 Found 749 hits with Last Name = 'hunter' and Initial = 'j'
Found 749 hits with Last Name = 'hunter' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281737
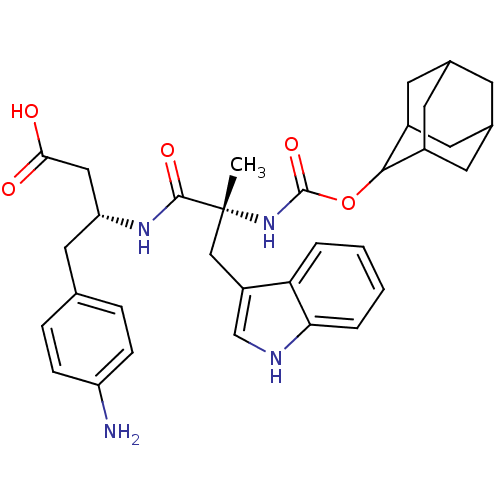
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N)cc1 |wU:1.0,wD:1.13,29.33,TLB:22:17:25:21.23.20,22:21:16.17.18:25,15:16:21.23.22:19.18.25,THB:20:21:16:19.18.25,20:19:16:21.23.22,15:16:25:21.23.20,(3.41,-9.92,;4.74,-10.71,;6.07,-9.95,;6.09,-8.41,;5.19,-7.17,;6.1,-5.93,;7.57,-6.4,;8.89,-5.63,;10.23,-6.4,;10.23,-7.94,;8.89,-8.71,;7.56,-7.94,;3.39,-11.46,;2.06,-10.69,;2.08,-9.15,;.73,-11.44,;-.81,-11.44,;-.62,-13,;-2,-13.53,;-3.42,-13.21,;-4.47,-14.66,;-3,-14.03,;-1.49,-14.43,;-3.21,-12.42,;-2.3,-11.04,;-3.61,-11.69,;6.07,-11.48,;6.05,-13.02,;7.4,-10.71,;8.73,-11.48,;8.73,-13.02,;10.06,-13.79,;10.06,-15.33,;8.73,-14.54,;10.06,-10.71,;11.39,-11.48,;11.39,-13.02,;12.72,-13.79,;14.05,-13.02,;15.38,-13.79,;14.05,-11.46,;12.72,-10.71,)| Show InChI InChI=1S/C33H40N4O5/c1-33(17-24-18-35-28-5-3-2-4-27(24)28,31(40)36-26(16-29(38)39)15-19-6-8-25(34)9-7-19)37-32(41)42-30-22-11-20-10-21(13-22)14-23(30)12-20/h2-9,18,20-23,26,30,35H,10-17,34H2,1H3,(H,36,40)(H,37,41)(H,38,39)/t20?,21?,22?,23?,26-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of binding of [3H]-PD 140376 to Cholecystokinin type B receptor in mouse cortex. |
Bioorg Med Chem Lett 3: 885-888 (1993)
Article DOI: 10.1016/S0960-894X(00)80686-1
BindingDB Entry DOI: 10.7270/Q2D79BWZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50008856
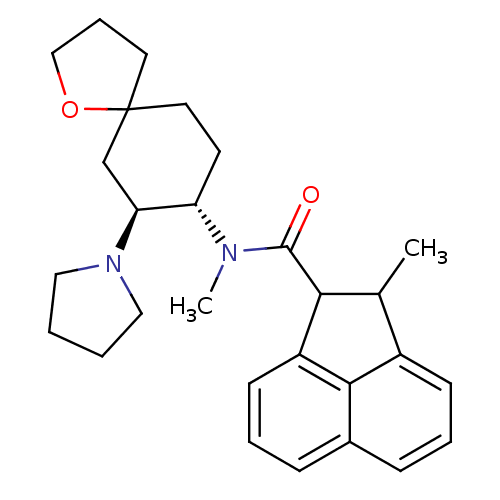
(2-Methyl-acenaphthene-1-carboxylic acid methyl-(7-...)Show SMILES CC1C(C(=O)N(C)[C@H]2CCC3(CCCO3)C[C@@H]2N2CCCC2)c2cccc3cccc1c23 Show InChI InChI=1S/C28H36N2O2/c1-19-21-10-5-8-20-9-6-11-22(26(20)21)25(19)27(31)29(2)23-12-14-28(13-7-17-32-28)18-24(23)30-15-3-4-16-30/h5-6,8-11,19,23-25H,3-4,7,12-18H2,1-2H3/t19?,23-,24-,25?,28?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Opioid receptor kappa affinity, determined with [3H]etorphine in the presence of excess unlabeled [D-Ala2-MePhe4-Glyol5]-enkephalin |
J Med Chem 34: 190-4 (1991)
BindingDB Entry DOI: 10.7270/Q2J103SG |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50285623
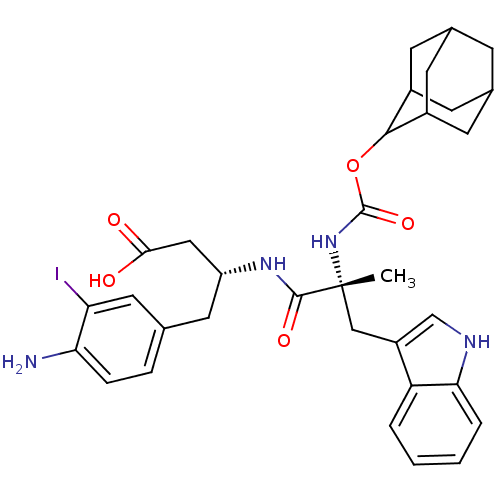
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N)c(I)c1 |wU:1.0,wD:29.33,1.13,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(8.02,-5.46,;9.37,-6.2,;10.7,-5.42,;10.68,-3.88,;11.96,-4.76,;13.17,-3.83,;12.66,-2.38,;13.41,-1.04,;12.62,.28,;11.08,.25,;10.33,-1.09,;11.13,-2.4,;9.4,-7.74,;8.09,-8.54,;8.12,-10.08,;6.74,-7.8,;5.46,-8.64,;5.45,-10.17,;4.05,-10.52,;2.72,-10.03,;1.53,-11.3,;3.03,-10.88,;4.43,-11.45,;3.02,-9.29,;4.06,-8.06,;2.72,-8.54,;10.47,-7.28,;12.01,-7.27,;10.51,-8.82,;11.86,-9.56,;11.89,-11.1,;10.57,-11.9,;10.61,-13.44,;9.22,-11.17,;13.17,-8.76,;14.52,-9.5,;14.55,-11.04,;15.9,-11.78,;17.22,-10.99,;18.57,-11.73,;17.18,-9.44,;18.5,-8.64,;15.83,-8.7,)| Show InChI InChI=1S/C33H39IN4O5/c1-33(16-23-17-36-28-5-3-2-4-25(23)28,38-32(42)43-30-21-9-19-8-20(11-21)12-22(30)10-19)31(41)37-24(15-29(39)40)13-18-6-7-27(35)26(34)14-18/h2-7,14,17,19-22,24,30,36H,8-13,15-16,35H2,1H3,(H,37,41)(H,38,42)(H,39,40)/t19?,20?,21?,22?,24-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]-PD 142308 to CCK-B receptor was determined |
Bioorg Med Chem Lett 5: 2501-2506 (1995)
Article DOI: 10.1016/0960-894X(95)00435-V
BindingDB Entry DOI: 10.7270/Q2DZ08SM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202991
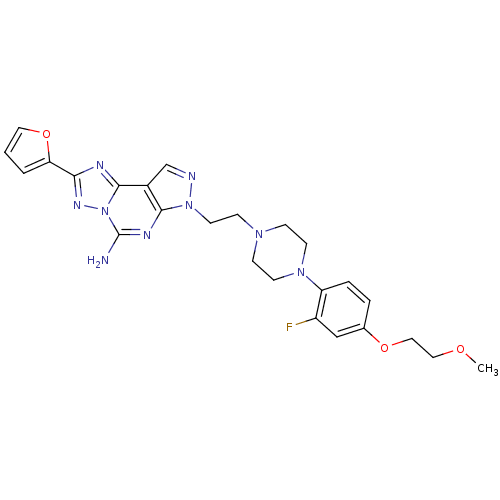
(7-(2-{4-[2-fluoro-4-(2-methoxy-ethoxy)-phenyl]-pip...)Show SMILES COCCOc1ccc(N2CCN(CCn3ncc4c3nc(N)n3nc(nc43)-c3ccco3)CC2)c(F)c1 Show InChI InChI=1S/C25H28FN9O3/c1-36-13-14-37-17-4-5-20(19(26)15-17)33-9-6-32(7-10-33)8-11-34-23-18(16-28-34)24-29-22(21-3-2-12-38-21)31-35(24)25(27)30-23/h2-5,12,15-16H,6-11,13-14H2,1H3,(H2,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202994
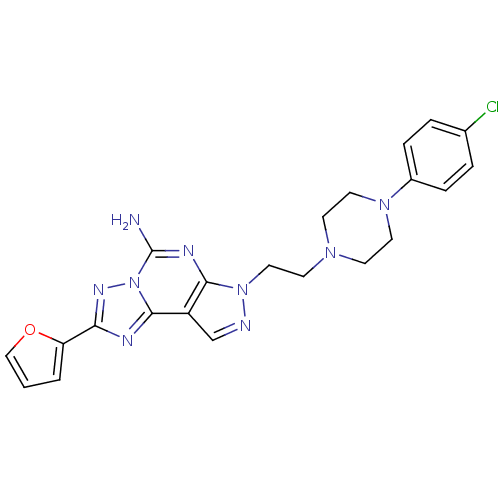
(7-{2-[4-(4-chloro-phenyl)-piperazin-1-yl]-ethyl}-2...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(Cl)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H22ClN9O/c23-15-3-5-16(6-4-15)30-10-7-29(8-11-30)9-12-31-20-17(14-25-31)21-26-19(18-2-1-13-33-18)28-32(21)22(24)27-20/h1-6,13-14H,7-12H2,(H2,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50285623

