

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094650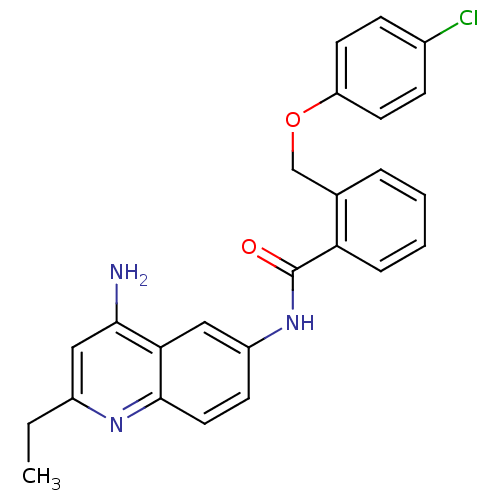 (CHEMBL434060 | N-(4-Amino-2-ethyl-quinolin-6-yl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094648 (CHEMBL139776 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094640 (CHEMBL140640 | N-(4-Amino-2-propyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094651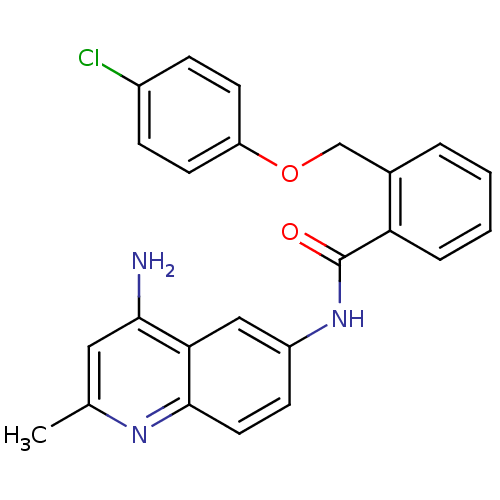 (CHEMBL342580 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094638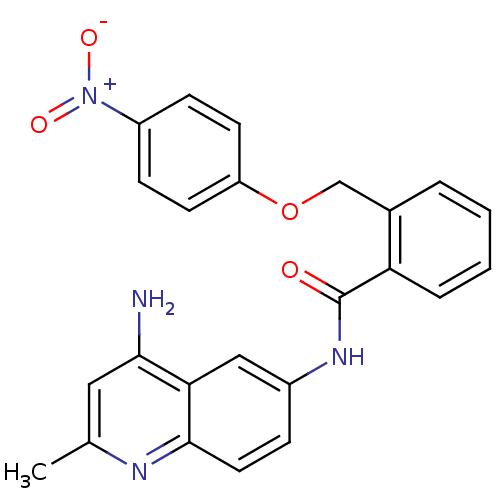 (CHEMBL337128 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094642 (CHEMBL142454 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094646 (CHEMBL139934 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094636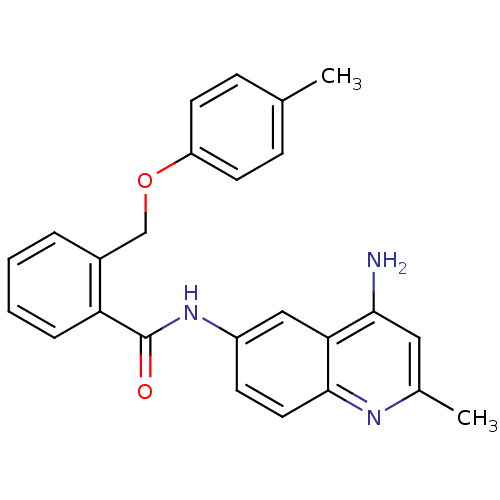 (CHEMBL140103 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094634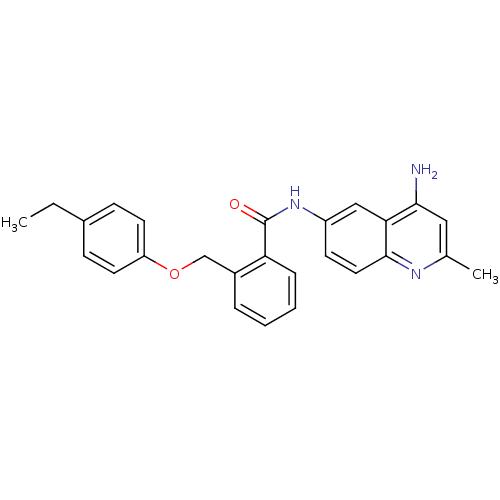 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay | Bioorg Med Chem 21: 979-92 (2013) Article DOI: 10.1016/j.bmc.2012.11.058 BindingDB Entry DOI: 10.7270/Q23F4R13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094644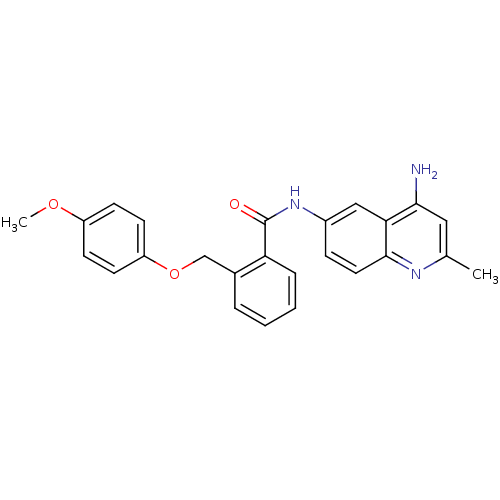 (CHEMBL140519 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094639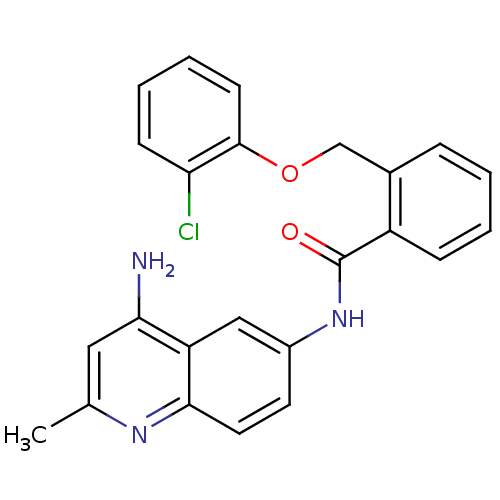 (CHEMBL142999 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094647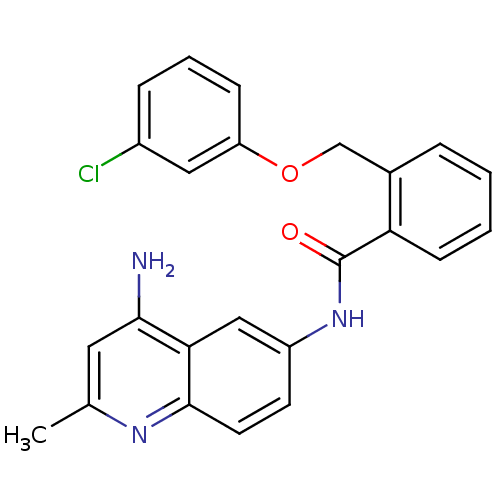 (CHEMBL143605 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094649 (CHEMBL140580 | N-(1-Amino-3-methyl-isoquinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094637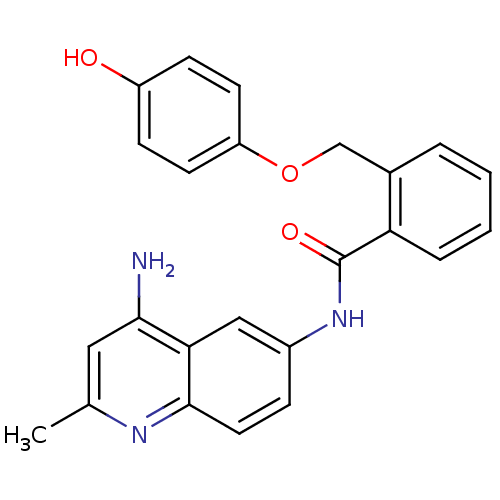 (CHEMBL139566 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094635 (CHEMBL336238 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513317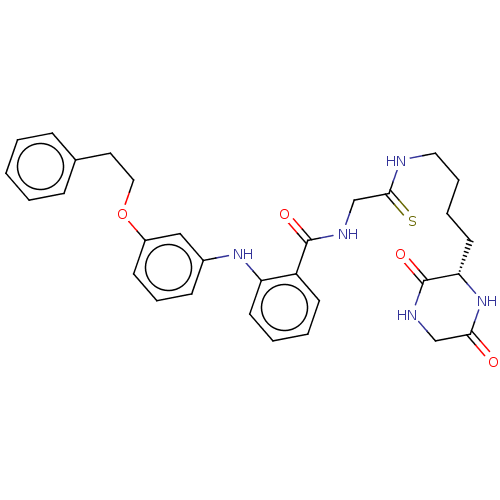 (CHEMBL4465620) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094643 (CHEMBL358306 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094641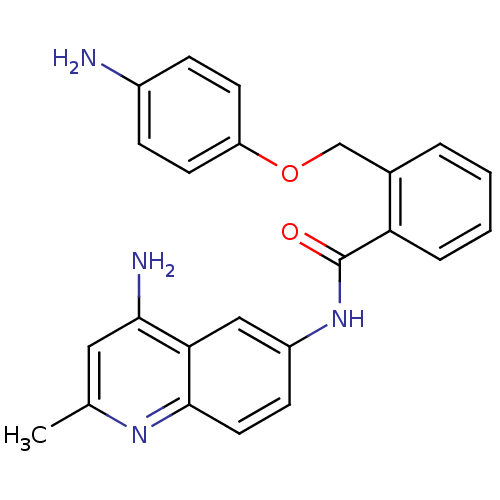 (CHEMBL422641 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094645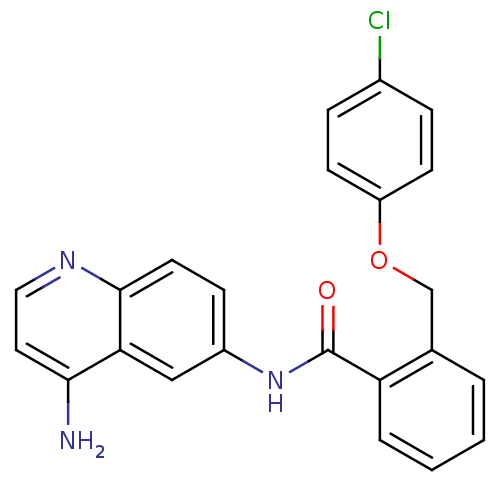 (CHEMBL143243 | N-(4-Amino-quinolin-6-yl)-2-(4-chlo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094652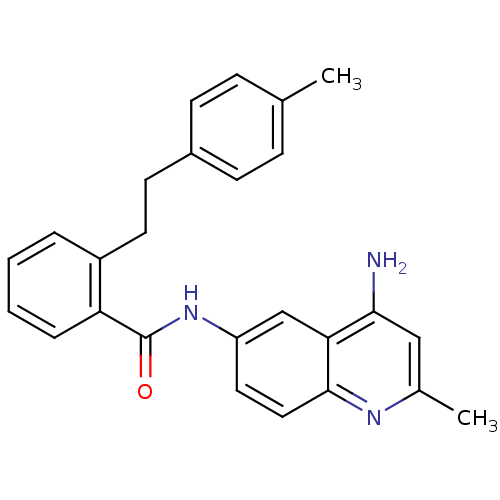 (CHEMBL343424 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50094634 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine (0.33 nM) binding from human Opioid receptor mu 1 expressed in CHO-K1 