 Found 417 hits with Last Name = 'hunter' and Initial = 'rn'
Found 417 hits with Last Name = 'hunter' and Initial = 'rn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Candida albicans) | BDBM50049912

(7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...)Show InChI InChI=1S/C16H21N5/c1-5-16(3,4)21-9(2)8-10-12(21)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224
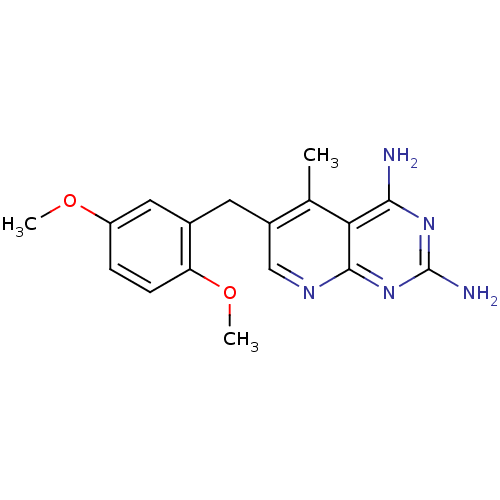
(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Candida albicans) | BDBM50049905
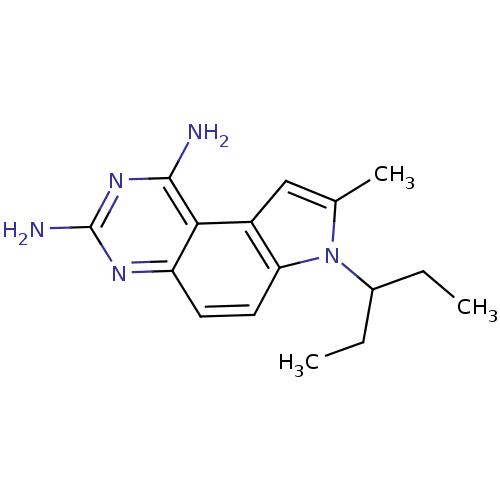
(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049901
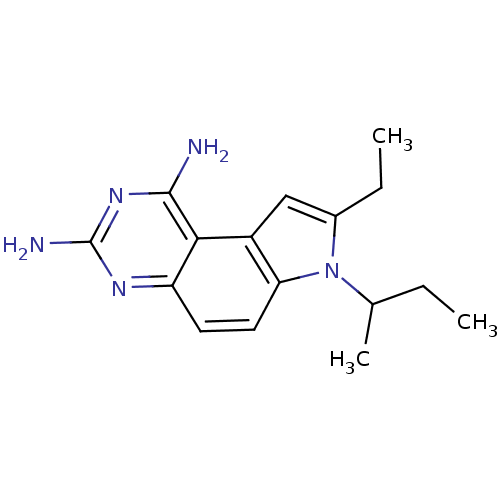
(7-sec-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-1...)Show InChI InChI=1S/C16H21N5/c1-4-9(3)21-10(5-2)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-9H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049907

(7-(1-Ethyl-propyl)-8-isopropyl-7H-pyrrolo[3,2-f]qu...)Show InChI InChI=1S/C18H25N5/c1-5-11(6-2)23-14-8-7-13-16(17(19)22-18(20)21-13)12(14)9-15(23)10(3)4/h7-11H,5-6H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049906
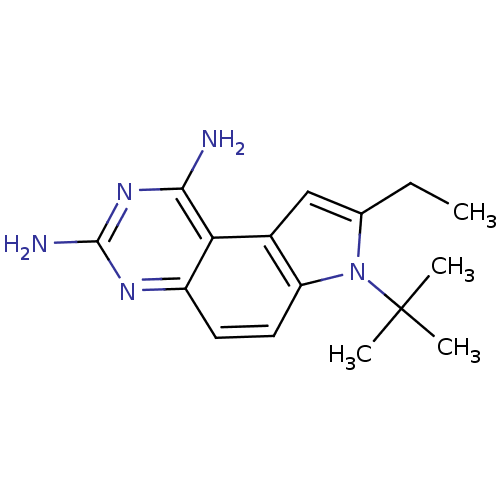
(7-tert-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C16H21N5/c1-5-9-8-10-12(21(9)16(2,3)4)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049911

(8-tert-Butyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diam...)Show InChI InChI=1S/C14H17N5/c1-14(2,3)10-6-7-8(17-10)4-5-9-11(7)12(15)19-13(16)18-9/h4-6,17H,1-3H3,(H4,15,16,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049898

(8-Isopropyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C13H15N5/c1-6(2)10-5-7-8(16-10)3-4-9-11(7)12(14)18-13(15)17-9/h3-6,16H,1-2H3,(H4,14,15,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049909

