

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V1a receptor (Microtus ochrogaster) | BDBM50380758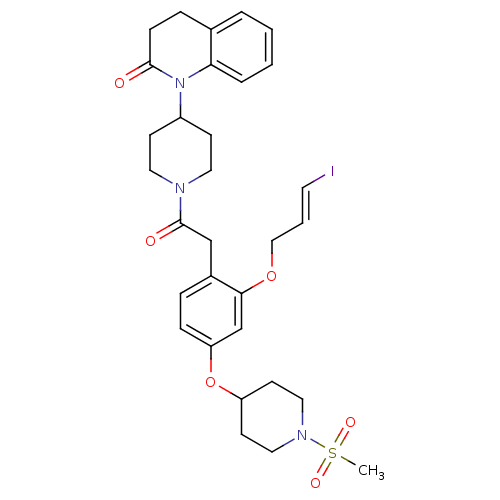 (CHEMBL2017867) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [125I]-LVA from prairie vole vasopressin V1A receptor after 72 hrs | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Microtus ochrogaster) | BDBM50380759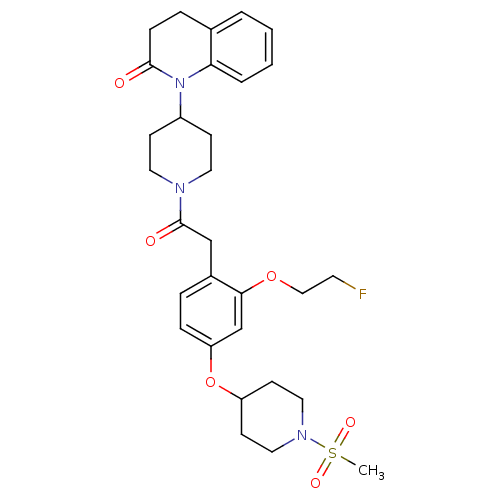 (CHEMBL2017869) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [125I]-OVTA from prairie vole Oxytocin receptor after 72 hrs | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Microtus ochrogaster) | BDBM50380758 (CHEMBL2017867) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [125I]-OVTA from prairie vole Oxytocin receptor after 72 hrs | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Microtus ochrogaster) | BDBM50380757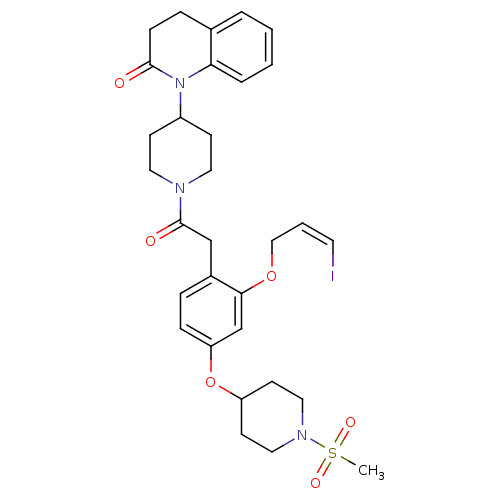 (CHEMBL2017868) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [125I]-OVTA from prairie vole Oxytocin receptor after 72 hrs | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50380757 (CHEMBL2017868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Microtus ochrogaster) | BDBM50380759 (CHEMBL2017869) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [125I]-LVA from prairie vole vasopressin V1A receptor after 72 hrs | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50380758 (CHEMBL2017867) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50380759 (CHEMBL2017869) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50380759 (CHEMBL2017869) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]Oxytocin from human OXTR expressed in CHO cell membranes after 90 mins by liquid scintillation counting method | Bioorg Med Chem 25: 