnn(c2N)-c2ccccc2)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H29F3N4O2/c1-2-24(14-8-10-18-9-6-7-13-21(18)24)16-25(35,26(27,28)29)17-31-23(34)20-15-32-33(22(20)30)19-11-4-3-5-12-19/h3-7,9,11-13,15,35H,2,8,10,14,16-17,30H2,1H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 17: 4737-45 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.066
BindingDB Entry DOI: 10.7270/Q2KD206G |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411489
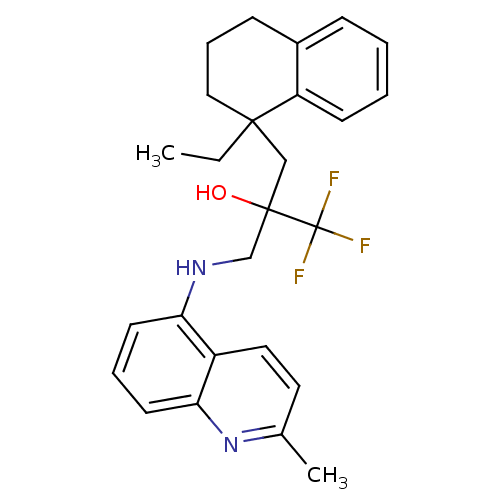
(CHEMBL238147)Show SMILES CCC1(CC(O)(CNc2cccc3nc(C)ccc23)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H29F3N2O/c1-3-24(15-7-9-19-8-4-5-10-21(19)24)16-25(32,26(27,28)29)17-30-22-11-6-12-23-20(22)14-13-18(2)31-23/h4-6,8,10-14,30,32H,3,7,9,15-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
J Med Chem 50: 6519-34 (2007)
Article DOI: 10.1021/jm070778w
BindingDB Entry DOI: 10.7270/Q21R6RRR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411024

(CHEMBL208996)Show SMILES Cc1noc(=O)c2ccc(NC(=O)[C@](O)(C[C@]3(CCCc4ccccc34)C3CCCC3)C(F)(F)F)cc12 Show InChI InChI=1S/C28H29F3N2O4/c1-17-22-15-20(12-13-21(22)24(34)37-33-17)32-25(35)27(36,28(29,30)31)16-26(19-9-3-4-10-19)14-6-8-18-7-2-5-11-23(18)26/h2,5,7,11-13,15,19,36H,3-4,6,8-10,14,16H2,1H3,(H,32,35)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50096487

((3R,6aS)-3-Allyl-2-oxo-hexahydro-pyrrolo[3,2-b]pyr...)Show SMILES [H][C@]12CCN(C(=O)OCc3ccccc3)[C@]1([H])[C@@H](CC=C)C(=O)N2C(=O)OCC Show InChI InChI=1S/C20H24N2O5/c1-3-8-15-17-16(22(18(15)23)20(25)26-4-2)11-12-21(17)19(24)27-13-14-9-6-5-7-10-14/h3,5-7,9-10,15-17H,1,4,8,11-13H2,2H3/t15-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human neutrophil elastase (HNE) |
Bioorg Med Chem Lett 11: 243-6 (2001)
BindingDB Entry DOI: 10.7270/Q2K074SH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411024

(CHEMBL208996)Show SMILES Cc1noc(=O)c2ccc(NC(=O)[C@](O)(C[C@]3(CCCc4ccccc34)C3CCCC3)C(F)(F)F)cc12 Show InChI InChI=1S/C28H29F3N2O4/c1-17-22-15-20(12-13-21(22)24(34)37-33-17)32-25(35)27(36,28(29,30)31)16-26(19-9-3-4-10-19)14-6-8-18-7-2-5-11-23(18)26/h2,5,7,11-13,15,19,36H,3-4,6,8-10,14,16H2,1H3,(H,32,35)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
J Med Chem 50: 6519-34 (2007)
Article DOI: 10.1021/jm070778w
BindingDB Entry DOI: 10.7270/Q21R6RRR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411487