((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N)c(I)c1 |wU:1.0,wD:29.33,1.13,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(8.02,-5.46,;9.37,-6.2,;10.7,-5.42,;10.68,-3.88,;11.96,-4.76,;13.17,-3.83,;12.66,-2.38,;13.41,-1.04,;12.62,.28,;11.08,.25,;10.33,-1.09,;11.13,-2.4,;9.4,-7.74,;8.09,-8.54,;8.12,-10.08,;6.74,-7.8,;5.46,-8.64,;5.45,-10.17,;4.05,-10.52,;2.72,-10.03,;1.53,-11.3,;3.03,-10.88,;4.43,-11.45,;3.02,-9.29,;4.06,-8.06,;2.72,-8.54,;10.47,-7.28,;12.01,-7.27,;10.51,-8.82,;11.86,-9.56,;11.89,-11.1,;10.57,-11.9,;10.61,-13.44,;9.22,-11.17,;13.17,-8.76,;14.52,-9.5,;14.55,-11.04,;15.9,-11.78,;17.22,-10.99,;18.57,-11.73,;17.18,-9.44,;18.5,-8.64,;15.83,-8.7,)| Show InChI InChI=1S/C33H39IN4O5/c1-33(16-23-17-36-28-5-3-2-4-25(23)28,38-32(42)43-30-21-9-19-8-20(11-21)12-22(30)10-19)31(41)37-24(15-29(39)40)13-18-6-7-27(35)26(34)14-18/h2-7,14,17,19-22,24,30,36H,8-13,15-16,35H2,1H3,(H,37,41)(H,38,42)(H,39,40)/t19?,20?,21?,22?,24-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards CCK-B receptor in mouse cerebral cortex membrane using [125I]bolton assay |
Bioorg Med Chem Lett 5: 2501-2506 (1995)
Article DOI: 10.1016/0960-894X(95)00435-V
BindingDB Entry DOI: 10.7270/Q2DZ08SM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50203000
(7-(2-{4-[4-(2-cyclopropoxy-ethoxy)-phenyl]-piperaz...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(OCCOC4CC4)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C27H31N9O3/c28-27-31-25-22(26-30-24(32-36(26)27)23-2-1-15-39-23)18-29-35(25)14-11-33-9-12-34(13-10-33)19-3-5-20(6-4-19)37-16-17-38-21-7-8-21/h1-6,15,18,21H,7-14,16-17H2,(H2,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202998