305-315 (2017) Article DOI: 10.1016/j.bmc.2016.10.035 BindingDB Entry DOI: 10.7270/Q24T6MNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Microtus ochrogaster) | BDBM50380757 (CHEMBL2017868) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [125I]-LVA from prairie vole vasopressin V1A receptor after 72 hrs | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50233853 (CHEMBL3392901) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]Oxytocin from human OXTR expressed in CHO cell membranes after 90 mins by liquid scintillation counting method | Bioorg Med Chem 25: 305-315 (2017) Article DOI: 10.1016/j.bmc.2016.10.035 BindingDB Entry DOI: 10.7270/Q24T6MNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Microtus ochrogaster) | BDBM50380760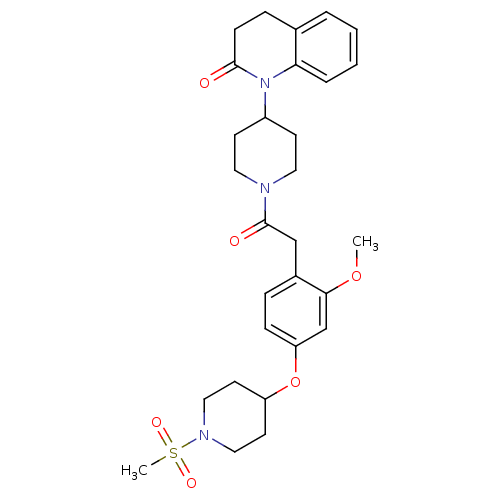 (CHEMBL2017979) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [125I]-OVTA from prairie vole Oxytocin receptor after 72 hrs | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50380760 (CHEMBL2017979) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50380761 (CHEMBL2017980) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Microtus ochrogaster) | BDBM50380760 (CHEMBL2017979) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [125I]-LVA from prairie vole vasopressin V1A receptor after 72 hrs | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50380757 (CHEMBL2017868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1A receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50380757 (CHEMBL2017868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2 receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50380758 (CHEMBL2017867) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1A receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50380761 (CHEMBL2017980) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1A receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50380758 (CHEMBL2017867) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1b receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50380761 (CHEMBL2017980) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1b receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50380761 (CHEMBL2017980) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2 receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50380760 (CHEMBL2017979) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1b receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50380759 (CHEMBL2017869) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1b receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50380758 (CHEMBL2017867) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2 receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50380759 (CHEMBL2017869) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2 receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50380760 (CHEMBL2017979) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2 receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50380760 (CHEMBL2017979) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1A receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50380759 (CHEMBL2017869) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1A receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50380757 (CHEMBL2017868) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1b receptor after 1.5 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2721-38 (2012) Article DOI: 10.1016/j.bmc.2012.02.019 BindingDB Entry DOI: 10.7270/Q29K4C7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366072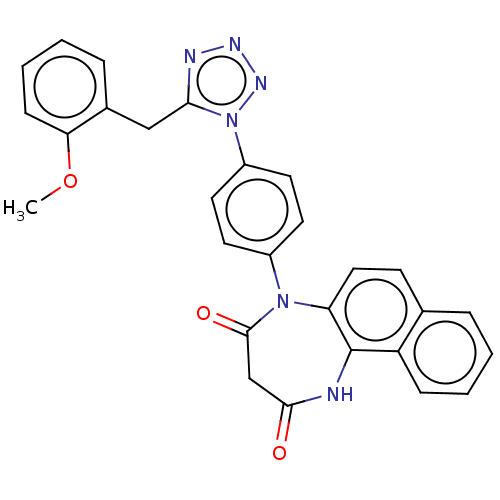 (5-[4-[5-(2-Methoxybenzyl)-1H-tetrazol-1-yl]phenyl]...