cells. | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094653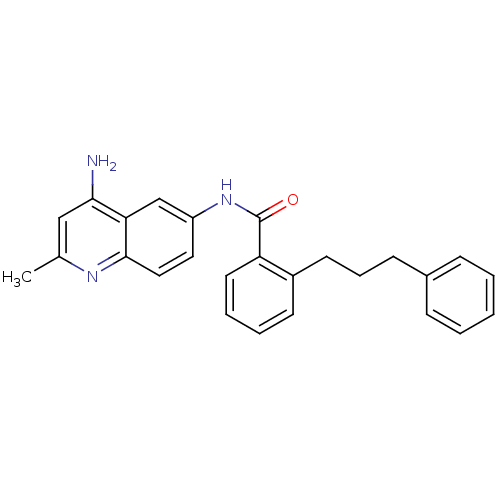 (CHEMBL141078 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094654 (Biphenyl-2-carboxylic acid (4-amino-2-methyl-quino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513318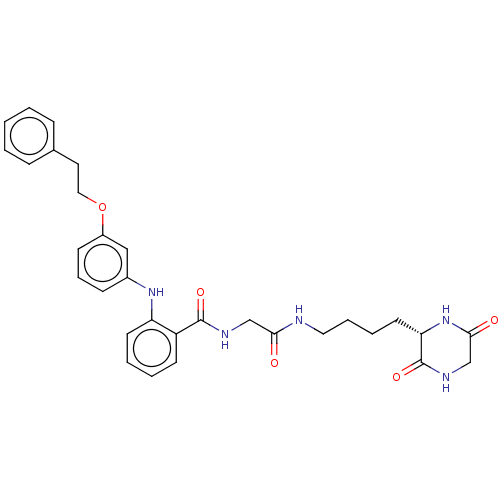 (CHEMBL4516553) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50094634 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor kappa 1 | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50094634 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 8.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor delta 1 expressed in CHO-K1 cells. | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493926 (US10988462, Example 37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50428877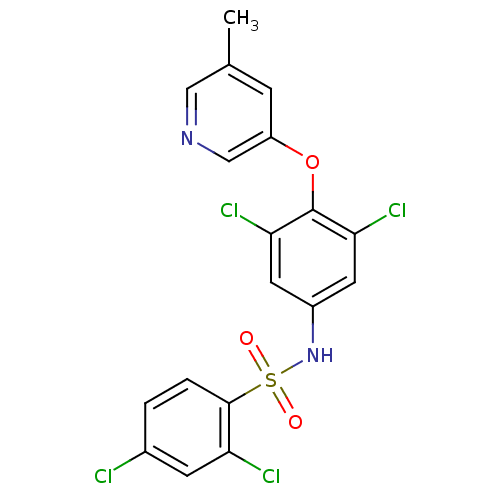 (CHEMBL2338480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem 21: 979-92 (2013) Article DOI: 10.1016/j.bmc.2012.11.058 BindingDB Entry DOI: 10.7270/Q23F4R13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493894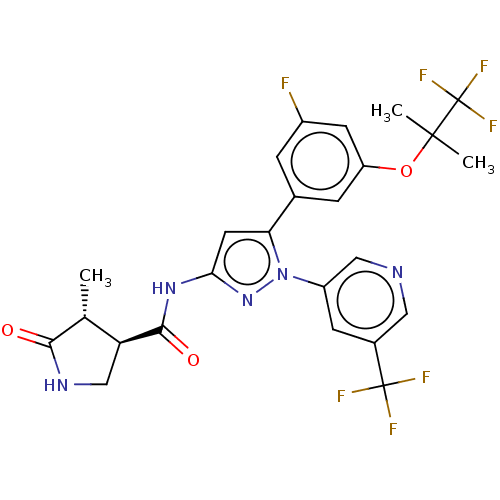 (US10988462, Example 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493906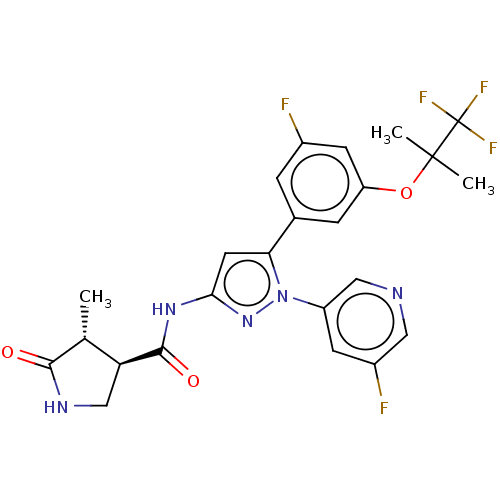 (US10988462, Example 