 Found 652 hits with Last Name = 'inoue' and Initial = 's'
Found 652 hits with Last Name = 'inoue' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063912
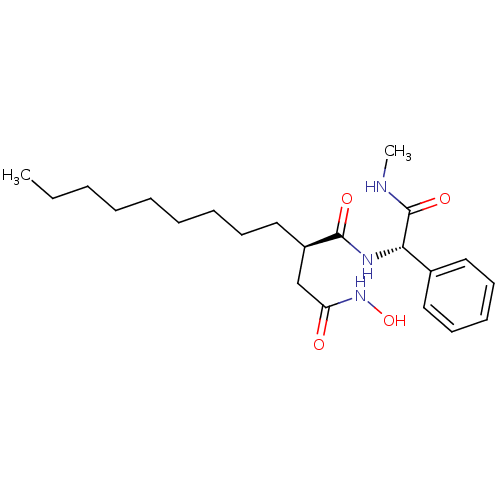
((R)-N*4*-Hydroxy-N*1*-((S)-methylcarbamoyl-phenyl-...)Show SMILES CCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)c1ccccc1 Show InChI InChI=1S/C22H35N3O4/c1-3-4-5-6-7-8-10-15-18(16-19(26)25-29)21(27)24-20(22(28)23-2)17-13-11-9-12-14-17/h9,11-14,18,20,29H,3-8,10,15-16H2,1-2H3,(H,23,28)(H,24,27)(H,25,26)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063915

((2R,3S)-N*4*-Hydroxy-2-isobutyl-3-methyl-N*1*-[(S)...)Show SMILES CNC(=O)[C@H](Cc1cccc2CCCCc12)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C23H35N3O4/c1-14(2)12-19(15(3)21(27)26-30)22(28)25-20(23(29)24-4)13-17-10-7-9-16-8-5-6-11-18(16)17/h7,9-10,14-15,19-20,30H,5-6,8,11-13H2,1-4H3,(H,24,29)(H,25,28)(H,26,27)/t15-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063915

((2R,3S)-N*4*-Hydroxy-2-isobutyl-3-methyl-N*1*-[(S)...)Show SMILES CNC(=O)[C@H](Cc1cccc2CCCCc12)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C23H35N3O4/c1-14(2)12-19(15(3)21(27)26-30)22(28)25-20(23(29)24-4)13-17-10-7-9-16-8-5-6-11-18(16)17/h7,9-10,14-15,19-20,30H,5-6,8,11-13H2,1-4H3,(H,24,29)(H,25,28)(H,26,27)/t15-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-1 (MMP-1). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063915

((2R,3S)-N*4*-Hydroxy-2-isobutyl-3-methyl-N*1*-[(S)...)Show SMILES CNC(=O)[C@H](Cc1cccc2CCCCc12)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C23H35N3O4/c1-14(2)12-19(15(3)21(27)26-30)22(28)25-20(23(29)24-4)13-17-10-7-9-16-8-5-6-11-18(16)17/h7,9-10,14-15,19-20,30H,5-6,8,11-13H2,1-4H3,(H,24,29)(H,25,28)(H,26,27)/t15-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-2 (MMP-2). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238110
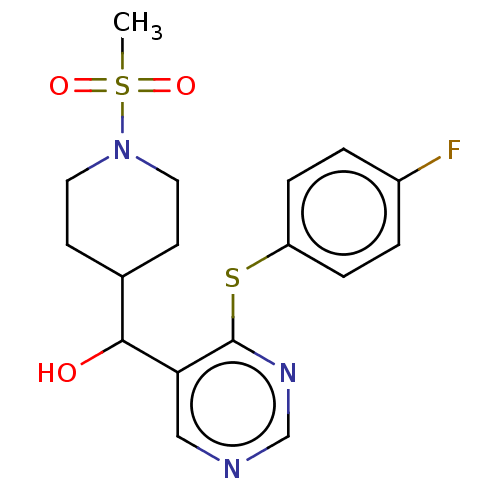
(CHEMBL4088766)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Sc1ccc(F)cc1 Show InChI InChI=1S/C17H20FN3O3S2/c1-26(23,24)21-8-6-12(7-9-21)16(22)15-10-19-11-20-17(15)25-14-4-2-13(18)3-5-14/h2-5,10-12,16,22H,6-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-1 (MMP-1). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50287290
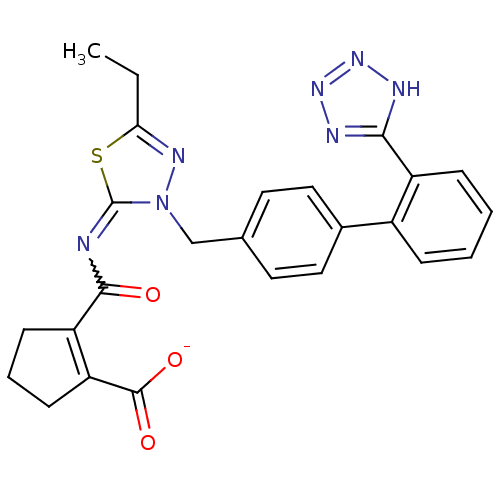
(CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C([O-])=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) |