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366073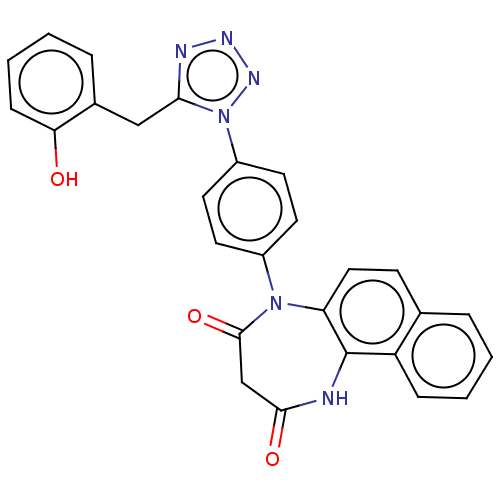 (5-[4-[5-(2-Hydroxybenzyl)-1H-tetrazol-1-yl]phenyl]...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366069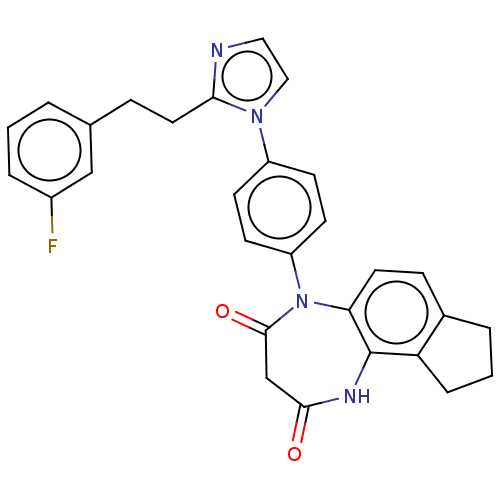 (US9873683, Example 79) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366075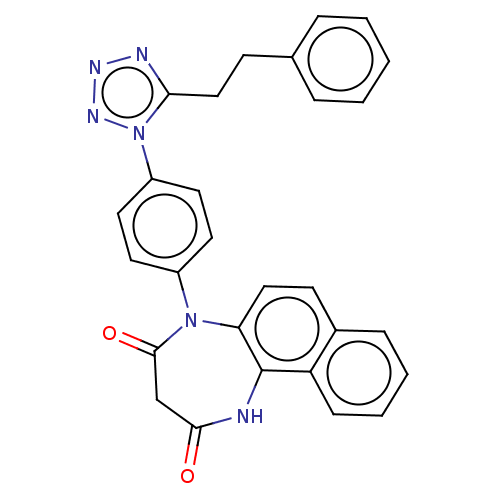 (5-[4-(5-Phenethyl-1H-tetrazol-1-yl)phenyl]-1H-naph...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50391931 (CHEMBL2147632) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of human BChE after 30 mins by microplate reader method | J Med Chem 55: 2649-71 (2012) Article DOI: 10.1021/jm201482p BindingDB Entry DOI: 10.7270/Q2T43V55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50391931 (CHEMBL2147632) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of human BChE after 30 mins by microplate reader method | J Med Chem 55: 2649-71 (2012) Article DOI: 10.1021/jm201482p BindingDB Entry DOI: 10.7270/Q2T43V55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM365870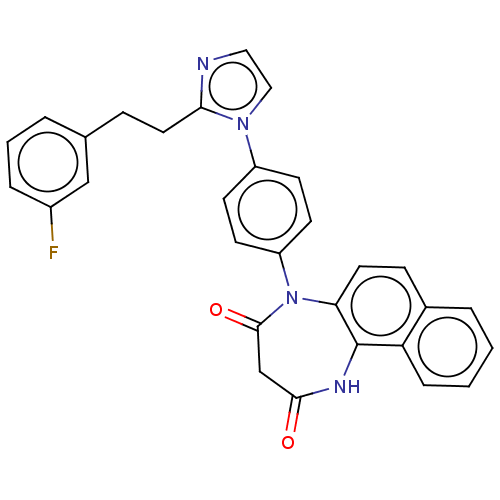 (US9873683, Example 31) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366062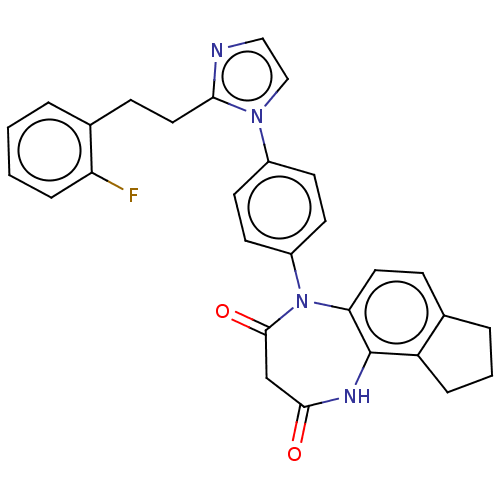 (US9873683, Example 67) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366049 (US9873683, Example 43) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50391933 (CHEMBL2147631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of human BChE after 30 mins by microplate reader method | J Med Chem 55: 2649-71 (2012) Article DOI: 