(8-tert-Butyl-7-isopropyl-7H-pyrrolo[3,2-f]quinazol...)Show InChI InChI=1S/C17H23N5/c1-9(2)22-12-7-6-11-14(15(18)21-16(19)20-11)10(12)8-13(22)17(3,4)5/h6-9H,1-5H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049897

(8-Ethyl-7-(1-ethyl-propyl)-7H-pyrrolo[3,2-f]quinaz...)Show InChI InChI=1S/C17H23N5/c1-4-10(5-2)22-11(6-3)9-12-14(22)8-7-13-15(12)16(18)21-17(19)20-13/h7-10H,4-6H2,1-3H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049899
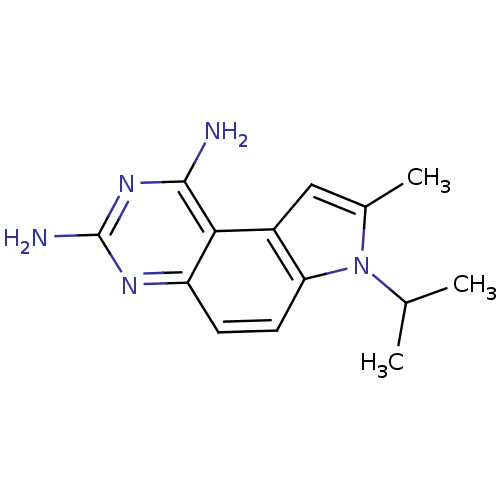
(7-Isopropyl-8-methyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C14H17N5/c1-7(2)19-8(3)6-9-11(19)5-4-10-12(9)13(15)18-14(16)17-10/h4-7H,1-3H3,(H4,15,16,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM18043
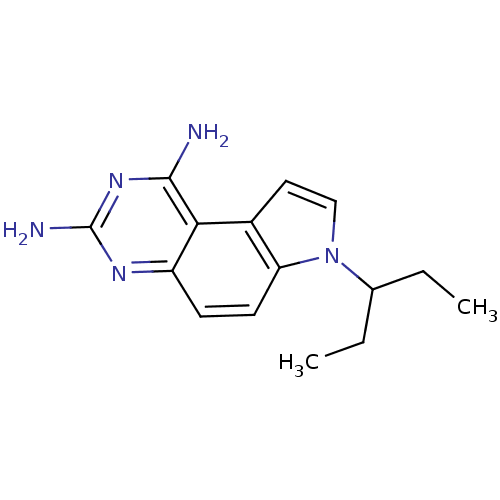
(1,3-DIAMINO-7-(1-ETHYEPROPYE)-7H-PYRRALO-[3,2-F]QU...)Show InChI InChI=1S/C15H19N5/c1-3-9(4-2)20-8-7-10-12(20)6-5-11-13(10)14(16)19-15(17)18-11/h5-9H,3-4H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049914

(7-(3,4,5-Trimethoxy-benzyl)-7H-pyrrolo[3,2-f]quina...)Show SMILES COc1cc(Cn2ccc3c2ccc2nc(N)nc(N)c32)cc(OC)c1OC Show InChI InChI=1S/C20H21N5O3/c1-26-15-8-11(9-16(27-2)18(15)28-3)10-25-7-6-12-14(25)5-4-13-17(12)19(21)24-20(22)23-13/h4-9H,10H2,1-3H3,(H4,21,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049905

(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049902

(7,8-Diethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C14H17N5/c1-3-8-7-9-11(19(8)4-2)6-5-10-12(9)13(15)18-14(16)17-10/h5-7H,3-4H2,1-2H3,(H4,15,16,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049913
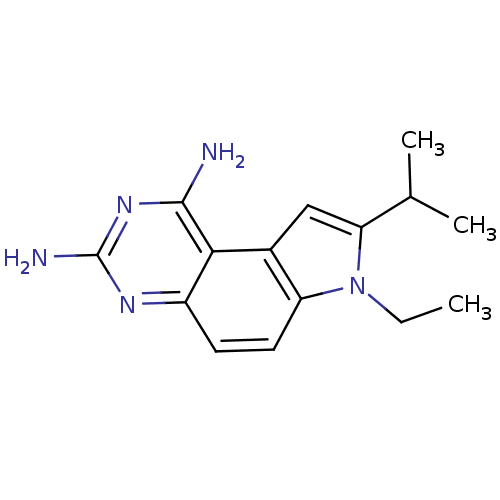
(7-Ethyl-8-isopropyl-7H-pyrrolo[3,2-f]quinazoline-1...)Show InChI InChI=1S/C15H19N5/c1-4-20-11-6-5-10-13(14(16)19-15(17)18-10)9(11)7-12(20)8(2)3/h5-8H,4H2,1-3H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049896
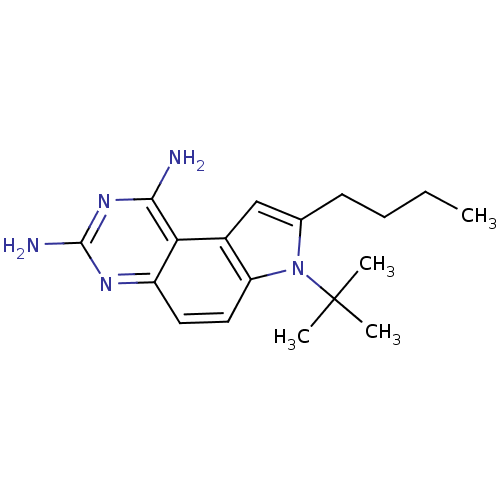
(8-Butyl-7-tert-butyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C18H25N5/c1-5-6-7-11-10-12-14(23(11)18(2,3)4)9-8-13-15(12)16(19)22-17(20)21-13/h8-10H,5-7H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049912