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493905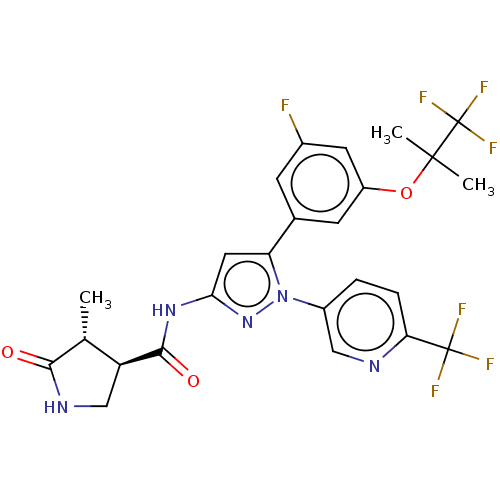 (US10988462, Example 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493901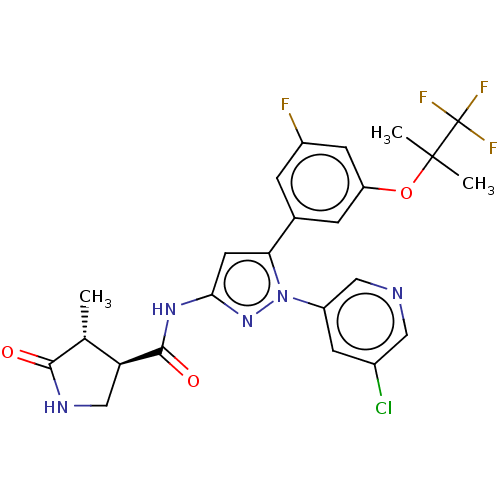 (US10988462, Example 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493879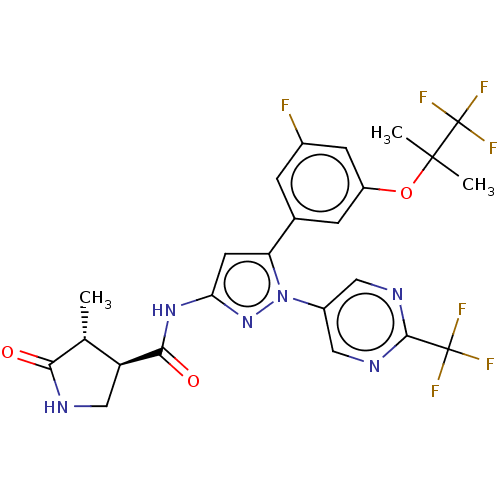 (US10988462, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493925 (US10988462, Example 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493917 (US10988462, Example 30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50467599 (CHEMBL4282070) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C... | Eur J Med Chem 158: 832-852 (2018) Article DOI: 10.1016/j.ejmech.2018.09.003 BindingDB Entry DOI: 10.7270/Q21V5HN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493897 (US10988462, Example 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50467583 (CHEMBL4295170) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C... | Eur J Med Chem 158: 832-852 (2018) Article DOI: 10.1016/j.ejmech.2018.09.003 BindingDB Entry DOI: 10.7270/Q21V5HN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50467595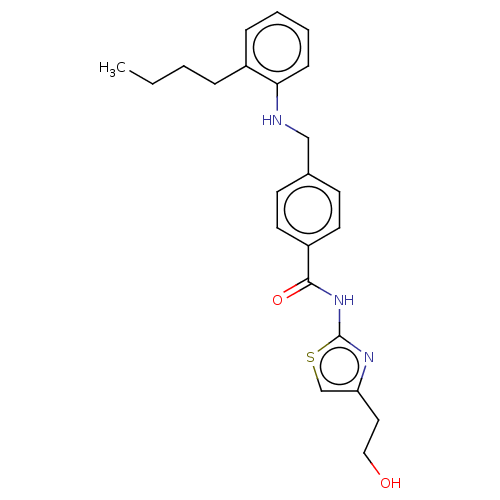 (CHEMBL4277121) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C... | Eur J