(CHEMBL236229)Show SMILES CCC1(CC(O)(CNc2cccc3cnccc23)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C25H27F3N2O/c1-2-23(13-6-9-18-7-3-4-10-21(18)23)16-24(31,25(26,27)28)17-30-22-11-5-8-19-15-29-14-12-20(19)22/h3-5,7-8,10-12,14-15,30-31H,2,6,9,13,16-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
J Med Chem 50: 6519-34 (2007)
Article DOI: 10.1021/jm070778w
BindingDB Entry DOI: 10.7270/Q21R6RRR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411487

(CHEMBL236229)Show SMILES CCC1(CC(O)(CNc2cccc3cnccc23)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C25H27F3N2O/c1-2-23(13-6-9-18-7-3-4-10-21(18)23)16-24(31,25(26,27)28)17-30-22-11-5-8-19-15-29-14-12-20(19)22/h3-5,7-8,10-12,14-15,30-31H,2,6,9,13,16-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
J Med Chem 50: 6519-34 (2007)
Article DOI: 10.1021/jm070778w
BindingDB Entry DOI: 10.7270/Q21R6RRR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414619
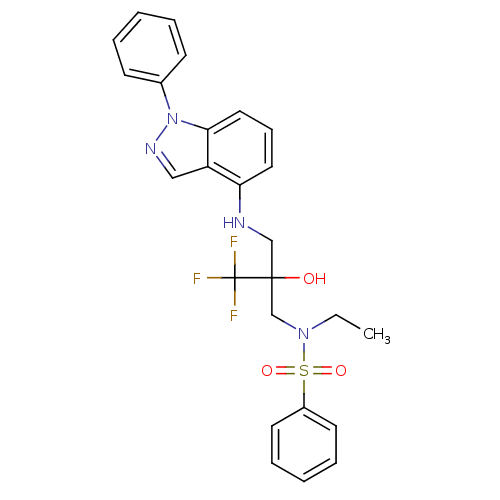
(CHEMBL559547)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccccc1)C(F)(F)F)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C25H25F3N4O3S/c1-2-31(36(34,35)20-12-7-4-8-13-20)18-24(33,25(26,27)28)17-29-22-14-9-15-23-21(22)16-30-32(23)19-10-5-3-6-11-19/h3-16,29,33H,2,17-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50423192
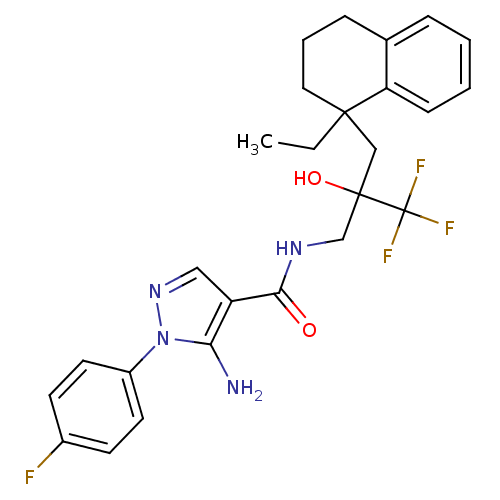
(CHEMBL237973)Show SMILES CCC1(CC(O)(CNC(=O)c2cnn(c2N)-c2ccc(F)cc2)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H28F4N4O2/c1-2-24(13-5-7-17-6-3-4-8-21(17)24)15-25(36,26(28,29)30)16-32-23(35)20-14-33-34(22(20)31)19-11-9-18(27)10-12-19/h3-4,6,8-12,14,36H,2,5,7,13,15-16,31H2,1H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 17: 4737-45 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.066
BindingDB Entry DOI: 10.7270/Q2KD206G |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50423192