(7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...)Show InChI InChI=1S/C16H21N5/c1-5-16(3,4)21-9(2)8-10-12(21)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049903
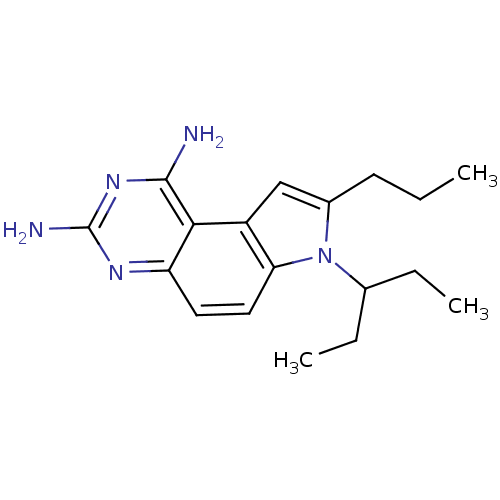
(7-(1-Ethyl-propyl)-8-propyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C18H25N5/c1-4-7-12-10-13-15(23(12)11(5-2)6-3)9-8-14-16(13)17(19)22-18(20)21-14/h8-11H,4-7H2,1-3H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049910

(7-Ethyl-8-propyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C15H19N5/c1-3-5-9-8-10-12(20(9)4-2)7-6-11-13(10)14(16)19-15(17)18-11/h6-8H,3-5H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049915
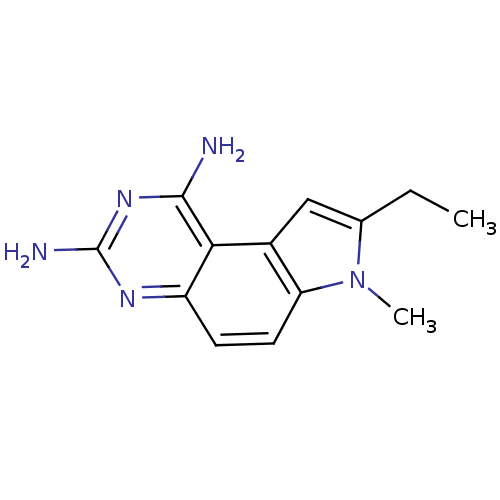
(8-Ethyl-7-methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C13H15N5/c1-3-7-6-8-10(18(7)2)5-4-9-11(8)12(14)17-13(15)16-9/h4-6H,3H2,1-2H3,(H4,14,15,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268
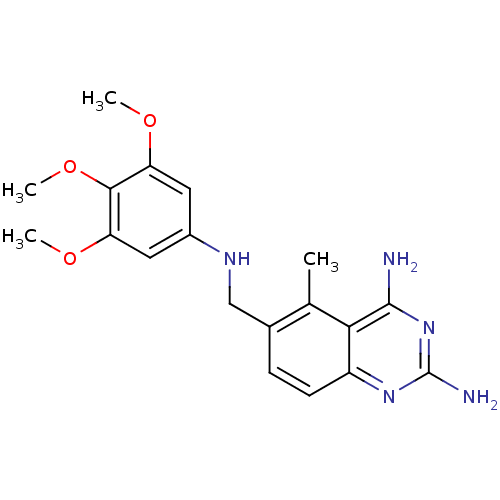
(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049908
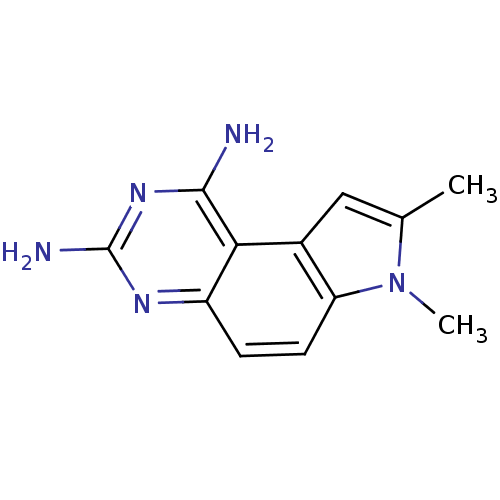
(7,8-Dimethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diam...)Show InChI InChI=1S/C12H13N5/c1-6-5-7-9(17(6)2)4-3-8-10(7)11(13)16-12(14)15-8/h3-5H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18043