Bioorg Med Chem Lett 6: 1469-1474 (1996)
Article DOI: 10.1016/S0960-894X(96)00250-8
BindingDB Entry DOI: 10.7270/Q2SN08XQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50287294
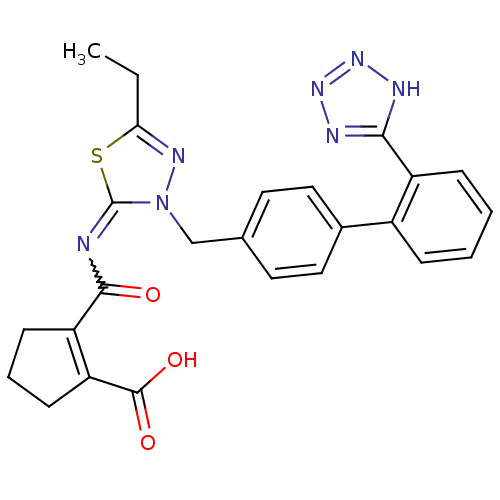
(2-[5-Ethyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C(O)=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) |
Bioorg Med Chem Lett 6: 1469-1474 (1996)
Article DOI: 10.1016/S0960-894X(96)00250-8
BindingDB Entry DOI: 10.7270/Q2SN08XQ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238110

(CHEMBL4088766)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Sc1ccc(F)cc1 Show InChI InChI=1S/C17H20FN3O3S2/c1-26(23,24)21-8-6-12(7-9-21)16(22)15-10-19-11-20-17(15)25-14-4-2-13(18)3-5-14/h2-5,10-12,16,22H,6-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063915

((2R,3S)-N*4*-Hydroxy-2-isobutyl-3-methyl-N*1*-[(S)...)Show SMILES CNC(=O)[C@H](Cc1cccc2CCCCc12)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C23H35N3O4/c1-14(2)12-19(15(3)21(27)26-30)22(28)25-20(23(29)24-4)13-17-10-7-9-16-8-5-6-11-18(16)17/h7,9-10,14-15,19-20,30H,5-6,8,11-13H2,1-4H3,(H,24,29)(H,25,28)(H,26,27)/t15-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-3 (MMP-3). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-3 (MMP-3). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50571009

(CHEMBL4865592)Show SMILES CC(C)n1cc(C(=O)Nc2ccc(Oc3ccnc(NC(=O)CC4CCN(C)CC4)c3)cc2)c(=O)n(-c2ccc(F)cc2)c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged Axl incubated for 1 hr by ADP-glo based luminometry analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116137
BindingDB Entry DOI: 10.7270/Q2PV6Q46 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50287291

(CHEMBL35381 | N-[5-Ethyl-3-[2'-(1H-tetrazol-5-yl)-...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)c2ccccc2C(O)=O)s1 |w:24.27| Show InChI InChI=1S/C26H21N7O3S/c1-2-22-30-33(26(37-22)27-24(34)20-9-5-6-10-21(20)25(35)36)15-16-11-13-17(14-12-16)18-7-3-4-8-19(18)23-28-31-32-29-23/h3-14H,2,15H2,1H3,(H,35,36)(H,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) |
Bioorg Med Chem Lett 6: 1469-1474 (1996)
Article DOI: 10.1016/S0960-894X(96)00250-8
BindingDB Entry DOI: 10.7270/Q2SN08XQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50571017

(CHEMBL4852684)Show SMILES CC(C)n1cc(C(=O)Nc2cc(F)c(Oc3ccnc(NC(=O)N4CCC(CC4)N4CCN(C)CC4)c3)cc2F)c(=O)n(-c2ccc(F)cc2)c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged Axl incubated for 1 hr by ADP-glo based luminometry analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116137