(2-(4-{4-[2-(5-amino-2-furan-2-yl-pyrazolo[4,3-e][1...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(OCCO)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C24H27N9O3/c25-24-28-22-19(23-27-21(29-33(23)24)20-2-1-14-36-20)16-26-32(22)12-9-30-7-10-31(11-8-30)17-3-5-18(6-4-17)35-15-13-34/h1-6,14,16,34H,7-13,15H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202996
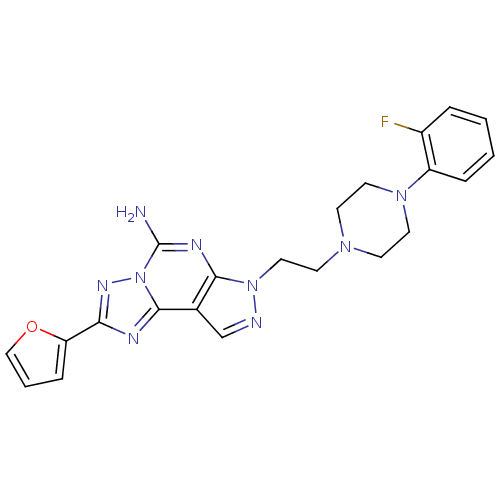
(7-{2-[4-(2-fluoro-phenyl)-piperazin-1-yl]-ethyl}-2...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccccc3F)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H22FN9O/c23-16-4-1-2-5-17(16)30-10-7-29(8-11-30)9-12-31-20-15(14-25-31)21-26-19(18-6-3-13-33-18)28-32(21)22(24)27-20/h1-6,13-14H,7-12H2,(H2,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202989
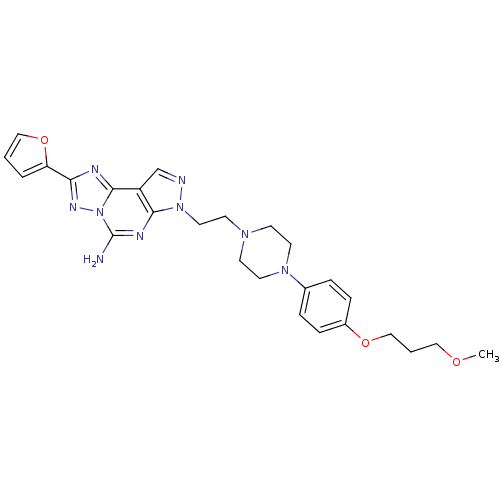
(2-furan-2-yl-7-(2-{4-[4-(3-methoxy-propoxy)-phenyl...)Show SMILES COCCCOc1ccc(cc1)N1CCN(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C26H31N9O3/c1-36-15-3-17-37-20-7-5-19(6-8-20)33-12-9-32(10-13-33)11-14-34-24-21(18-28-34)25-29-23(22-4-2-16-38-22)31-35(25)26(27)30-24/h2,4-8,16,18H,3,9-15,17H2,1H3,(H2,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202990
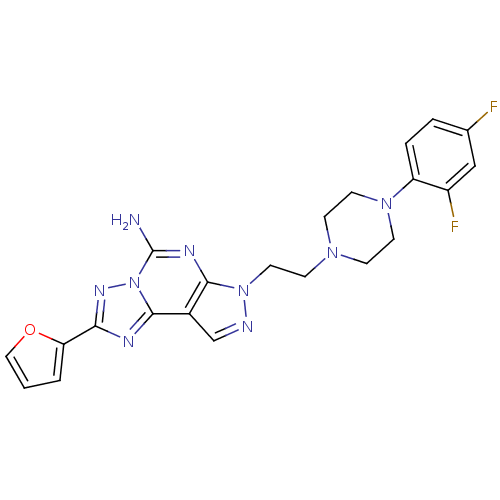
(2-(furan-2-yl)-7-(2-(4-(4-(2-methoxyethoxy)phenyl)...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(F)cc3F)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H21F2N9O/c23-14-3-4-17(16(24)12-14)31-8-5-30(6-9-31)7-10-32-20-15(13-26-32)21-27-19(18-2-1-11-34-18)29-33(21)22(25)28-20/h1-4,11-13H,5-10H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202985
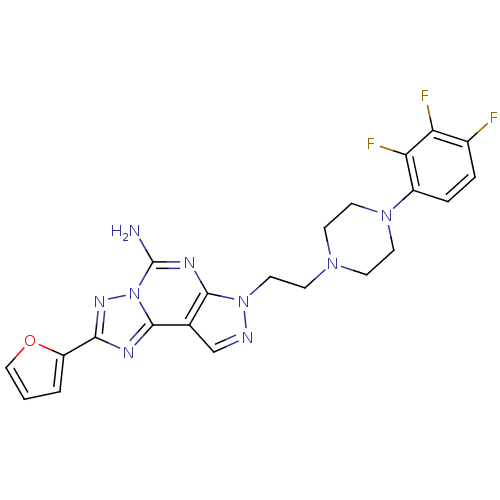
(2-furan-2-yl-7-{2-[4-(2,3,4-trifluoro-phenyl)-pipe...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(F)c(F)c3F)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H20F3N9O/c23-14-3-4-15(18(25)17(14)24)32-8-5-31(6-9-32)7-10-33-20-13(12-27-33)21-28-19(16-2-1-11-35-16)30-34(21)22(26)29-20/h1-4,11-12H,5-10H2,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of binding of [3H]-PD 140376 to Cholecystokinin type B receptor in guinea pig cortex. |
Bioorg Med Chem Lett 3: 885-888 (1993)
Article DOI: 10.1016/S0960-894X(00)80686-1
BindingDB Entry DOI: 10.7270/Q2D79BWZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50203001
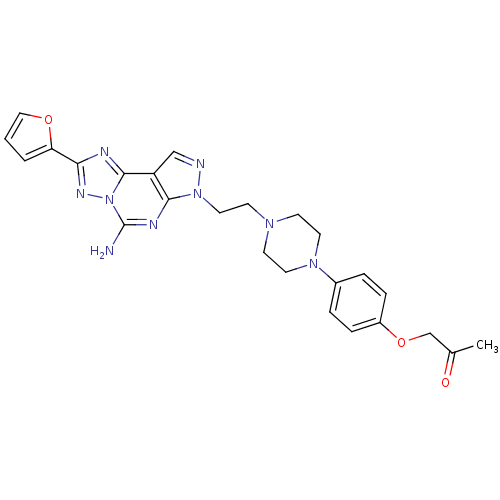
(1-(4-{4-[2-(5-amino-2-furan-2-yl-pyrazolo[4,3-e][1...)Show SMILES CC(=O)COc1ccc(cc1)N1CCN(CCn2ncc3c4nc(nn4c(N)nc23)-c2ccco2)CC1 Show InChI InChI=1S/C25H27N9O3/c1-17(35)16-37-19-6-4-18(5-7-19)32-11-8-31(9-12-32)10-13-33-23-20(15-27-33)24-28-22(21-3-2-14-36-21)30-34(24)25(26)29-23/h2-7,14-15H,8-13,16H2,1H3,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50367937