(1,3-DIAMINO-7-(1-ETHYEPROPYE)-7H-PYRRALO-[3,2-F]QU...)Show InChI InChI=1S/C15H19N5/c1-3-9(4-2)20-8-7-10-12(20)6-5-11-13(10)14(16)19-15(17)18-11/h5-9H,3-4H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049914

(7-(3,4,5-Trimethoxy-benzyl)-7H-pyrrolo[3,2-f]quina...)Show SMILES COc1cc(Cn2ccc3c2ccc2nc(N)nc(N)c32)cc(OC)c1OC Show InChI InChI=1S/C20H21N5O3/c1-26-15-8-11(9-16(27-2)18(15)28-3)10-25-7-6-12-14(25)5-4-13-17(12)19(21)24-20(22)23-13/h4-9H,10H2,1-3H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049899

(7-Isopropyl-8-methyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C14H17N5/c1-7(2)19-8(3)6-9-11(19)5-4-10-12(9)13(15)18-14(16)17-10/h4-7H,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049898

(8-Isopropyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C13H15N5/c1-6(2)10-5-7-8(16-10)3-4-9-11(7)12(14)18-13(15)17-9/h3-6,16H,1-2H3,(H4,14,15,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049902

(7,8-Diethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C14H17N5/c1-3-8-7-9-11(19(8)4-2)6-5-10-12(9)13(15)18-14(16)17-10/h5-7H,3-4H2,1-2H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049904
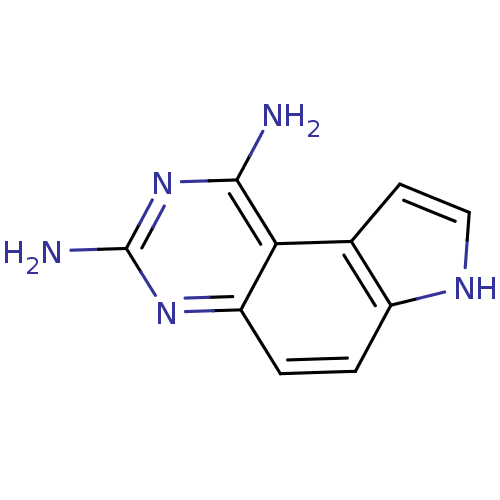
(7H-Pyrrolo[3,2-f]quinazoline-1,3-diamine | CHEMBL3...)Show InChI InChI=1S/C10H9N5/c11-9-8-5-3-4-13-6(5)1-2-7(8)14-10(12)15-9/h1-4,13H,(H4,11,12,14,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049910

(7-Ethyl-8-propyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C15H19N5/c1-3-5-9-8-10-12(20(9)4-2)7-6-11-13(10)14(16)19-15(17)18-11/h6-8H,3-5H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049900
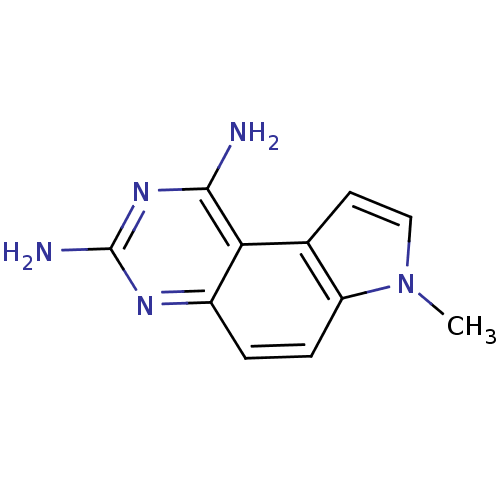
(7-Methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine ...)Show InChI InChI=1S/C11H11N5/c1-16-5-4-6-8(16)3-2-7-9(6)10(12)15-11(13)14-7/h2-5H,1H3,(H4,12,13,14,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049915

(8-Ethyl-7-methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C13H15N5/c1-3-7-6-8-10(18(7)2)5-4-9-11(8)12(14)17-13(15)16-9/h4-6H,3H2,1-2H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049896

(8-Butyl-7-tert-butyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C18H25N5/c1-5-6-7-11-10-12-14(23(11)18(2,3)4)9-8-13-15(12)16(19)22-17(20)21-13/h8-10H,5-7H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049908