BindingDB Entry DOI: 10.7270/Q2PV6Q46 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434413
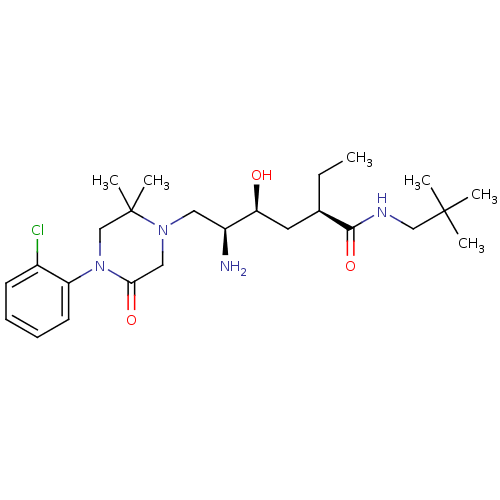
(CHEMBL2387447)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NCC(C)(C)C |r| Show InChI InChI=1S/C25H41ClN4O3/c1-7-17(23(33)28-15-24(2,3)4)12-21(31)19(27)13-29-14-22(32)30(16-25(29,5)6)20-11-9-8-10-18(20)26/h8-11,17,19,21,31H,7,12-16,27H2,1-6H3,(H,28,33)/t17-,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50571010

(CHEMBL4848604)Show SMILES CC(C)n1cc(C(=O)Nc2ccc(Oc3ccnc(NC(=O)CC4CCN(C)CC4)c3)cc2F)c(=O)n(-c2ccc(F)cc2)c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged Axl incubated for 1 hr by ADP-glo based luminometry analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116137
BindingDB Entry DOI: 10.7270/Q2PV6Q46 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50571019

(CHEMBL4845957)Show SMILES CC(C)n1cc(C(=O)Nc2ccc(Oc3cc(NC(=O)C4CC4)ncn3)cc2)c(=O)n(-c2ccc(F)cc2)c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged Axl incubated for 1 hr by ADP-glo based luminometry analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116137
BindingDB Entry DOI: 10.7270/Q2PV6Q46 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434425
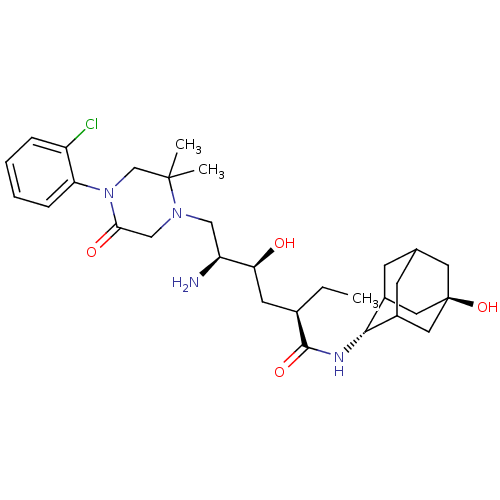
(CHEMBL2387567)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:4.4,2.2,28.29,wD:6.6,35.38,TLB:27:28:38.34.35:32.31.30,27:28:30:38.35.37,36:35:28:32.31.30,THB:34:33:30:38.35.37,34:35:28.33.32:30,37:35:28:32.31.30,37:31:28:38.34.35,(14.26,-52.83,;12.92,-53.6,;12.92,-55.14,;11.59,-55.9,;10.25,-55.13,;10.26,-53.59,;8.92,-55.9,;7.59,-55.13,;8.92,-57.44,;7.58,-58.21,;6.25,-57.44,;4.91,-58.21,;3.57,-57.44,;4.92,-59.75,;6.25,-60.52,;7.58,-59.75,;9.06,-60.14,;7.97,-61.23,;3.58,-60.53,;2.24,-59.76,;.92,-60.53,;.91,-62.08,;2.25,-62.85,;3.58,-62.08,;4.92,-62.85,;14.25,-55.91,;14.25,-57.45,;15.59,-55.14,;16.92,-55.91,;18.12,-54.64,;18.11,-53.15,;19.46,-52.67,;18.42,-53.9,;18.42,-55.49,;19.83,-56.06,;20.84,-54.78,;22.17,-55.55,;20.85,-53.25,;19.45,-55.13,)| Show InChI InChI=1S/C30H45ClN4O4/c1-4-19(28(38)33-27-20-9-18-10-21(27)14-30(39,12-18)13-20)11-25(36)23(32)15-34-16-26(37)35(17-29(34,2)3)24-8-6-5-7-22(24)31/h5-8,18-21,23,25,27,36,39H,4,9-17,32H2,1-3H3,(H,33,38)/t18?,19-,20?,21?,23+,25+,27-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063921