(CHEMBL237973)Show SMILES CCC1(CC(O)(CNC(=O)c2cnn(c2N)-c2ccc(F)cc2)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H28F4N4O2/c1-2-24(13-5-7-17-6-3-4-8-21(17)24)15-25(36,26(28,29)30)16-32-23(35)20-14-33-34(22(20)31)19-11-9-18(27)10-12-19/h3-4,6,8-12,14,36H,2,5,7,13,15-16,31H2,1H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 17: 4737-45 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.066
BindingDB Entry DOI: 10.7270/Q2KD206G |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50096483

((3R,6aS)-1-Methanesulfonyl-4-(3-piperidin-1-yl-pro...)Show SMILES Cl.[H][C@]12CCN(C(=O)CCN3CCCCC3)[C@]1([H])[C@@H](CCC)C(=O)N2S(C)(=O)=O |r| Show InChI InChI=1S/C18H31N3O4S.ClH/c1-3-7-14-17-15(21(18(14)23)26(2,24)25)8-13-20(17)16(22)9-12-19-10-5-4-6-11-19;/h14-15,17H,3-13H2,1-2H3;1H/t14-,15+,17-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 uM |
Bioorg Med Chem Lett 11: 243-6 (2001)
BindingDB Entry DOI: 10.7270/Q2K074SH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411026
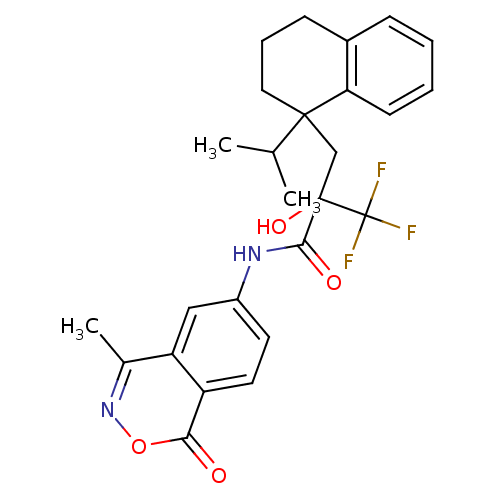
(CHEMBL214336)Show SMILES CC(C)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H27F3N2O4/c1-15(2)24(12-6-8-17-7-4-5-9-21(17)24)14-25(34,26(27,28)29)23(33)30-18-10-11-19-20(13-18)16(3)31-35-22(19)32/h4-5,7,9-11,13,15,34H,6,8,12,14H2,1-3H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411477

(CHEMBL393551)Show SMILES CC1(CC(O)(CNc2cccc3ncccc23)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C24H25F3N2O/c1-22(13-5-8-17-7-2-3-10-19(17)22)15-23(30,24(25,26)27)16-29-21-12-4-11-20-18(21)9-6-14-28-20/h2-4,6-7,9-12,14,29-30H,5,8,13,15-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.09 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
J Med Chem 50: 6519-34 (2007)
Article DOI: 10.1021/jm070778w
BindingDB Entry DOI: 10.7270/Q21R6RRR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411039

(CHEMBL385450)Show SMILES CCCCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C27H29F3N2O4/c1-3-4-13-25(14-7-9-18-8-5-6-10-22(18)25)16-26(35,27(28,29)30)24(34)31-19-11-12-20-21(15-19)17(2)32-36-23(20)33/h5-6,8,10-12,15,35H,3-4,7,9,13-14,16H2,1-2H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414616

(CHEMBL558716)Show SMILES CCCN(CC(O)(CNc1cccc2n(ncc12)-c1ccccc1)C(F)(F)F)C(=O)c1ccccc1 Show InChI InChI=1S/C27H27F3N4O2/c1-2-16-33(25(35)20-10-5-3-6-11-20)19-26(36,27(28,29)30)18-31-23-14-9-15-24-22(23)17-32-34(24)21-12-7-4-8-13-21/h3-15,17,31,36H,2,16,18-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data