(7,8-Dimethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diam...)Show InChI InChI=1S/C12H13N5/c1-6-5-7-9(17(6)2)4-3-8-10(7)11(13)16-12(14)15-8/h3-5H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049900

(7-Methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine ...)Show InChI InChI=1S/C11H11N5/c1-16-5-4-6-8(16)3-2-7-9(6)10(12)15-11(13)14-7/h2-5H,1H3,(H4,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049904

(7H-Pyrrolo[3,2-f]quinazoline-1,3-diamine | CHEMBL3...)Show InChI InChI=1S/C10H9N5/c11-9-8-5-3-4-13-6(5)1-2-7(8)14-10(12)15-9/h1-4,13H,(H4,11,12,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7751
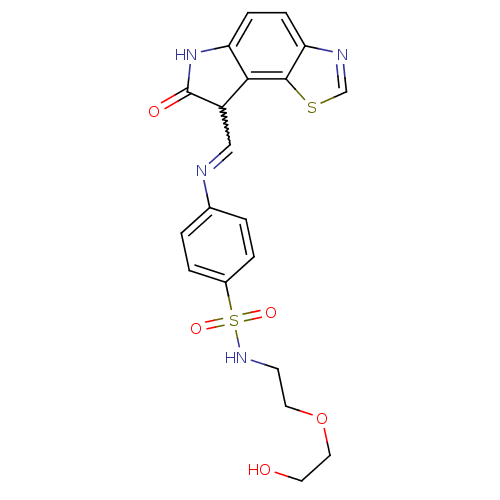
((12Z)-12-{[(4-{[2-(2-hydroxyethoxy)ethyl]sulfamoyl...)Show SMILES OCCOCCNS(=O)(=O)c1ccc(cc1)N=CC1C(=O)Nc2ccc3ncsc3c12 |w:17.18| Show InChI InChI=1S/C20H20N4O5S2/c25-8-10-29-9-7-23-31(27,28)14-3-1-13(2-4-14)21-11-15-18-16(24-20(15)26)5-6-17-19(18)30-12-22-17/h1-6,11-12,15,23,25H,7-10H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... |
J Med Chem 44: 4339-58 (2001)
Article DOI: 10.1021/jm010117d
BindingDB Entry DOI: 10.7270/Q2ST7N10 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM26477
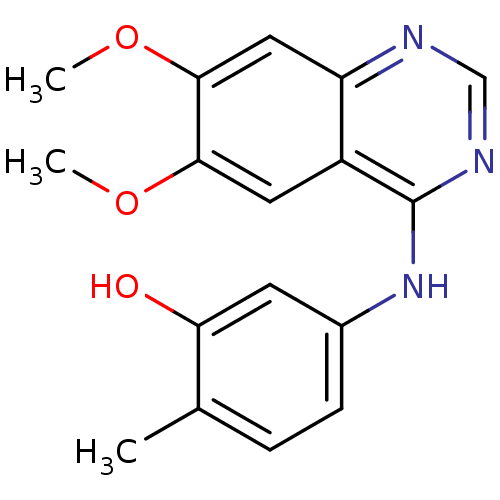
(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-2-methylph...)Show InChI InChI=1S/C17H17N3O3/c1-10-4-5-11(6-14(10)21)20-17-12-7-15(22-2)16(23-3)8-13(12)18-9-19-17/h4-9,21H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
The assay uses purified baculovirus-expressed GST-VEGFR2 interacting with biotinylated peptide substrates. HTRF is based on the proximity of europium... |
J Med Chem 51: 4632-40 (2008)
Article DOI: 10.1021/jm800566m
BindingDB Entry DOI: 10.7270/Q26971XR |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM26467
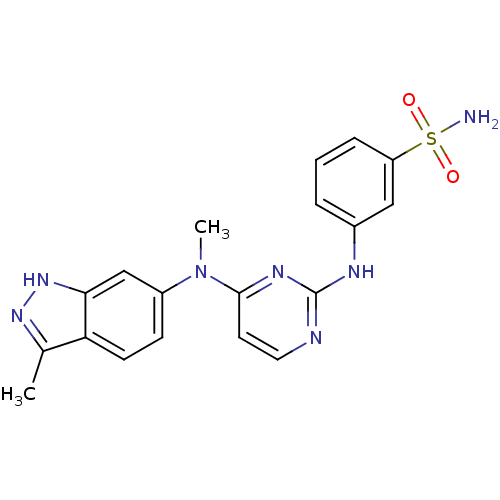
