

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11626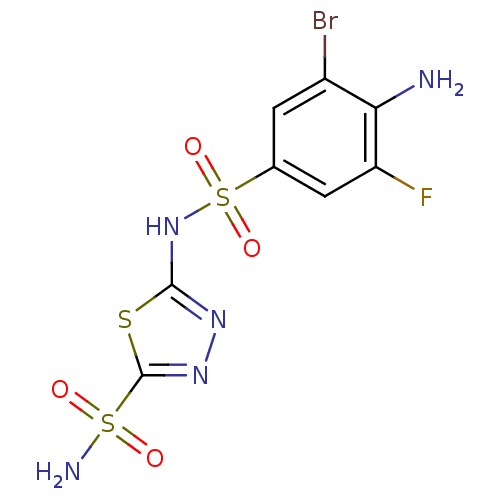 (β-CA inhibitor, 2 | 2-N-(4-amino-3-bromo-5-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11627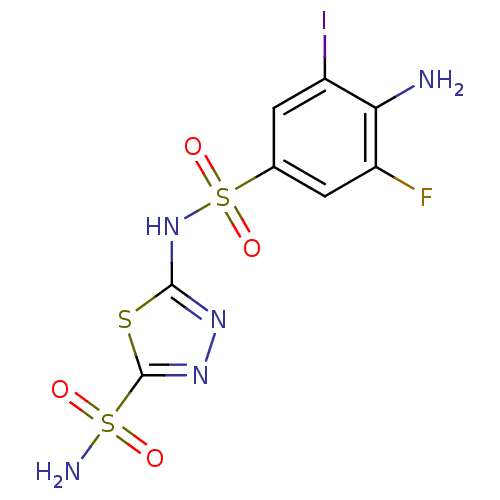 (β-CA inhibitor, 3 | 2-N-(4-amino-3-fluoro-5-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11625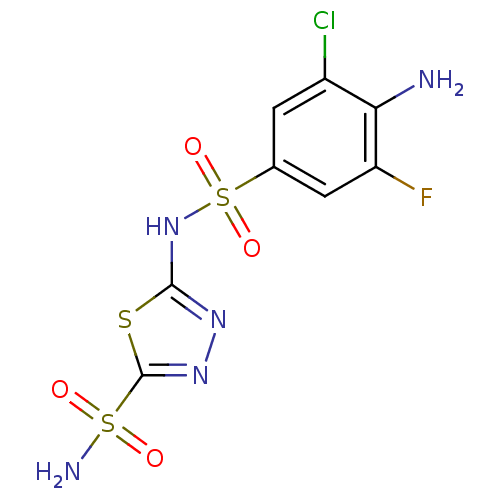 (2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50547697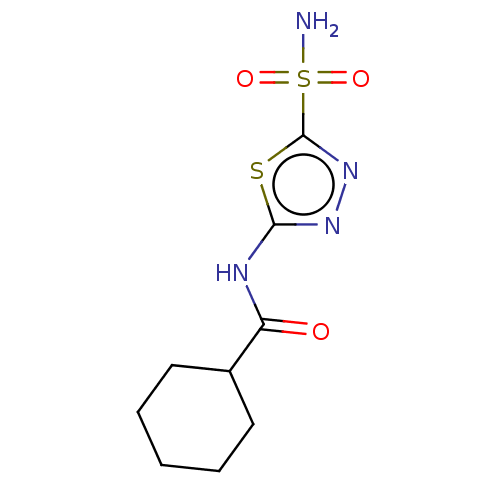 (CHEMBL4739913) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50331834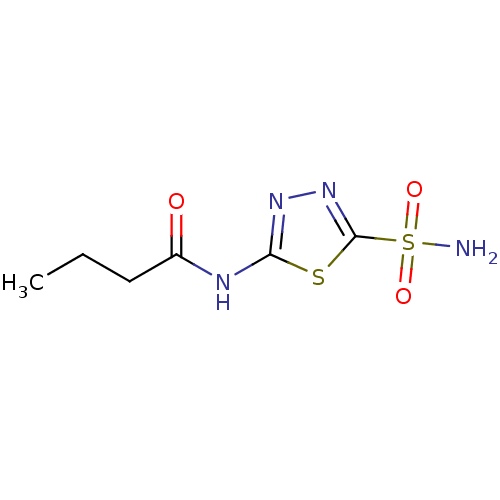 (5-butyramido-2-sulfamoyl-1,3,4-thiadiazole | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM16668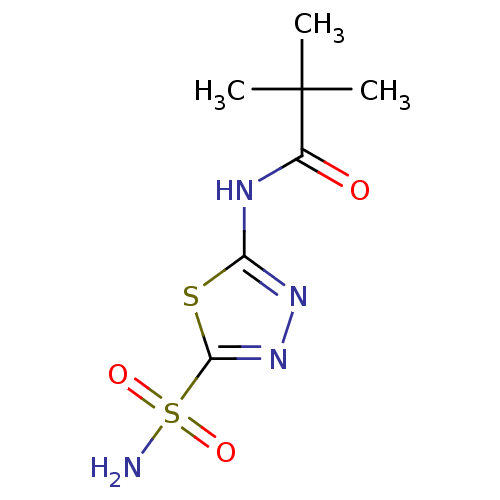 (2,2-dimethyl-N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50331835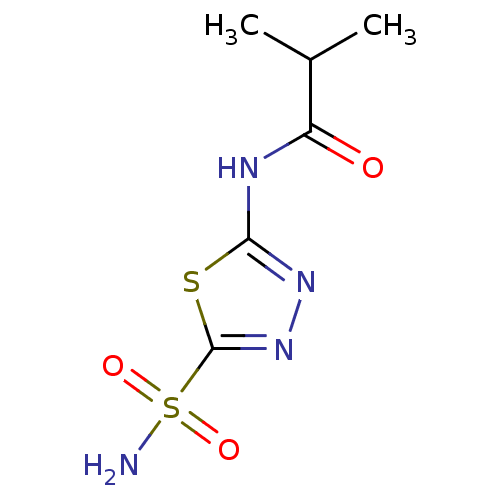 (5-(2-methyl-propylamido)-2-sulfamoyl-1,3,4-thiadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11628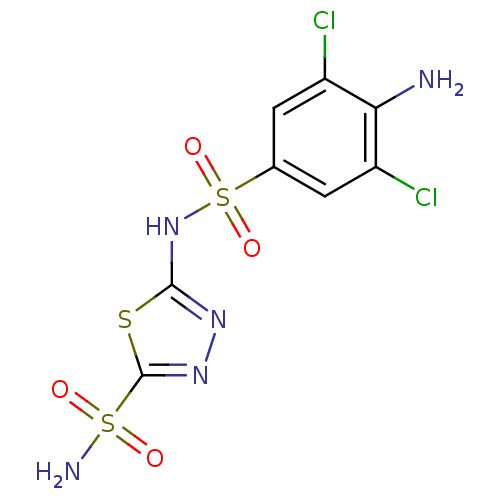 (2-N-(4-amino-3,5-dichlorobenzene)-1,3,4-thiadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11631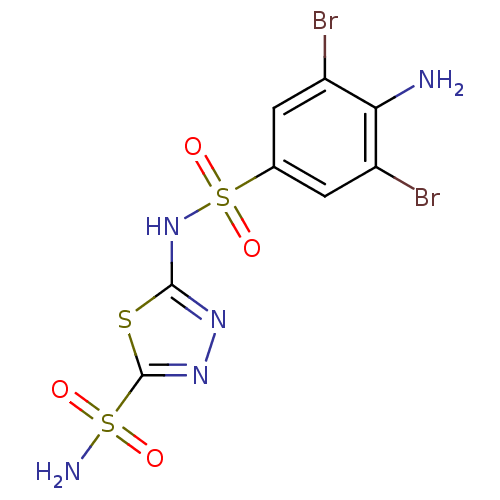 (2-N-(4-amino-3,5-dibromobenzene)-1,3,4-thiadiazole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50331834 (5-butyramido-2-sulfamoyl-1,3,4-thiadiazole | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50185304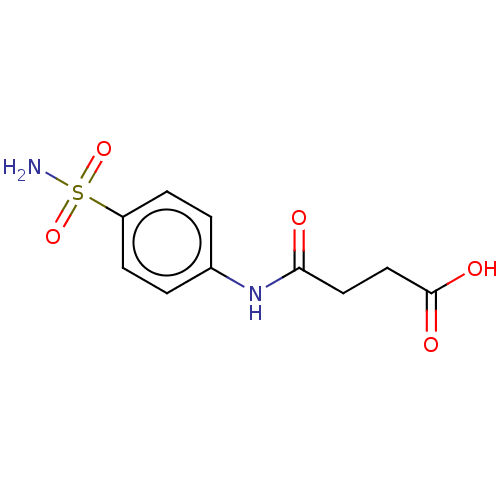 (Sulfasuccinamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50547697 (CHEMBL4739913) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50331835 (5-(2-methyl-propylamido)-2-sulfamoyl-1,3,4-thiadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50084563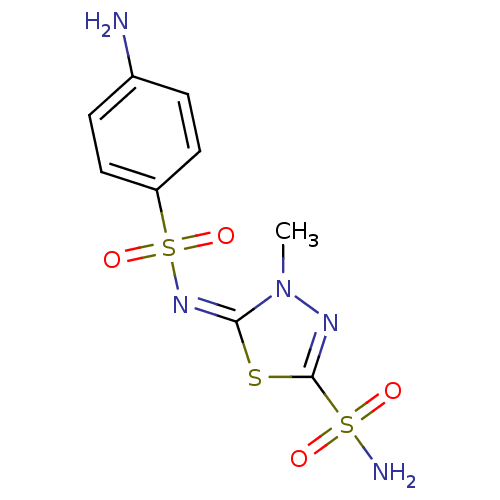 (5-(4-Amino-benzenesulfonylimino)-4-methyl-4,5-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human carbonic anhydrase II (CA2) | J Med Chem 43: 292-300 (2000) BindingDB Entry DOI: 10.7270/Q22806T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11632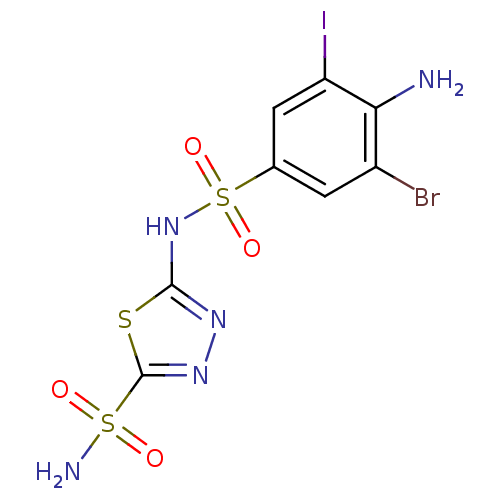 (2-N-(4-amino-3-bromo-5-iodobenzene)-1,3,4-thiadiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11629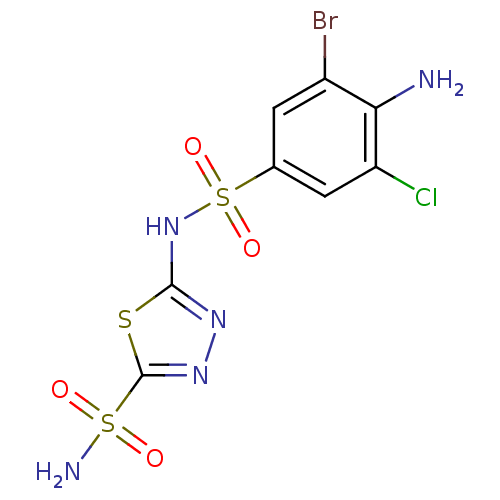 (2-N-(4-amino-3-bromo-5-chlorobenzene)-1,3,4-thiadi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11624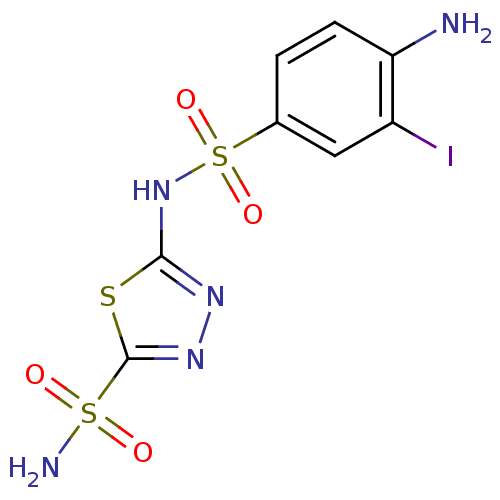 (2-N-(4-amino-3-iodobenzene)-1,3,4-thiadiazole-2,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11622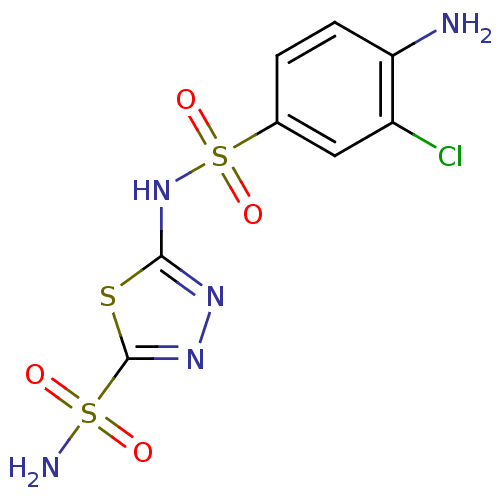 (β-CA inhibitor, 1 | 2-N-(4-amino-3-chlorobenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16668 (2,2-dimethyl-N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11623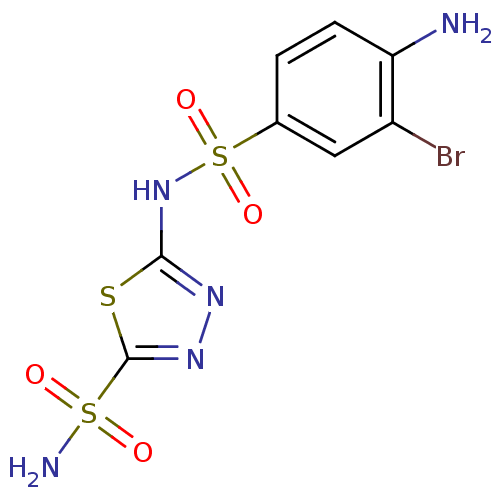 (2-N-(4-amino-3-bromobenzene)-1,3,4-thiadiazole-2,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50331833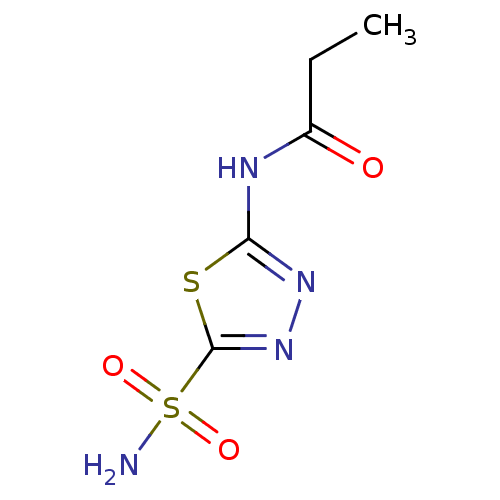 (5-propylamido-2-sulfamoyl-1,3,4-thiadiazole | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01390 BindingDB Entry DOI: 10.7270/Q2BC435H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50084563 (5-(4-Amino-benzenesulfonylimino)-4-methyl-4,5-dihy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes | J Med Chem 43: 292-300 (2000) BindingDB Entry DOI: 10.7270/Q22806T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11630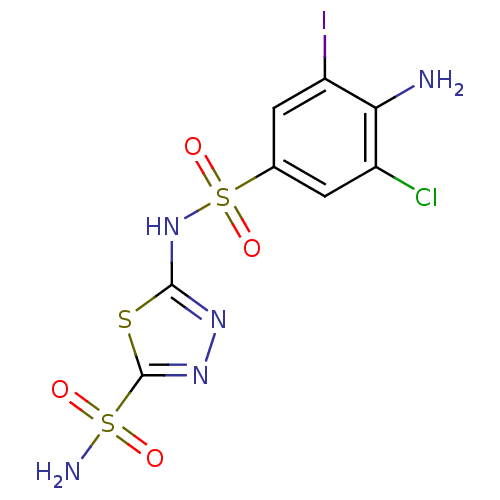 (2-N-(4-amino-3-chloro-5-iodobenzene)-1,3,4-thiadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11621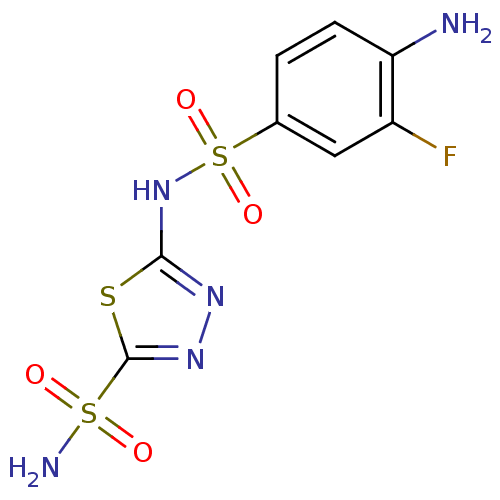 (2-N-(4-amino-3-fluorobenzene)-1,3,4-thiadiazole-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11633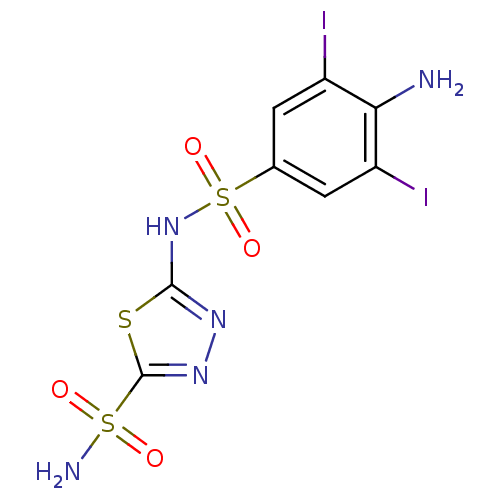 (2-N-(4-amino-3,5-diiodobenzene)-1,3,4-thiadiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11626 (β-CA inhibitor, 2 | 2-N-(4-amino-3-bromo-5-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50094834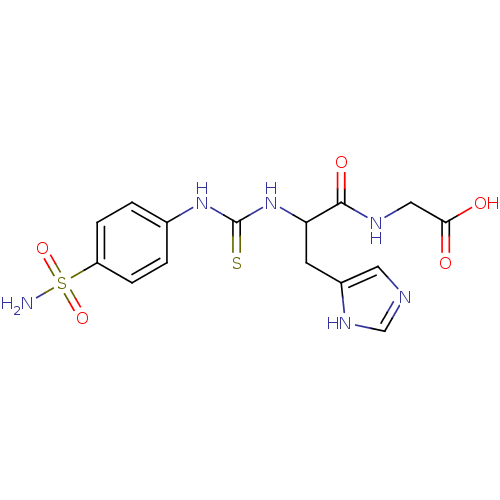 (CHEMBL148167 | {3-(1H-Imidazol-4-yl)-2-[3-(4-sulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 43: 4884-92 (2000) BindingDB Entry DOI: 10.7270/Q22B8ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11631 (2-N-(4-amino-3,5-dibromobenzene)-1,3,4-thiadiazole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50084563 (5-(4-Amino-benzenesulfonylimino)-4-methyl-4,5-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human carbonic anhydrase I (CA1) | J Med Chem 43: 292-300 (2000) BindingDB Entry DOI: 10.7270/Q22806T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11628 (2-N-(4-amino-3,5-dichlorobenzene)-1,3,4-thiadiazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11616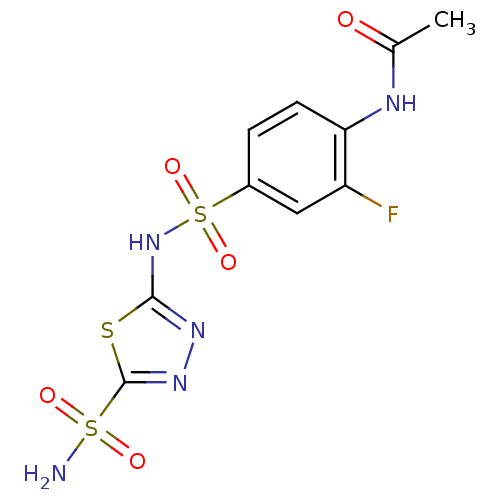 (5-(4-Acetamido-3-fluorobenzenesulfonamido)-1,3,4-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11629 (2-N-(4-amino-3-bromo-5-chlorobenzene)-1,3,4-thiadi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11617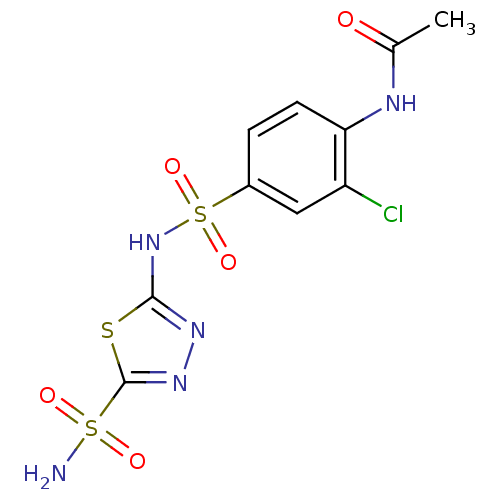 (5-(4-Acetamido-3-chlorobenzenesulfonamido)-1,3,4-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11625 (2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11618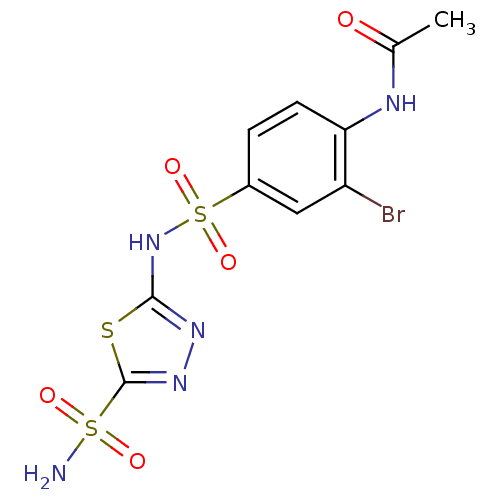 (5-(4-Acetamido-3-bromobenzenesulfonamido)-1,3,4-th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11630 (2-N-(4-amino-3-chloro-5-iodobenzene)-1,3,4-thiadia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11627 (β-CA inhibitor, 3 | 2-N-(4-amino-3-fluoro-5-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11631 (2-N-(4-amino-3,5-dibromobenzene)-1,3,4-thiadiazole...