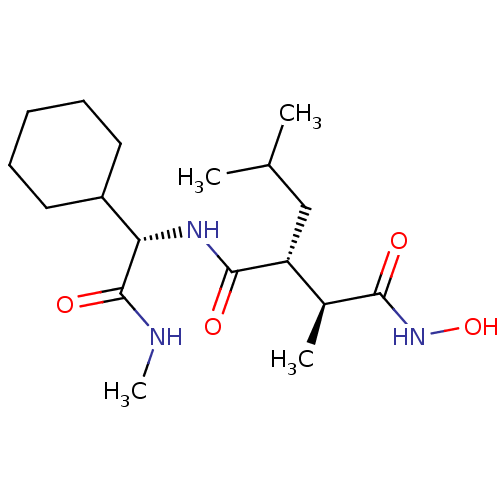
((2R,3S)-N*1*-((S)-Cyclohexyl-methylcarbamoyl-methy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO)C1CCCCC1 Show InChI InChI=1S/C18H33N3O4/c1-11(2)10-14(12(3)16(22)21-25)17(23)20-15(18(24)19-4)13-8-6-5-7-9-13/h11-15,25H,5-10H2,1-4H3,(H,19,24)(H,20,23)(H,21,22)/t12-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434435
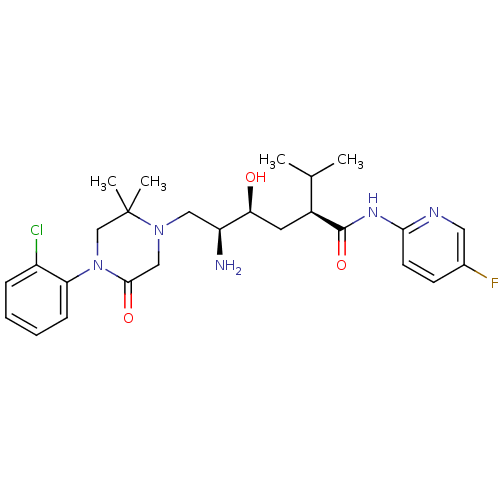
(CHEMBL2387557)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)Nc1ccc(F)cn1 |r| Show InChI InChI=1S/C26H35ClFN5O3/c1-16(2)18(25(36)31-23-10-9-17(28)12-30-23)11-22(34)20(29)13-32-14-24(35)33(15-26(32,3)4)21-8-6-5-7-19(21)27/h5-10,12,16,18,20,22,34H,11,13-15,29H2,1-4H3,(H,30,31,36)/t18-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434412
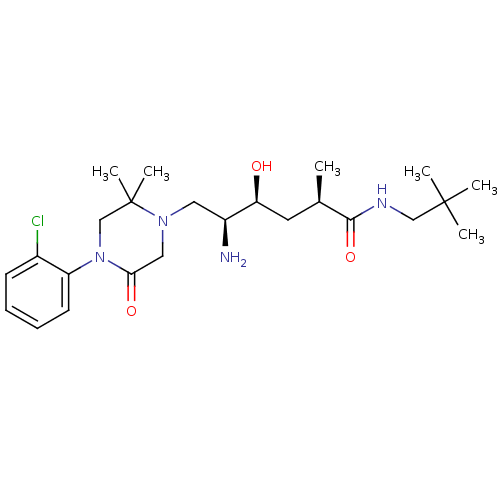
(CHEMBL2387448)Show SMILES C[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NCC(C)(C)C |r| Show InChI InChI=1S/C24H39ClN4O3/c1-16(22(32)27-14-23(2,3)4)11-20(30)18(26)12-28-13-21(31)29(15-24(28,5)6)19-10-8-7-9-17(19)25/h7-10,16,18,20,30H,11-15,26H2,1-6H3,(H,27,32)/t16-,18+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434414
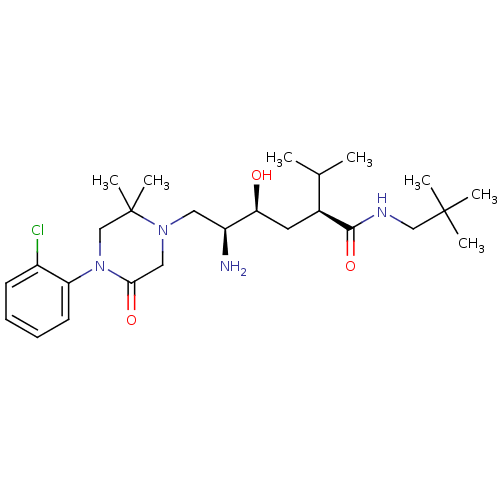
(CHEMBL2387446)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NCC(C)(C)C |r| Show InChI InChI=1S/C26H43ClN4O3/c1-17(2)18(24(34)29-15-25(3,4)5)12-22(32)20(28)13-30-14-23(33)31(16-26(30,6)7)21-11-9-8-10-19(21)27/h8-11,17-18,20,22,32H,12-16,28H2,1-7H3,(H,29,34)/t18-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387262
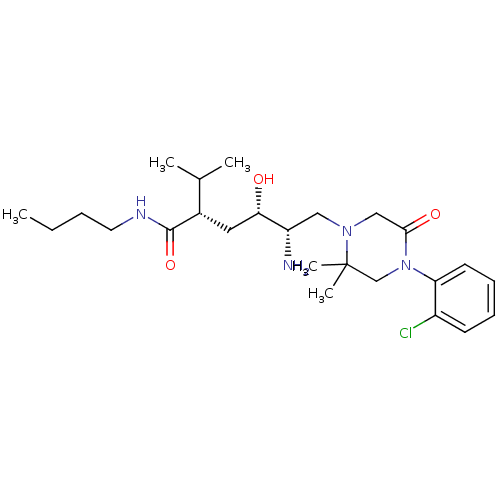
(CHEMBL2048702)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(C)C |r| Show InChI InChI=1S/C25H41ClN4O3/c1-6-7-12-28-24(33)18(17(2)3)13-22(31)20(27)14-29-15-23(32)30(16-25(29,4)5)21-11-9-8-10-19(21)26/h8-11,17-18,20,22,31H,6-7,12-16,27H2,1-5H3,(H,28,33)/t18-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381600
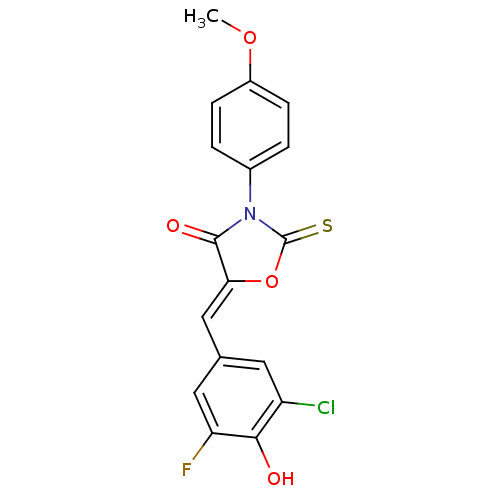
(CHEMBL2018254)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2cc(F)c(O)c(Cl)c2)C1=O Show InChI InChI=1S/C17H11ClFNO4S/c1-23-11-4-2-10(3-5-11)20-16(22)14(24-17(20)25)8-9-6-12(18)15(21)13(19)7-9/h2-8,21H,1H3/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434434
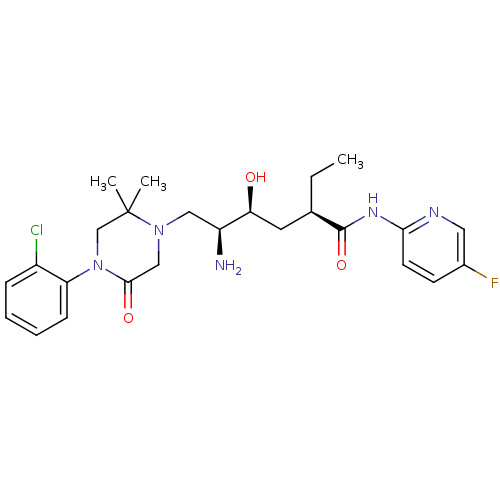