Med Chem 158: 832-852 (2018) Article DOI: 10.1016/j.ejmech.2018.09.003 BindingDB Entry DOI: 10.7270/Q21V5HN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50467619 (CHEMBL4278602) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C... | Eur J Med Chem 158: 832-852 (2018) Article DOI: 10.1016/j.ejmech.2018.09.003 BindingDB Entry DOI: 10.7270/Q21V5HN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493907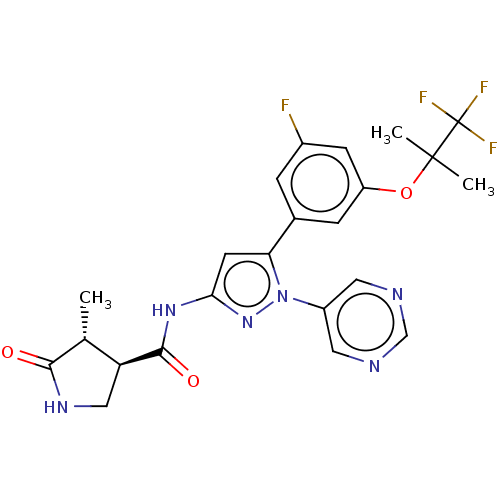 (US10988462, Example 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493898 (US10988462, Example 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50467602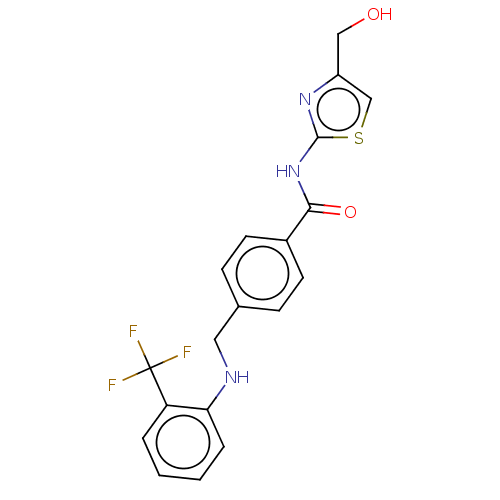 (CHEMBL4277371) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C... | Eur J Med Chem 158: 832-852 (2018) Article DOI: 10.1016/j.ejmech.2018.09.003 BindingDB Entry DOI: 10.7270/Q21V5HN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50467579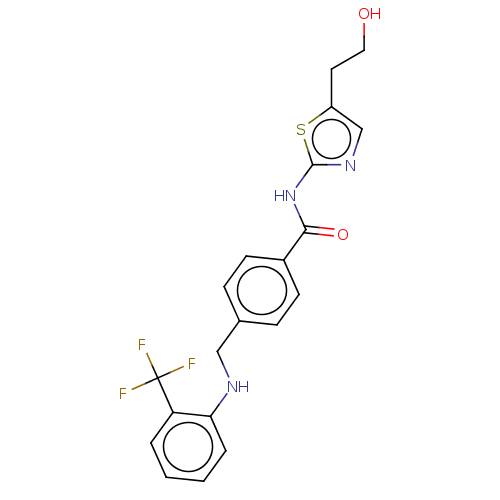 (CHEMBL4289946) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C... | Eur J Med Chem 158: 832-852 (2018) Article DOI: 10.1016/j.ejmech.2018.09.003 BindingDB Entry DOI: 10.7270/Q21V5HN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50467593 (CHEMBL4284259) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of