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50185295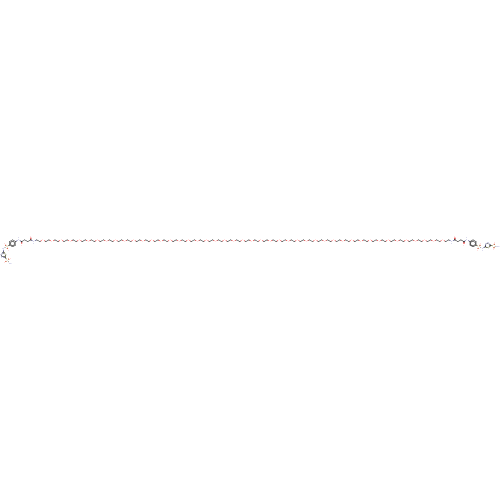 (CHEMBL3822883) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11619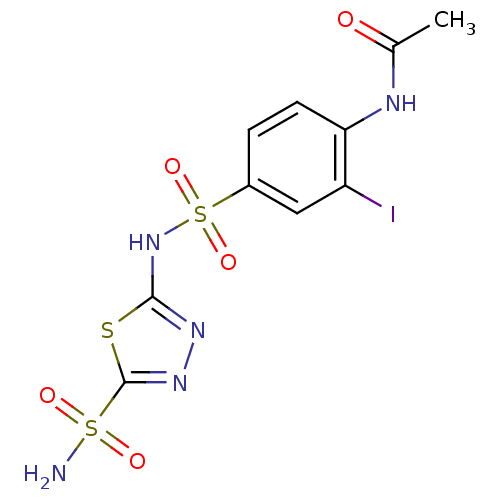 (5-(4-Acetamido-3-iodobenzenesulfonamido)-1,3,4-thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11628 (2-N-(4-amino-3,5-dichlorobenzene)-1,3,4-thiadiazol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11626 (β-CA inhibitor, 2 | 2-N-(4-amino-3-bromo-5-fl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11623 (2-N-(4-amino-3-bromobenzene)-1,3,4-thiadiazole-2,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11632 (2-N-(4-amino-3-bromo-5-iodobenzene)-1,3,4-thiadiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10870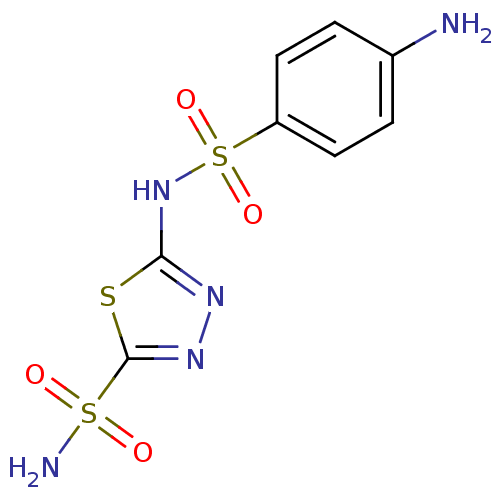 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human carbonic anhydrase II (CA2) | J Med Chem 43: 292-300 (2000) BindingDB Entry DOI: 10.7270/Q22806T7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50084561 (2,6-Dimethyl-1-{[4-(3-methyl-5-sulfamoyl-3H-[1,3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human carbonic anhydrase II (CA2) | J Med Chem 43: 292-300 (2000) BindingDB Entry DOI: 10.7270/Q22806T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50094828 (3-Hydroxy-3-phenyl-2-[3-(4-sulfamoyl-phenyl)-thiou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 43: 4884-92 (2000) BindingDB Entry DOI: 10.7270/Q22B8ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50084560 (2,6-Dimethyl-4-phenyl-1-{[4-(5-sulfamoyl-[1,3,4]th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human carbonic anhydrase II (CA2) | J Med Chem 43: 292-300 (2000) BindingDB Entry DOI: 10.7270/Q22806T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11629 (2-N-(4-amino-3-bromo-5-chlorobenzene)-1,3,4-thiadi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11625 (2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 790 total ) | Next | Last >> |