(CHEMBL2387558)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)Nc1ccc(F)cn1 |r| Show InChI InChI=1S/C25H33ClFN5O3/c1-4-16(24(35)30-22-10-9-17(27)12-29-22)11-21(33)19(28)13-31-14-23(34)32(15-25(31,2)3)20-8-6-5-7-18(20)26/h5-10,12,16,19,21,33H,4,11,13-15,28H2,1-3H3,(H,29,30,35)/t16-,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434429
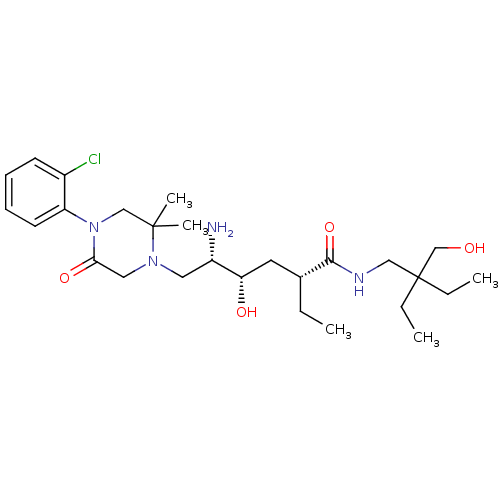
(CHEMBL2387563)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NCC(CC)(CC)CO |r| Show InChI InChI=1S/C27H45ClN4O4/c1-6-19(25(36)30-16-27(7-2,8-3)18-33)13-23(34)21(29)14-31-15-24(35)32(17-26(31,4)5)22-12-10-9-11-20(22)28/h9-12,19,21,23,33-34H,6-8,13-18,29H2,1-5H3,(H,30,36)/t19-,21+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50439255
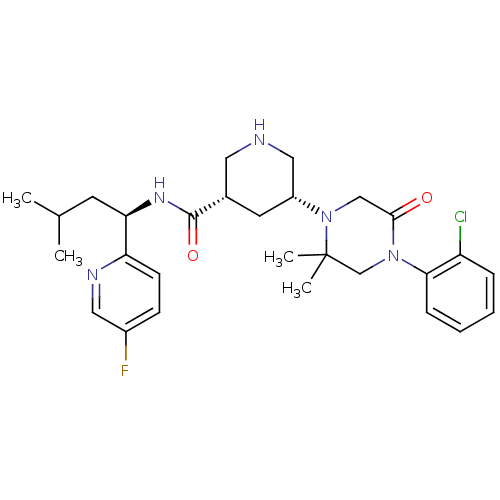
(CHEMBL2419040)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H]1CNC[C@@H](C1)N1CC(=O)N(CC1(C)C)c1ccccc1Cl)c1ccc(F)cn1 |r| Show InChI InChI=1S/C28H37ClFN5O2/c1-18(2)11-24(23-10-9-20(30)14-32-23)33-27(37)19-12-21(15-31-13-19)35-16-26(36)34(17-28(35,3)4)25-8-6-5-7-22(25)29/h5-10,14,18-19,21,24,31H,11-13,15-17H2,1-4H3,(H,33,37)/t19-,21+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin using (Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg) as substrate i... |
Bioorg Med Chem 21: 5907-22 (2013)
Article DOI: 10.1016/j.bmc.2013.06.057
BindingDB Entry DOI: 10.7270/Q2TH8P4B |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-2 (MMP-2). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434424
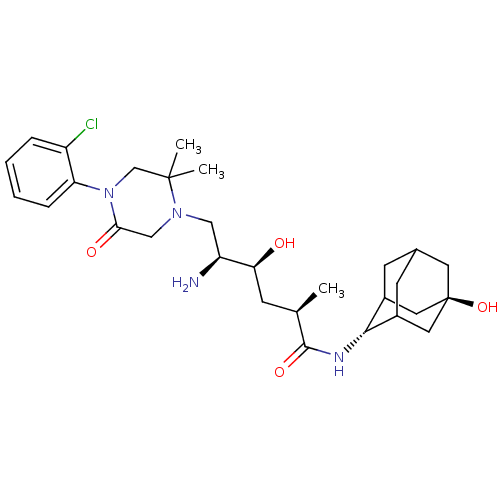
(CHEMBL2387568)Show SMILES C[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:3.3,1.0,27.28,wD:5.5,34.37,TLB:26:27:37.33.34:31.30.29,26:27:29:37.34.36,35:34:27:31.30.29,THB:33:32:29:37.34.36,33:34:27.32.31:29,36:34:27:31.30.29,36:30:27:37.33.34,(36.05,-54.18,;36.05,-55.72,;34.71,-56.49,;33.38,-55.72,;33.38,-54.18,;32.04,-56.49,;30.71,-55.71,;32.04,-58.03,;30.71,-58.79,;29.37,-58.03,;28.03,-58.8,;26.7,-58.03,;28.04,-60.33,;29.38,-61.1,;30.7,-60.33,;32.19,-60.73,;31.1,-61.82,;26.7,-61.11,;25.37,-60.35,;24.04,-61.12,;24.04,-62.66,;25.37,-63.43,;26.71,-62.66,;28.04,-63.43,;37.38,-56.49,;37.38,-58.03,;38.71,-55.73,;40.05,-56.5,;41.24,-55.22,;41.23,-53.74,;42.58,-53.26,;41.54,-54.49,;41.55,-56.08,;42.95,-56.64,;43.97,-55.37,;45.29,-56.13,;43.98,-53.84,;42.57,-55.71,)| Show InChI InChI=1S/C29H43ClN4O4/c1-17(27(37)32-26-19-9-18-10-20(26)13-29(38,11-18)12-19)8-24(35)22(31)14-33-15-25(36)34(16-28(33,2)3)23-7-5-4-6-21(23)30/h4-7,17-20,22,24,26,35,38H,8-16,31H2,1-3H3,(H,32,37)/t17-,18?,19?,20?,22+,24+,26-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434428