(CHEMBL1202931)Show SMILES CN([C@@H]1CC[C@]2(CCCO2)C[C@H]1N1CCCC1)C(=O)Cc1cccc2occc12 |r| Show InChI InChI=1S/C24H32N2O3/c1-25(23(27)16-18-6-4-7-22-19(18)9-15-28-22)20-8-11-24(10-5-14-29-24)17-21(20)26-12-2-3-13-26/h4,6-7,9,15,20-21H,2-3,5,8,10-14,16-17H2,1H3/t20-,21-,24-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 |
J Med Chem 33: 286-91 (1990)
BindingDB Entry DOI: 10.7270/Q2DJ5G7P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50008858
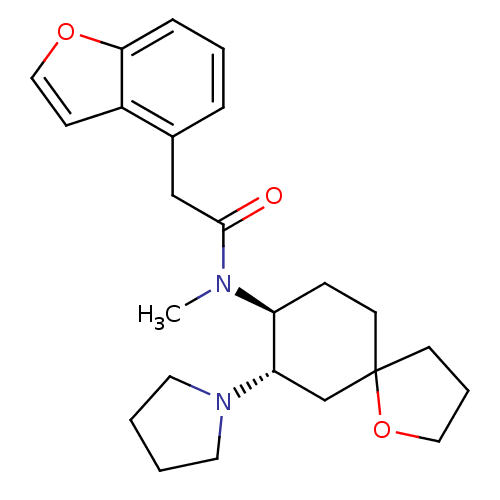
(2-Benzofuran-4-yl-N-methyl-N-(7-pyrrolidin-1-yl-1-...)Show SMILES CN([C@H]1CCC2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1cccc2occc12 Show InChI InChI=1S/C24H32N2O3/c1-25(23(27)16-18-6-4-7-22-19(18)9-15-28-22)20-8-11-24(10-5-14-29-24)17-21(20)26-12-2-3-13-26/h4,6-7,9,15,20-21H,2-3,5,8,10-14,16-17H2,1H3/t20-,21-,24?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Compound was evaluated for Opioid receptor kappa 1 affinity,determined with [3H]etorphine in the presence of excess unlabeled [D-Ala2-MePhe4-Glyol5]-... |
J Med Chem 34: 190-4 (1991)
BindingDB Entry DOI: 10.7270/Q2J103SG |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50285625
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccc(N=[N+]=[N-])c(I)c1 |wU:1.0,wD:29.33,1.13,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(7.11,-5.46,;8.46,-6.2,;9.79,-5.42,;9.77,-3.88,;11.05,-4.76,;12.26,-3.83,;11.76,-2.38,;12.5,-1.04,;11.71,.28,;10.17,.25,;9.42,-1.09,;10.22,-2.4,;8.5,-7.74,;7.18,-8.54,;7.21,-10.08,;5.83,-7.8,;4.55,-8.64,;4.54,-10.17,;3.14,-10.52,;1.81,-10.03,;.62,-11.31,;2.12,-10.89,;3.52,-11.45,;2.11,-9.29,;3.15,-8.07,;1.81,-8.54,;9.57,-7.28,;11.1,-7.28,;9.6,-8.82,;10.95,-9.56,;10.98,-11.1,;9.67,-11.9,;9.7,-13.44,;8.31,-11.17,;12.27,-8.76,;13.62,-9.5,;13.64,-11.04,;14.99,-11.79,;16.31,-10.99,;17.66,-11.73,;18.98,-10.93,;20.3,-10.13,;16.28,-9.44,;17.59,-8.64,;14.93,-8.7,)| Show InChI InChI=1S/C33H37IN6O5/c1-33(16-23-17-36-27-5-3-2-4-25(23)27,38-32(44)45-30-21-9-19-8-20(11-21)12-22(30)10-19)31(43)37-24(15-29(41)42)13-18-6-7-28(39-40-35)26(34)14-18/h2-7,14,17,19-22,24,30,36H,8-13,15-16H2,1H3,(H,37,43)(H,38,44)(H,41,42)/t19?,20?,21?,22?,24-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards CCK-B receptor in mouse cerebral cortex membrane using [125I]bolton assay |
Bioorg Med Chem Lett 5: 2501-2506 (1995)
Article DOI: 10.1016/0960-894X(95)00435-V
BindingDB Entry DOI: 10.7270/Q2DZ08SM |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50449787
(CHEMBL2062154 | PD-134308)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)N[C@](C)(Cc1c[nH]c4ccccc14)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1)[C@@]([H])(C2)C3 |wU:30.41,14.15,45.49,3.3,wD:6.6,1.0,TLB:5:3:47:9.6.8,10:9:47:3.48.2,THB:5:6:47:3.48.2,10:9:1.47.8:3.5.48,2:3:9:1.47.8,2:1:9:3.5.48,(-14.99,-2.1,;-13.56,-2.66,;-14.77,-3.94,;-13.26,-3.53,;-13.35,-5.06,;-11.86,-4.09,;-10.83,-2.82,;-9.38,-3.33,;-12.24,-3.16,;-10.83,-1.28,;-9.29,-1.31,;-8.5,.01,;-9.25,1.36,;-6.96,-.01,;-6.19,1.3,;-5.42,-.02,;-7.44,2.2,;-7.28,3.74,;-8.44,4.76,;-7.81,6.18,;-6.29,6.03,;-5.14,7.07,;-3.69,6.6,;-3.34,5.08,;-4.49,4.06,;-5.94,4.52,;-4.66,1.42,;-3.99,2.8,;-3.79,.15,;-2.27,.27,;-1.4,-1.02,;-2.08,-2.4,;-1.22,-3.69,;.32,-3.58,;-1.9,-5.07,;-3.43,-5.16,;-4.1,-6.57,;-5.64,-6.67,;-3.25,-7.83,;.14,-.91,;.99,-2.2,;2.51,-2.08,;3.19,-.7,;2.32,.59,;.8,.47,;-12.23,-.7,;-12.2,.82,;-13.58,-1.18,;-13.27,-1.94,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/t21-,22+,24-,25+,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of binding of [3H]-PD 140376 to Cholecystokinin type B receptor in guinea pig cortex. |
Bioorg Med Chem Lett 3: 885-888 (1993)
Article DOI: 10.1016/S0960-894X(00)80686-1
BindingDB Entry DOI: 10.7270/Q2D79BWZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202992