(3-({4-[methyl(3-methyl-1H-indazol-6-yl)amino]pyrim...)Show SMILES CN(c1ccc2c(C)n[nH]c2c1)c1ccnc(Nc2cccc(c2)S(N)(=O)=O)n1 Show InChI InChI=1S/C19H19N7O2S/c1-12-16-7-6-14(11-17(16)25-24-12)26(2)18-8-9-21-19(23-18)22-13-4-3-5-15(10-13)29(20,27)28/h3-11H,1-2H3,(H,24,25)(H2,20,27,28)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
The assay uses purified baculovirus-expressed GST-VEGFR2 interacting with biotinylated peptide substrates. HTRF is based on the proximity of europium... |
J Med Chem 51: 4632-40 (2008)
Article DOI: 10.1021/jm800566m
BindingDB Entry DOI: 10.7270/Q26971XR |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7753
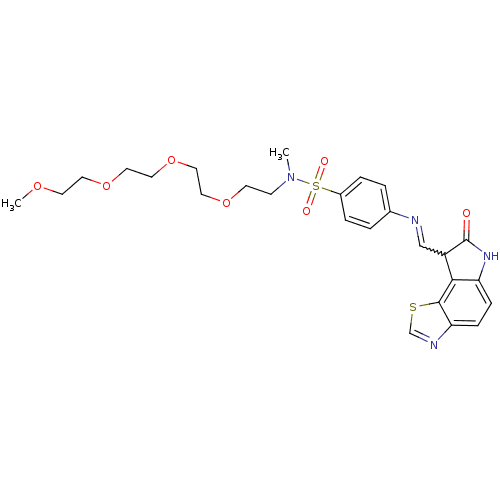
(N-methyl-4-({[(12Z)-11-oxo-3-thia-5,10-diazatricyc...)Show SMILES COCCOCCOCCOCCN(C)S(=O)(=O)c1ccc(cc1)N=CC1C(=O)Nc2ccc3ncsc3c12 |w:25.26| Show InChI InChI=1S/C26H32N4O7S2/c1-30(9-10-35-13-14-37-16-15-36-12-11-34-2)39(32,33)20-5-3-19(4-6-20)27-17-21-24-22(29-26(21)31)7-8-23-25(24)38-18-28-23/h3-8,17-18,21H,9-16H2,1-2H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... |
J Med Chem 44: 4339-58 (2001)
Article DOI: 10.1021/jm010117d
BindingDB Entry DOI: 10.7270/Q2ST7N10 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7692
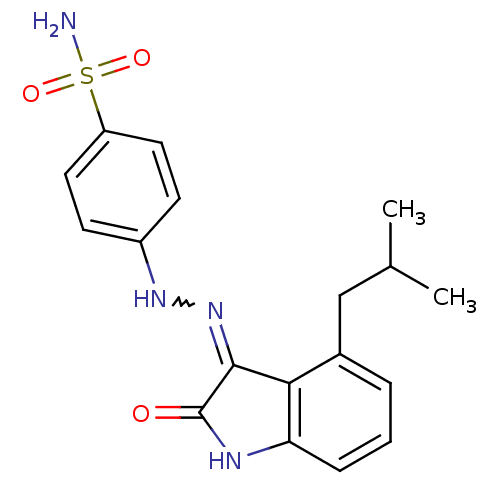
(4-[N -(4-Isobutyl-2-oxo-1,2-dihydro-indol-3-yliden...)Show SMILES CC(C)Cc1cccc2NC(=O)C(=NNc3ccc(cc3)S(N)(=O)=O)c12 |w:13.13| Show InChI InChI=1S/C18H20N4O3S/c1-11(2)10-12-4-3-5-15-16(12)17(18(23)20-15)22-21-13-6-8-14(9-7-13)26(19,24)25/h3-9,11,21H,10H2,1-2H3,(H2,19,24,25)(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... |
J Med Chem 44: 4339-58 (2001)
Article DOI: 10.1021/jm010117d
BindingDB Entry DOI: 10.7270/Q2ST7N10 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7746
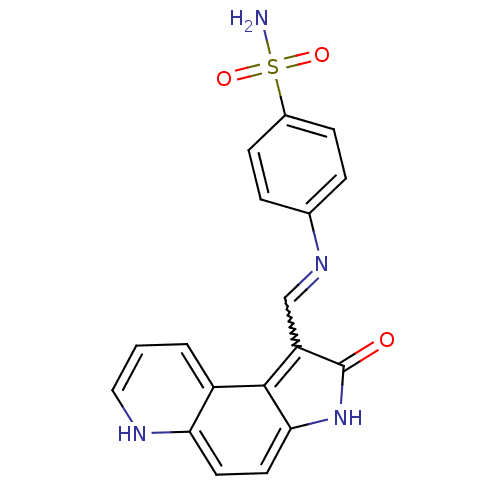