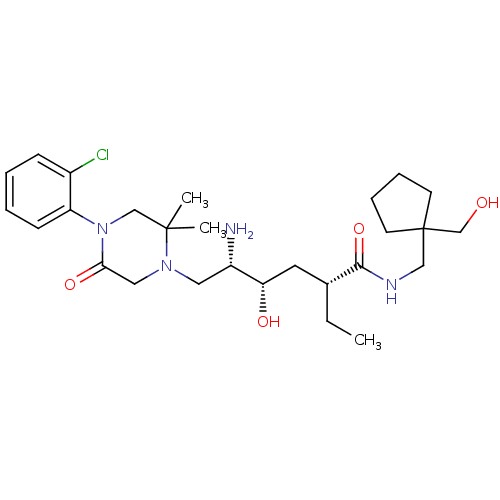
(CHEMBL2387564)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NCC1(CO)CCCC1 |r| Show InChI InChI=1S/C27H43ClN4O4/c1-4-19(25(36)30-16-27(18-33)11-7-8-12-27)13-23(34)21(29)14-31-15-24(35)32(17-26(31,2)3)22-10-6-5-9-20(22)28/h5-6,9-10,19,21,23,33-34H,4,7-8,11-18,29H2,1-3H3,(H,30,36)/t19-,21+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434423
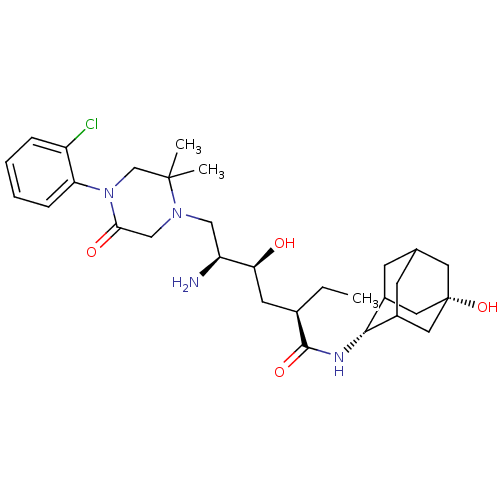
(CHEMBL2387569)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@@](O)(C3)C2 |r,wU:4.4,2.2,28.29,35.38,wD:6.6,TLB:32:33:38:30.31.37,32:31:28.33.34:38,36:35:28:30.32.31,THB:27:28:30.32.31:34.35.38,27:28:38:30.31.37,37:31:28:34.35.38,37:35:28:30.32.31,(14.52,-3.69,;13.18,-4.46,;13.18,-6,;11.84,-6.77,;10.51,-5.99,;10.51,-4.45,;9.18,-6.76,;7.84,-5.99,;9.17,-8.3,;7.84,-9.07,;6.5,-8.3,;5.16,-9.07,;3.83,-8.31,;5.17,-10.61,;6.51,-11.38,;7.83,-10.61,;9.32,-11.01,;8.23,-12.09,;3.84,-11.39,;2.5,-10.62,;1.17,-11.4,;1.17,-12.94,;2.5,-13.71,;3.84,-12.94,;5.18,-13.71,;14.51,-6.77,;14.51,-8.31,;15.85,-6,;17.18,-6.77,;18.38,-5.5,;19.7,-5.99,;21.1,-5.64,;20.09,-6.92,;18.68,-6.35,;18.68,-4.76,;19.71,-3.53,;19.7,-1.99,;21.11,-4.11,;18.37,-4.01,)| Show InChI InChI=1S/C30H45ClN4O4/c1-4-19(28(38)33-27-20-9-18-10-21(27)14-30(39,12-18)13-20)11-25(36)23(32)15-34-16-26(37)35(17-29(34,2)3)24-8-6-5-7-22(24)31/h5-8,18-21,23,25,27,36,39H,4,9-17,32H2,1-3H3,(H,33,38)/t18?,19-,20?,21?,23+,25+,27-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434411
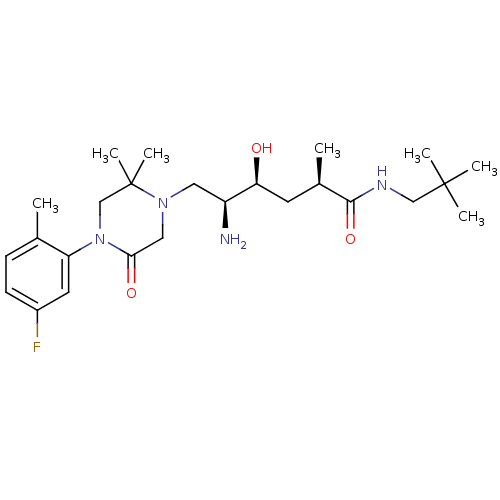
(CHEMBL2387449)Show SMILES C[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cc(F)ccc1C)C(=O)NCC(C)(C)C |r| Show InChI InChI=1S/C25H41FN4O3/c1-16-8-9-18(26)11-20(16)30-15-25(6,7)29(13-22(30)32)12-19(27)21(31)10-17(2)23(33)28-14-24(3,4)5/h8-9,11,17,19,21,31H,10,12-15,27H2,1-7H3,(H,28,33)/t17-,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434427
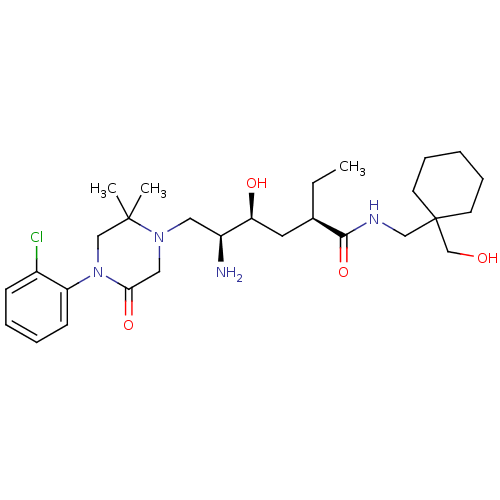
(CHEMBL2387565)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NCC1(CO)CCCCC1 |r| Show InChI InChI=1S/C28H45ClN4O4/c1-4-20(26(37)31-17-28(19-34)12-8-5-9-13-28)14-24(35)22(30)15-32-16-25(36)33(18-27(32,2)3)23-11-7-6-10-21(23)29/h6-7,10-11,20,22,24,34-35H,4-5,8-9,12-19,30H2,1-3H3,(H,31,37)/t20-,22+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50439253
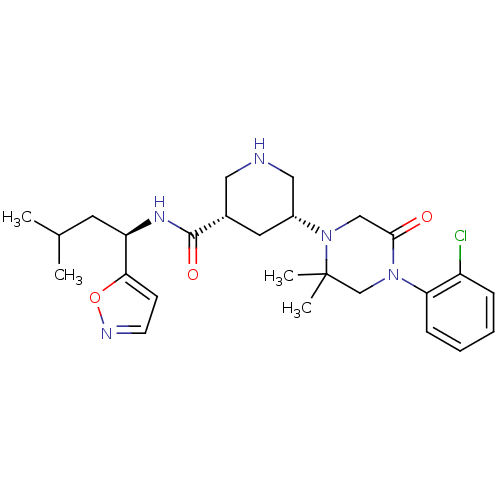