(7-{2-[4-(4-fluoro-phenyl)-piperazin-1-yl]-ethyl}-2...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(F)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H22FN9O/c23-15-3-5-16(6-4-15)30-10-7-29(8-11-30)9-12-31-20-17(14-25-31)21-26-19(18-2-1-13-33-18)28-32(21)22(24)27-20/h1-6,13-14H,7-12H2,(H2,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202999

(2-furan-2-yl-7-{2-[4-(2,4,6-trifluoro-phenyl)-pipe...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3c(F)cc(F)cc3F)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H20F3N9O/c23-13-10-15(24)18(16(25)11-13)32-6-3-31(4-7-32)5-8-33-20-14(12-27-33)21-28-19(17-2-1-9-35-17)30-34(21)22(26)29-20/h1-2,9-12H,3-8H2,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202986
(2-furan-2-yl-7-(2-{4-[4-(2-methoxy-ethoxy)-phenyl]...)Show SMILES COCCOc1ccc(cc1)N1CCN(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C25H29N9O3/c1-35-15-16-36-19-6-4-18(5-7-19)32-11-8-31(9-12-32)10-13-33-23-20(17-27-33)24-28-22(21-3-2-14-37-21)30-34(24)25(26)29-23/h2-7,14,17H,8-13,15-16H2,1H3,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50008852
(2-Methyl-acenaphthene-1-carboxylic acid (7-imidazo...)Show SMILES CC1C(C(=O)N(C)[C@H]2CCC3(CCCO3)C[C@@H]2N2CCNC2)c2cccc3cccc1c23 Show InChI InChI=1S/C27H35N3O2/c1-18-20-8-3-6-19-7-4-9-21(25(19)20)24(18)26(31)29(2)22-10-12-27(11-5-15-32-27)16-23(22)30-14-13-28-17-30/h3-4,6-9,18,22-24,28H,5,10-17H2,1-2H3/t18?,22-,23-,24?,27?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Opioid receptor kappa affinity, determined with [3H]etorphine in the presence of excess unlabeled [D-Ala2-MePhe4-Glyol5]-enkephalin |
J Med Chem 34: 190-4 (1991)
BindingDB Entry DOI: 10.7270/Q2J103SG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202997
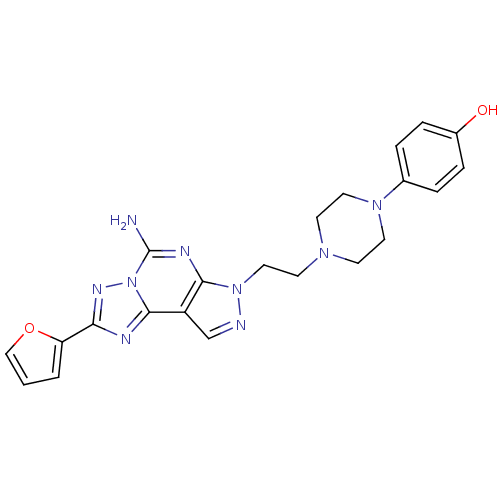
(4-{4-[2-(5-amino-2-furan-2-yl-pyrazolo[4,3-e][1,2,...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(O)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H23N9O2/c23-22-26-20-17(21-25-19(27-31(21)22)18-2-1-13-33-18)14-24-30(20)12-9-28-7-10-29(11-8-28)15-3-5-16(32)6-4-15/h1-6,13-14,32H,7-12H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202987
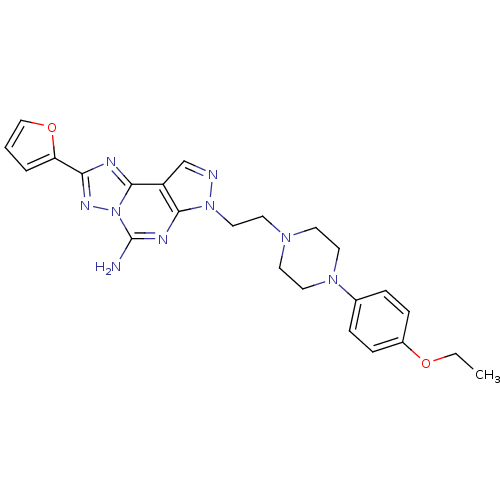
(7-{2-[4-(4-ethoxy-phenyl)-piperazin-1-yl]-ethyl}-2...)Show SMILES CCOc1ccc(cc1)N1CCN(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C24H27N9O2/c1-2-34-18-7-5-17(6-8-18)31-12-9-30(10-13-31)11-14-32-22-19(16-26-32)23-27-21(20-4-3-15-35-20)29-33(23)24(25)28-22/h3-8,15-16H,2,9-14H2,1H3,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202988

(7-{2-[4-(3,4-difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(F)c(F)c3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H21F2N9O/c23-16-4-3-14(12-17(16)24)31-8-5-30(6-9-31)7-10-32-20-15(13-26-32)21-27-19(18-2-1-11-34-18)29-33(21)22(25)28-20/h1-4,11-13H,5-10H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202993
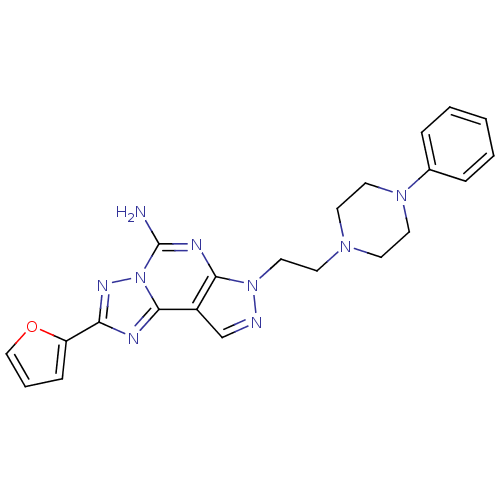
(2-furan-2-yl-7-[2-(4-phenyl-piperazin-1-yl)-ethyl]...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccccc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H23N9O/c23-22-26-20-17(21-25-19(27-31(21)22)18-7-4-14-32-18)15-24-30(20)13-10-28-8-11-29(12-9-28)16-5-2-1-3-6-16/h1-7,14-15H,8-13H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202983