(4-({[(3Z)-4-oxo-5,10-diazatricyclo[7.4.0.0^{2,6}]t...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Cc1c2c(ccc3[nH]cccc23)[nH]c1=O |w:11.12| Show InChI InChI=1S/C18H14N4O3S/c19-26(24,25)12-5-3-11(4-6-12)21-10-14-17-13-2-1-9-20-15(13)7-8-16(17)22-18(14)23/h1-10,20H,(H,22,23)(H2,19,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... |
J Med Chem 44: 4339-58 (2001)
Article DOI: 10.1021/jm010117d
BindingDB Entry DOI: 10.7270/Q2ST7N10 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7693
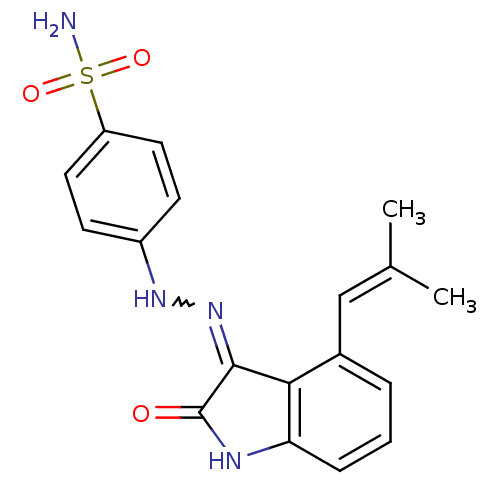
(4-{2-[(3Z)-4-(2-methylprop-1-en-1-yl)-2-oxo-2,3-di...)Show SMILES [#6]\[#6](-[#6])=[#6]\c1cccc2-[#7]-[#6](=O)-[#6](=[#7]-[#7]-c3ccc(cc3)S([#7])(=O)=O)-c12 |w:13.13| Show InChI InChI=1S/C18H18N4O3S/c1-11(2)10-12-4-3-5-15-16(12)17(18(23)20-15)22-21-13-6-8-14(9-7-13)26(19,24)25/h3-10,21H,1-2H3,(H2,19,24,25)(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... |
J Med Chem 44: 4339-58 (2001)
Article DOI: 10.1021/jm010117d
BindingDB Entry DOI: 10.7270/Q2ST7N10 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7745
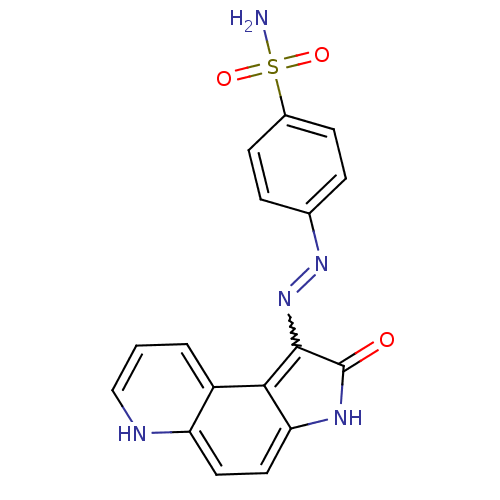
(4-[N -2-Oxo-2,3-dihydropyrrolo[3,2-f]quinolin-1-yl...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1c2c(ccc3[nH]cccc23)[nH]c1=O |w:11.12| Show InChI InChI=1S/C17H13N5O3S/c18-26(24,25)11-5-3-10(4-6-11)21-22-16-15-12-2-1-9-19-13(12)7-8-14(15)20-17(16)23/h1-9,19H,(H,20,23)(H2,18,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... |
J Med Chem 44: 4339-58 (2001)
Article DOI: 10.1021/jm010117d
BindingDB Entry DOI: 10.7270/Q2ST7N10 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM26478
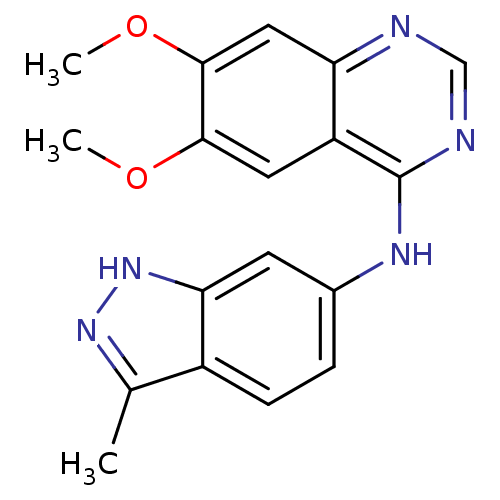
(6,7-dimethoxy-N-(3-methyl-1H-indazol-6-yl)quinazol...)Show InChI InChI=1S/C18H17N5O2/c1-10-12-5-4-11(6-15(12)23-22-10)21-18-13-7-16(24-2)17(25-3)8-14(13)19-9-20-18/h4-9H,1-3H3,(H,22,23)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
The assay uses purified baculovirus-expressed GST-VEGFR2 interacting with biotinylated peptide substrates. HTRF is based on the proximity of europium... |