(CHEMBL2419042)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H]1CNC[C@@H](C1)N1CC(=O)N(CC1(C)C)c1ccccc1Cl)c1ccno1 |r| Show InChI InChI=1S/C26H36ClN5O3/c1-17(2)11-21(23-9-10-29-35-23)30-25(34)18-12-19(14-28-13-18)32-15-24(33)31(16-26(32,3)4)22-8-6-5-7-20(22)27/h5-10,17-19,21,28H,11-16H2,1-4H3,(H,30,34)/t18-,19+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin using (Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg) as substrate i... |
Bioorg Med Chem 21: 5907-22 (2013)
Article DOI: 10.1016/j.bmc.2013.06.057
BindingDB Entry DOI: 10.7270/Q2TH8P4B |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-2 (MMP-2). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50571012

(CHEMBL4867696)Show SMILES CN1CCC(CC(=O)Nc2cc(Oc3ccc(NC(=O)c4cn(C5CCCC5)c(=O)n(-c5ccc(F)cc5)c4=O)cc3F)ccn2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged Axl incubated for 1 hr by ADP-glo based luminometry analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116137
BindingDB Entry DOI: 10.7270/Q2PV6Q46 |
More data for this
Ligand-Target Pair | |
Renin
(Macaca fascicularis) | BDBM50434421
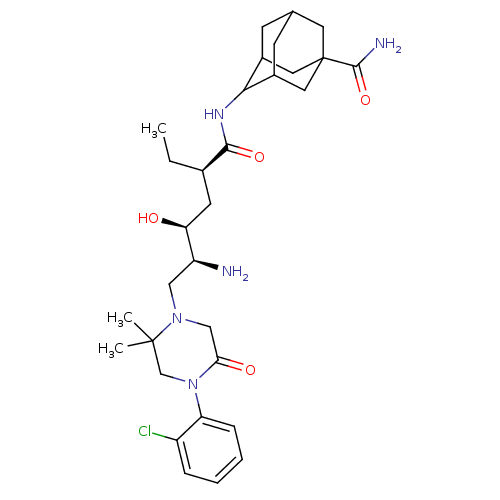
(CHEMBL2387571)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NC1C2CC3CC1CC(C3)(C2)C(N)=O |r,wU:4.4,2.2,wD:6.6,TLB:27:28:34.37.35:30.31.32,27:28:32:34.35.36,37:35:28.29.30:32,38:35:28:30.31.32,THB:37:29:32:34.35.36,36:35:28:30.31.32,36:31:28:34.37.35,(14.47,-18.32,;13.13,-19.09,;13.13,-20.63,;11.8,-21.4,;10.46,-20.63,;10.47,-19.09,;9.13,-21.39,;7.8,-20.62,;9.13,-22.93,;7.79,-23.7,;6.46,-22.93,;5.12,-23.7,;3.78,-22.94,;5.13,-25.24,;6.46,-26.01,;7.79,-25.24,;9.27,-25.64,;8.19,-26.73,;3.79,-26.02,;2.45,-25.26,;1.13,-26.03,;1.12,-27.57,;2.46,-28.34,;3.79,-27.57,;5.13,-28.34,;14.47,-21.4,;14.46,-22.94,;15.8,-20.63,;17.13,-21.41,;18.63,-20.99,;18.63,-19.39,;19.67,-18.17,;18.32,-18.64,;18.33,-20.13,;19.66,-20.62,;21.05,-20.27,;21.07,-18.74,;20.04,-21.55,;22.38,-21.04,;23.71,-20.27,;22.38,-22.58,)| Show InChI InChI=1S/C31H46ClN5O4/c1-4-19(28(40)35-27-20-9-18-10-21(27)14-31(12-18,13-20)29(34)41)11-25(38)23(33)15-36-16-26(39)37(17-30(36,2)3)24-8-6-5-7-22(24)32/h5-8,18-21,23,25,27,38H,4,9-17,33H2,1-3H3,(H2,34,41)(H,35,40)/t18?,19-,20?,21?,23+,25+,27?,31?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in cynomolgus monkey plasma after 1 hr by RIA |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434421