(2-furan-2-yl-7-(2-{4-[4-(2-methoxy-propoxy)-phenyl...)Show SMILES COC(C)COc1ccc(cc1)N1CCN(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 |w:2.2| Show InChI InChI=1S/C26H31N9O3/c1-18(36-2)17-38-20-7-5-19(6-8-20)33-12-9-32(10-13-33)11-14-34-24-21(16-28-34)25-29-23(22-4-3-15-37-22)31-35(25)26(27)30-24/h3-8,15-16,18H,9-14,17H2,1-2H3,(H2,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity towards mu opioid receptor was determined |
J Med Chem 32: 1620-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GH9JJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM81962

(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of binding of [3H]-PD 140376 to Cholecystokinin type B receptor in guinea pig cortex. |
Bioorg Med Chem Lett 3: 885-888 (1993)
Article DOI: 10.1016/S0960-894X(00)80686-1
BindingDB Entry DOI: 10.7270/Q2D79BWZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202984
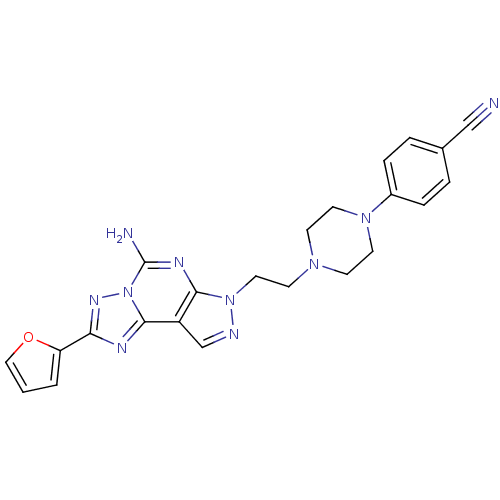
(4-{4-[2-(5-amino-2-furan-2-yl-pyrazolo[4,3-e][1,2,...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(cc3)C#N)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C23H22N10O/c24-14-16-3-5-17(6-4-16)31-10-7-30(8-11-31)9-12-32-21-18(15-26-32)22-27-20(19-2-1-13-34-19)29-33(22)23(25)28-21/h1-6,13,15H,7-12H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202995

(2-furan-2-yl-7-{2-[4-(4-methanesulfinylmethoxy-phe...)Show SMILES CS(=O)COc1ccc(cc1)N1CCN(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C24H27N9O3S/c1-37(34)16-36-18-6-4-17(5-7-18)31-11-8-30(9-12-31)10-13-32-22-19(15-26-32)23-27-21(20-3-2-14-35-20)29-33(23)24(25)28-22/h2-7,14-15H,8-13,16H2,1H3,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50202986

(2-furan-2-yl-7-(2-{4-[4-(2-methoxy-ethoxy)-phenyl]...)Show SMILES COCCOc1ccc(cc1)N1CCN(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C25H29N9O3/c1-35-15-16-36-19-6-4-18(5-7-19)32-11-8-31(9-12-32)10-13-33-23-20(17-27-33)24-28-22(21-3-2-14-37-21)30-34(24)25(26)29-23/h2-7,14,17H,8-13,15-16H2,1H3,(H2,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to rat adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50008857
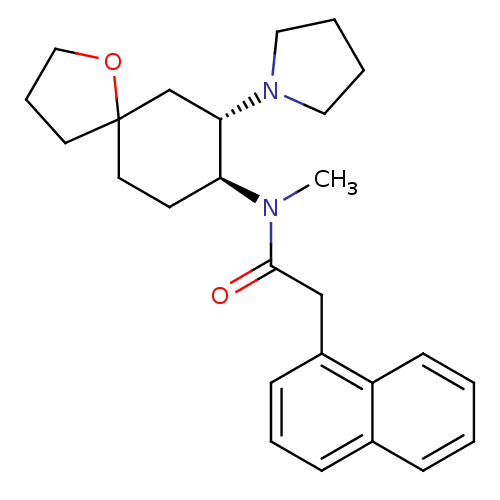
(CHEMBL543329 | N-Methyl-2-naphthalen-1-yl-N-(7-pyr...)Show SMILES CN([C@H]1CCC2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1cccc2ccccc12 Show InChI InChI=1S/C26H34N2O2/c1-27(25(29)18-21-10-6-9-20-8-2-3-11-22(20)21)23-12-14-26(13-7-17-30-26)19-24(23)28-15-4-5-16-28/h2-3,6,8-11,23-24H,4-5,7,12-19H2,1H3/t23-,24-,26?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Compound was evaluated for Opioid receptor kappa 1 affinity,determined with [3H]etorphine in the presence of excess unlabeled [D-Ala2-MePhe4-Glyol5]-... |
J Med Chem 34: 190-4 (1991)
BindingDB Entry DOI: 10.7270/Q2J103SG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50008854
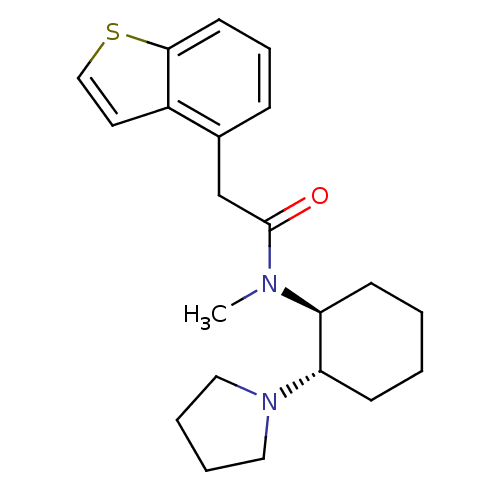
(2-Benzo[b]thiophen-4-yl-N-methyl-N-(2-pyrrolidin-1...)Show SMILES CN([C@H]1CCCC[C@@H]1N1CCCC1)C(=O)Cc1cccc2sccc12 Show InChI InChI=1S/C21H28N2OS/c1-22(18-8-2-3-9-19(18)23-12-4-5-13-23)21(24)15-16-7-6-10-20-17(16)11-14-25-20/h6-7,10-11,14,18-19H,2-5,8-9,12-13,15H2,1H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity towards opioid receptor kappa was determined |
J Med Chem 32: 1620-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GH9JJB |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50008854