J Med Chem 51: 4632-40 (2008)
Article DOI: 10.1021/jm800566m
BindingDB Entry DOI: 10.7270/Q26971XR |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7727
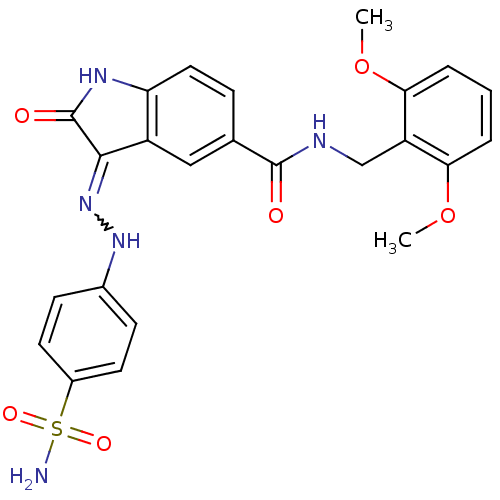
((3Z)-N-[(2,6-dimethoxyphenyl)methyl]-2-oxo-3-[2-(4...)Show SMILES COc1cccc(OC)c1CNC(=O)c1ccc2NC(=O)C(=NNc3ccc(cc3)S(N)(=O)=O)c2c1 |w:22.23| Show InChI InChI=1S/C24H23N5O6S/c1-34-20-4-3-5-21(35-2)18(20)13-26-23(30)14-6-11-19-17(12-14)22(24(31)27-19)29-28-15-7-9-16(10-8-15)36(25,32)33/h3-12,28H,13H2,1-2H3,(H,26,30)(H2,25,32,33)(H,27,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... |
J Med Chem 44: 4339-58 (2001)
Article DOI: 10.1021/jm010117d
BindingDB Entry DOI: 10.7270/Q2ST7N10 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7719
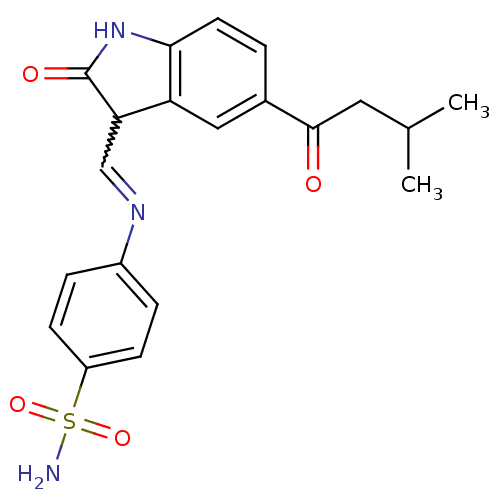
(4-({[(3Z)-5-(3-methylbutanoyl)-2-oxo-2,3-dihydro-1...)Show SMILES CC(C)CC(=O)c1ccc2NC(=O)C(C=Nc3ccc(cc3)S(N)(=O)=O)c2c1 |w:14.13| Show InChI InChI=1S/C20H21N3O4S/c1-12(2)9-19(24)13-3-8-18-16(10-13)17(20(25)23-18)11-22-14-4-6-15(7-5-14)28(21,26)27/h3-8,10-12,17H,9H2,1-2H3,(H,23,25)(H2,21,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... |
J Med Chem 44: 4339-58 (2001)
Article DOI: 10.1021/jm010117d
BindingDB Entry DOI: 10.7270/Q2ST7N10 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50142026
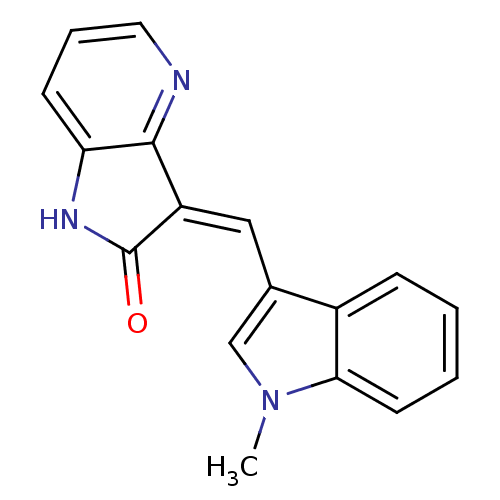
(3-((1-methyl-1H-indol-3-yl)methylene)-1H-pyrrolo[3...)Show InChI InChI=1S/C17H13N3O/c1-20-10-11(12-5-2-3-7-15(12)20)9-13-16-14(19-17(13)21)6-4-8-18-16/h2-10H,1H3,(H,19,21)/b13-9- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of receptor Tyrosine kinase A, TrkA (nerve growth factor receptor) |
Bioorg Med Chem Lett 14: 953-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.002
BindingDB Entry DOI: 10.7270/Q23R0TDF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data