mouse liver microsome SCD assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]CoA as substrate in presence of NADH incu... | Eur J Med Chem 158: 832-852 (2018) Article DOI: 10.1016/j.ejmech.2018.09.003 BindingDB Entry DOI: 10.7270/Q21V5HN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50467578 (CHEMBL4293631) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C... | Eur J Med Chem 158: 832-852 (2018) Article DOI: 10.1016/j.ejmech.2018.09.003 BindingDB Entry DOI: 10.7270/Q21V5HN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493927 (US10988462, Example 38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50467624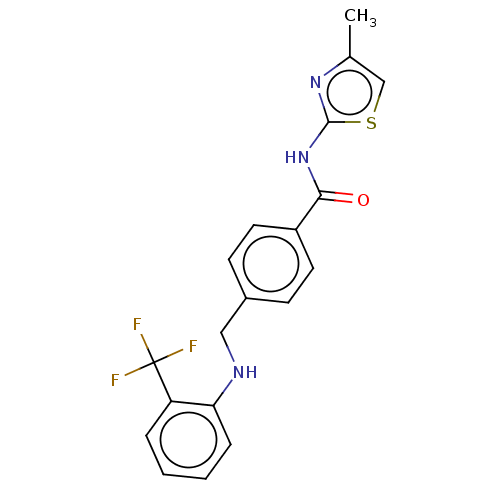 (CHEMBL4282359) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C... | Eur J Med Chem 158: 832-852 (2018) Article DOI: 10.1016/j.ejmech.2018.09.003 BindingDB Entry DOI: 10.7270/Q21V5HN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50467621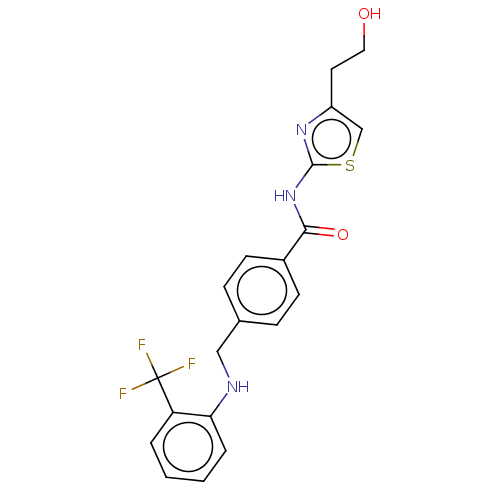 (CHEMBL4288013) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C... | Eur J Med Chem 158: 832-852 (2018) Article DOI: 10.1016/j.ejmech.2018.09.003 BindingDB Entry DOI: 10.7270/Q21V5HN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 223 total ) | Next | Last >> |