(2-Benzo[b]thiophen-4-yl-N-methyl-N-(2-pyrrolidin-1...)Show SMILES CN([C@H]1CCCC[C@@H]1N1CCCC1)C(=O)Cc1cccc2sccc12 Show InChI InChI=1S/C21H28N2OS/c1-22(18-8-2-3-9-19(18)23-12-4-5-13-23)21(24)15-16-7-6-10-20-17(16)11-14-25-20/h6-7,10-11,14,18-19H,2-5,8-9,12-13,15H2,1H3/t18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Opioid receptor kappa affinity, determined with [3H]etorphine in the presence of excess unlabeled [D-Ala2-MePhe4-Glyol5]-enkephalin |
J Med Chem 34: 190-4 (1991)
BindingDB Entry DOI: 10.7270/Q2J103SG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50008854

(2-Benzo[b]thiophen-4-yl-N-methyl-N-(2-pyrrolidin-1...)Show SMILES CN([C@H]1CCCC[C@@H]1N1CCCC1)C(=O)Cc1cccc2sccc12 Show InChI InChI=1S/C21H28N2OS/c1-22(18-8-2-3-9-19(18)23-12-4-5-13-23)21(24)15-16-7-6-10-20-17(16)11-14-25-20/h6-7,10-11,14,18-19H,2-5,8-9,12-13,15H2,1H3/t18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 |
J Med Chem 33: 286-91 (1990)
BindingDB Entry DOI: 10.7270/Q2DJ5G7P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50017495
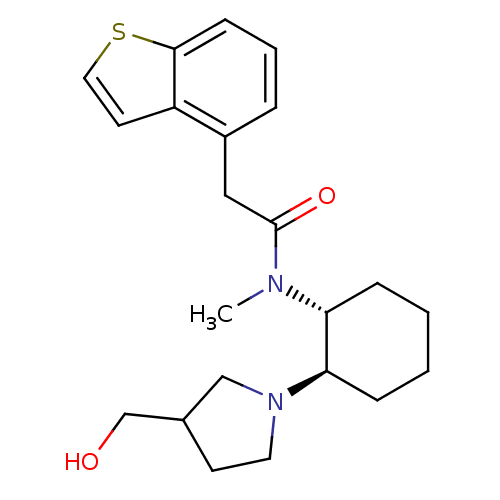
(2-Benzo[b]thiophen-4-yl-N-[2-(3-hydroxymethyl-pyrr...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCC(CO)C1)C(=O)Cc1cccc2sccc12 Show InChI InChI=1S/C22H30N2O2S/c1-23(22(26)13-17-5-4-8-21-18(17)10-12-27-21)19-6-2-3-7-20(19)24-11-9-16(14-24)15-25/h4-5,8,10,12,16,19-20,25H,2-3,6-7,9,11,13-15H2,1H3/t16?,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity towards opioid receptor kappa was determined |
J Med Chem 32: 1620-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GH9JJB |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501345

(CHEMBL3958857)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H21ClN6O12P2/c15-3-7(22)17-1-2-30-34(26,27)33-35(28,29)31-4-6-9(23)10(24)13(32-6)21-5-18-8-11(21)19-14(16)20-12(8)25/h5-6,9-10,13,23-24H,1-4H2,(H,17,22)(H,26,27)(H,28,29)(H3,16,19,20,25)/t6-,9-,10-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50000296

(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Opioid receptor kappa affinity, determined with [3H]etorphine in the presence of excess unlabeled [D-Ala2-MePhe4-Glyol5]-enkephalin |
J Med Chem 34: 190-4 (1991)
BindingDB Entry DOI: 10.7270/Q2J103SG |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50167898
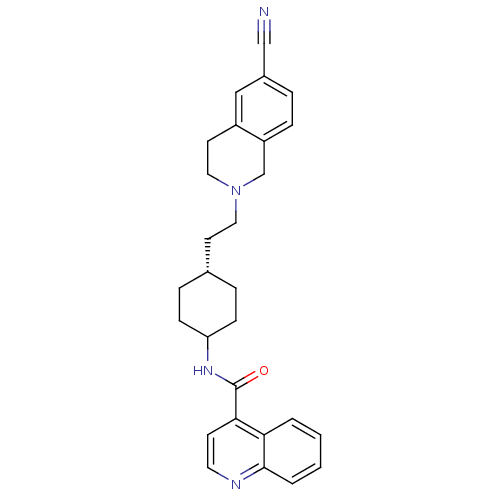
(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50008855
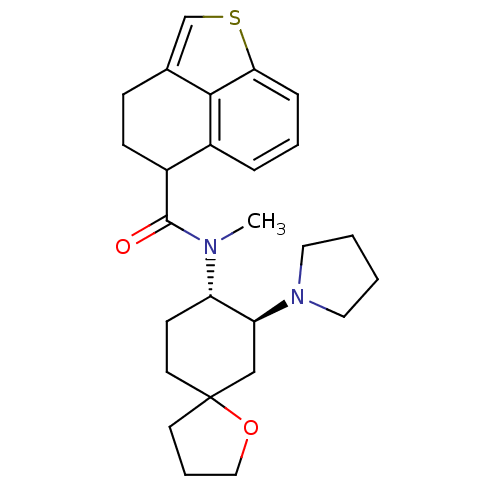
(4,5-Dihydro-3H-naphtho[1,8-bc]thiophene-5-carboxyl...)Show SMILES CN([C@H]1CCC2(CCCO2)C[C@@H]1N1CCCC1)C(=O)C1CCc2csc3cccc1c23 Show InChI InChI=1S/C26H34N2O2S/c1-27(25(29)20-9-8-18-17-31-23-7-4-6-19(20)24(18)23)21-10-12-26(11-5-15-30-26)16-22(21)28-13-2-3-14-28/h4,6-7,17,20-22H,2-3,5,8-16H2,1H3/t20?,21-,22-,26?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Opioid receptor kappa affinity, determined with [3H]etorphine in the presence of excess unlabeled [D-Ala2-MePhe4-Glyol5]-enkephalin |
J Med Chem 34: 190-4 (1991)
BindingDB Entry DOI: 10.7270/Q2J103SG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50008855