10.1021/jm201482p BindingDB Entry DOI: 10.7270/Q2T43V55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM393615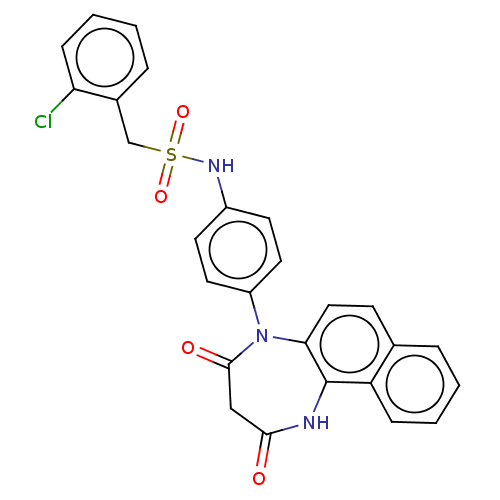 (US09969700, 173) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita del Piemonte Orientale | Assay Description The P2X4 receptor antagonist activity of the compounds of the present invention was measured as follows. The 1321N1 cells stably expressing human P2X... | J Med Chem 52: 3001-9 (2009) BindingDB Entry DOI: 10.7270/Q2542QX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366057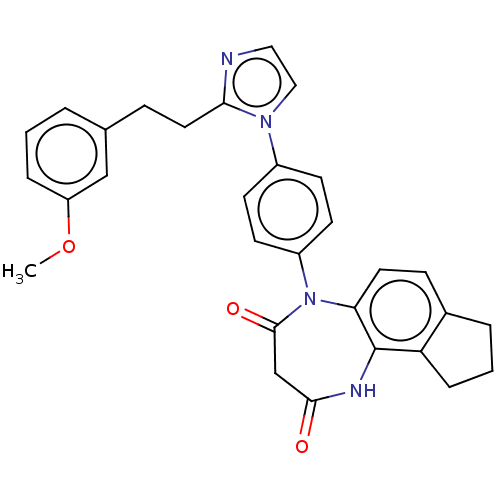 (US9873683, Example 61) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366078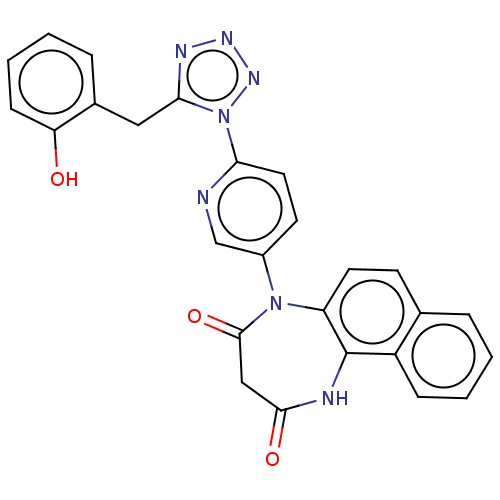 (5-[6-[5-(2-Hydroxybenzyl)-1H-tetrazol-1-yl]pyridin...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM365827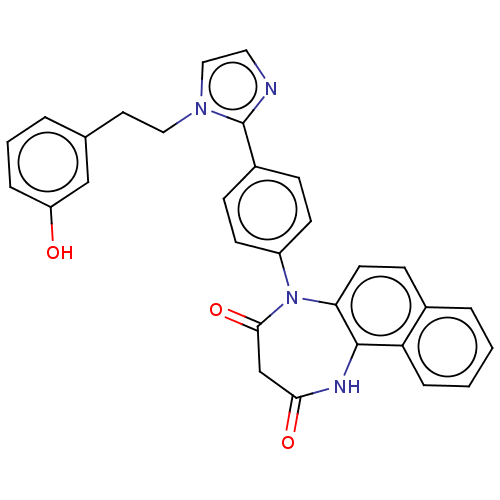 (US9873683, Example 27) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366077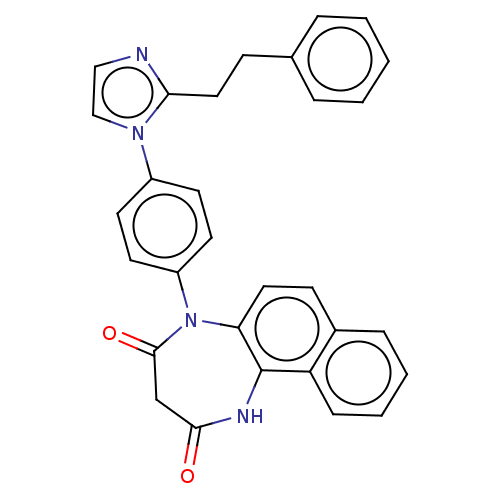 (5-[4-(2-Phenethyl-1H-imidazol-1-yl)phenyl]-1H-naph...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM365950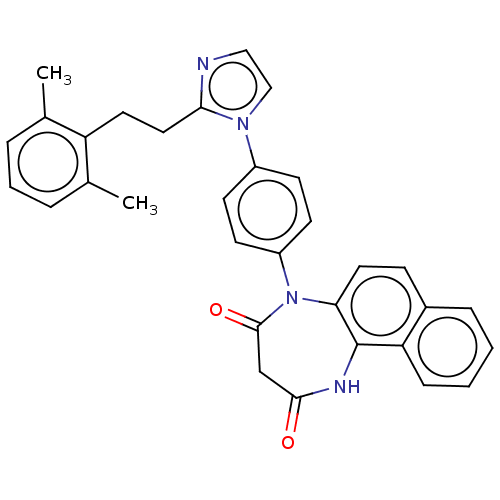 (US9873683, Example 37) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM365816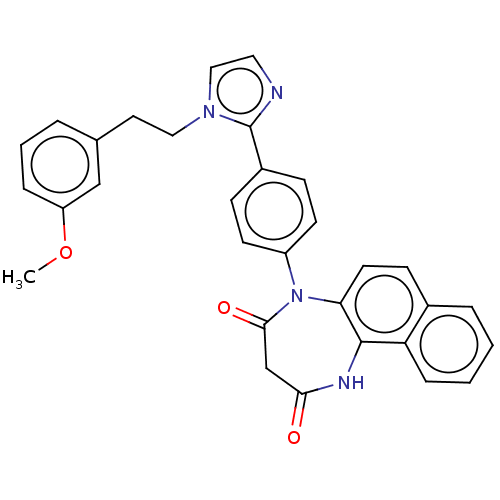 (US9873683, Example 25) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366071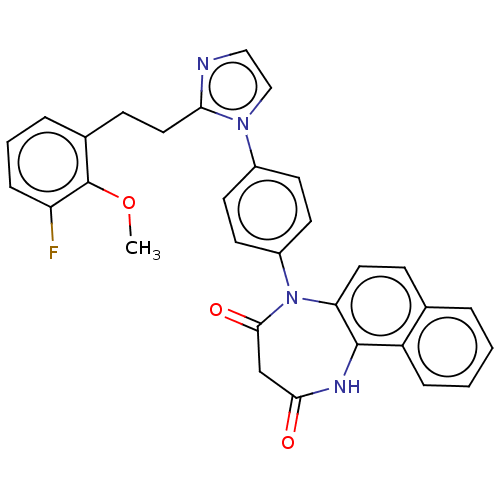 (US9873683, Example 83) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366058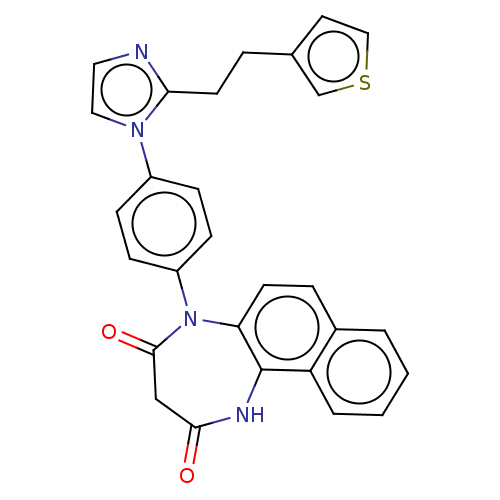 (US9873683, Example 62) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM365756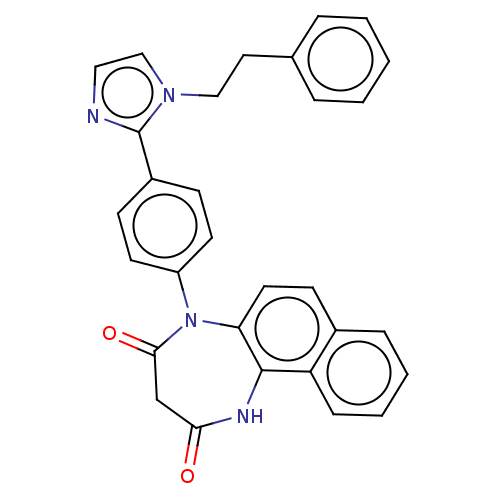 (US9873683, Example 22) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 240 total ) | Next | Last >> |