(4,5-Dihydro-3H-naphtho[1,8-bc]thiophene-5-carboxyl...)Show SMILES CN([C@H]1CCC2(CCCO2)C[C@@H]1N1CCCC1)C(=O)C1CCc2csc3cccc1c23 Show InChI InChI=1S/C26H34N2O2S/c1-27(25(29)20-9-8-18-17-31-23-7-4-6-19(20)24(18)23)21-10-12-26(11-5-15-30-26)16-22(21)28-13-2-3-14-28/h4,6-7,17,20-22H,2-3,5,8-16H2,1H3/t20?,21-,22-,26?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Opioid receptor kappa affinity, determined with [3H]etorphine in the presence of excess unlabeled [D-Ala2-MePhe4-Glyol5]-enkephalin |
J Med Chem 34: 190-4 (1991)
BindingDB Entry DOI: 10.7270/Q2J103SG |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50017500
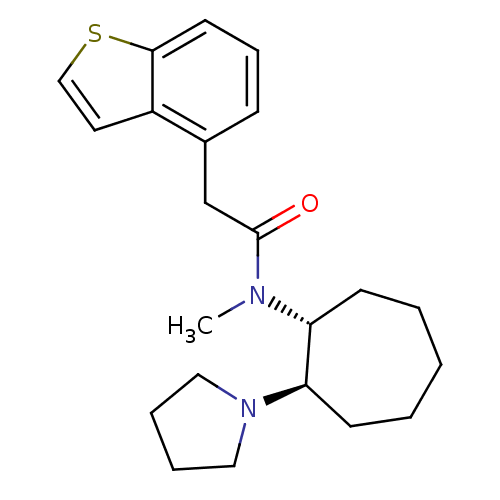
(2-Benzo[b]thiophen-4-yl-N-methyl-N-(2-pyrrolidin-1...)Show SMILES CN([C@@H]1CCCCC[C@H]1N1CCCC1)C(=O)Cc1cccc2sccc12 Show InChI InChI=1S/C22H30N2OS/c1-23(19-9-3-2-4-10-20(19)24-13-5-6-14-24)22(25)16-17-8-7-11-21-18(17)12-15-26-21/h7-8,11-12,15,19-20H,2-6,9-10,13-14,16H2,1H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor kappa 1 was determined |
J Med Chem 32: 1620-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GH9JJB |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50024321
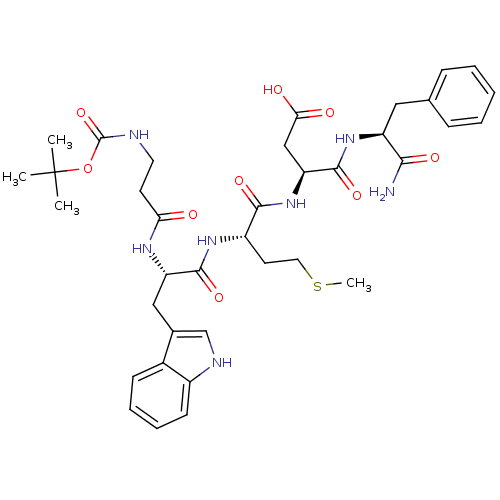
(3-{2-[2-(3-tert-Butoxycarbonylamino-propionylamino...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H49N7O9S/c1-37(2,3)53-36(52)39-16-14-30(45)41-28(19-23-21-40-25-13-9-8-12-24(23)25)34(50)42-26(15-17-54-4)33(49)44-29(20-31(46)47)35(51)43-27(32(38)48)18-22-10-6-5-7-11-22/h5-13,21,26-29,40H,14-20H2,1-4H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]-PD 142308 to CCK-B receptor was determined |
Bioorg Med Chem Lett 5: 2501-2506 (1995)
Article DOI: 10.1016/0960-894X(95)00435-V
BindingDB Entry DOI: 10.7270/Q2DZ08SM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50017488
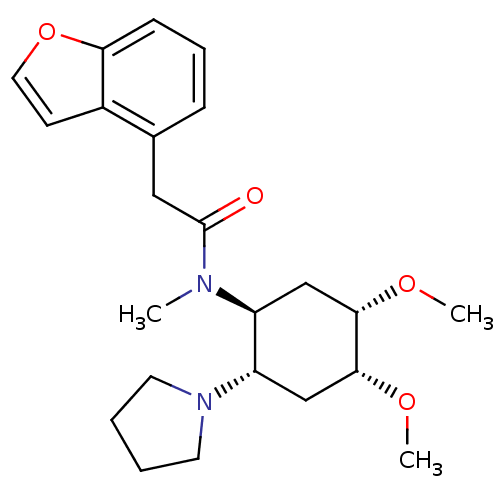
(2-Benzofuran-4-yl-N-(4,5-dimethoxy-2-pyrrolidin-1-...)Show SMILES CO[C@H]1C[C@@H]([C@H](C[C@H]1OC)N1CCCC1)N(C)C(=O)Cc1cccc2occc12 Show InChI InChI=1S/C23H32N2O4/c1-24(23(26)13-16-7-6-8-20-17(16)9-12-29-20)18-14-21(27-2)22(28-3)15-19(18)25-10-4-5-11-25/h6-9,12,18-19,21-22H,4-5,10-11,13-15H2,1-3H3/t18-,19-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor kappa 1 was determined |
J Med Chem 32: 1620-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GH9JJB |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50013147

(2-Benzo[b]thiophen-4-yl-N-(4,5-dimethoxy-2-pyrroli...)Show SMILES CO[C@H]1CC([C@H](C[C@H]1OC)N1CCCC1)N(C)C(=O)Cc1cccc2sccc12 Show InChI InChI=1S/C23H32N2O3S/c1-24(23(26)13-16-7-6-8-22-17(16)9-12-29-22)18-14-20(27-2)21(28-3)15-19(18)25-10-4-5-11-25/h6-9,12,18-21H,4-5,10-11,13-15H2,1-3H3/t18?,19-,20-,21+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 |
J Med Chem 33: 286-91 (1990)
BindingDB Entry DOI: 10.7270/Q2DJ5G7P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50017494

(2-Benzo[b]thiophen-7-yl-N-methyl-N-(2-pyrrolidin-1...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1cccc2ccsc12 Show InChI InChI=1S/C21H28N2OS/c1-22(18-9-2-3-10-19(18)23-12-4-5-13-23)20(24)15-17-8-6-7-16-11-14-25-21(16)17/h6-8,11,14,18-19H,2-5,9-10,12-13,15H2,1H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity towards opioid receptor kappa was determined |
J Med Chem 32: 1620-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GH9JJB |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50017498

(2-Benzofuran-4-yl-N-[2-(3-hydroxymethyl-pyrrolidin...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCC(CO)C1)C(=O)Cc1cccc2occc12 Show InChI InChI=1S/C22H30N2O3/c1-23(22(26)13-17-5-4-8-21-18(17)10-12-27-21)19-6-2-3-7-20(19)24-11-9-16(14-24)15-25/h4-5,8,10,12,16,19-20,25H,2-3,6-7,9,11,13-15H2,1H3/t16?,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor kappa 1 was determined |
J Med Chem 32: 1620-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GH9JJB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data