(CHEMBL2387571)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NC1C2CC3CC1CC(C3)(C2)C(N)=O |r,wU:4.4,2.2,wD:6.6,TLB:27:28:34.37.35:30.31.32,27:28:32:34.35.36,37:35:28.29.30:32,38:35:28:30.31.32,THB:37:29:32:34.35.36,36:35:28:30.31.32,36:31:28:34.37.35,(14.47,-18.32,;13.13,-19.09,;13.13,-20.63,;11.8,-21.4,;10.46,-20.63,;10.47,-19.09,;9.13,-21.39,;7.8,-20.62,;9.13,-22.93,;7.79,-23.7,;6.46,-22.93,;5.12,-23.7,;3.78,-22.94,;5.13,-25.24,;6.46,-26.01,;7.79,-25.24,;9.27,-25.64,;8.19,-26.73,;3.79,-26.02,;2.45,-25.26,;1.13,-26.03,;1.12,-27.57,;2.46,-28.34,;3.79,-27.57,;5.13,-28.34,;14.47,-21.4,;14.46,-22.94,;15.8,-20.63,;17.13,-21.41,;18.63,-20.99,;18.63,-19.39,;19.67,-18.17,;18.32,-18.64,;18.33,-20.13,;19.66,-20.62,;21.05,-20.27,;21.07,-18.74,;20.04,-21.55,;22.38,-21.04,;23.71,-20.27,;22.38,-22.58,)| Show InChI InChI=1S/C31H46ClN5O4/c1-4-19(28(40)35-27-20-9-18-10-21(27)14-31(12-18,13-20)29(34)41)11-25(38)23(33)15-36-16-26(39)37(17-30(36,2)3)24-8-6-5-7-22(24)32/h5-8,18-21,23,25,27,38H,4,9-17,33H2,1-3H3,(H2,34,41)(H,35,40)/t18?,19-,20?,21?,23+,25+,27?,31?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50402219
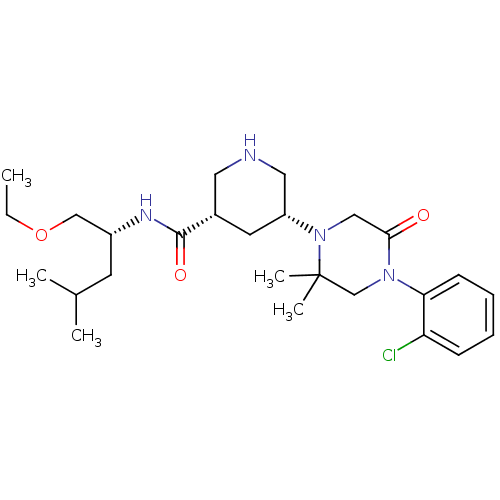
(CHEMBL2204732)Show SMILES CCOC[C@@H](CC(C)C)NC(=O)[C@@H]1CNC[C@@H](C1)N1CC(=O)N(CC1(C)C)c1ccccc1Cl |r| Show InChI InChI=1S/C26H41ClN4O3/c1-6-34-16-20(11-18(2)3)29-25(33)19-12-21(14-28-13-19)31-15-24(32)30(17-26(31,4)5)23-10-8-7-9-22(23)27/h7-10,18-21,28H,6,11-17H2,1-5H3,(H,29,33)/t19-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin using (Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg) as substrate i... |
Bioorg Med Chem 21: 5907-22 (2013)
Article DOI: 10.1016/j.bmc.2013.06.057
BindingDB Entry DOI: 10.7270/Q2TH8P4B |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50439251
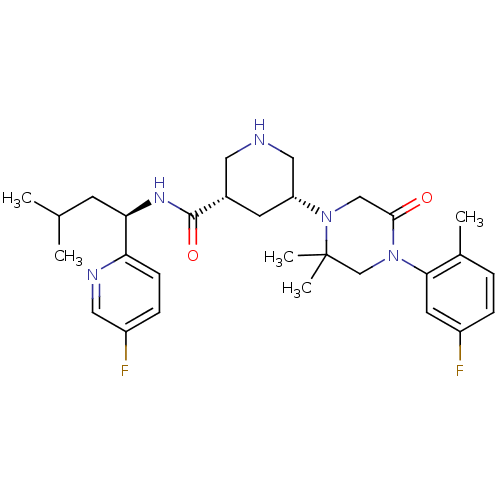
(CHEMBL2419045)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H]1CNC[C@@H](C1)N1CC(=O)N(CC1(C)C)c1cc(F)ccc1C)c1ccc(F)cn1 |r| Show InChI InChI=1S/C29H39F2N5O2/c1-18(2)10-25(24-9-8-22(31)14-33-24)34-28(38)20-11-23(15-32-13-20)36-16-27(37)35(17-29(36,4)5)26-12-21(30)7-6-19(26)3/h6-9,12,14,18,20,23,25,32H,10-11,13,15-17H2,1-5H3,(H,34,38)/t20-,23+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin using (Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg) as substrate i... |
Bioorg Med Chem 21: 5907-22 (2013)
Article DOI: 10.1016/j.bmc.2013.06.057
BindingDB Entry DOI: 10.7270/Q2TH8P4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50571018
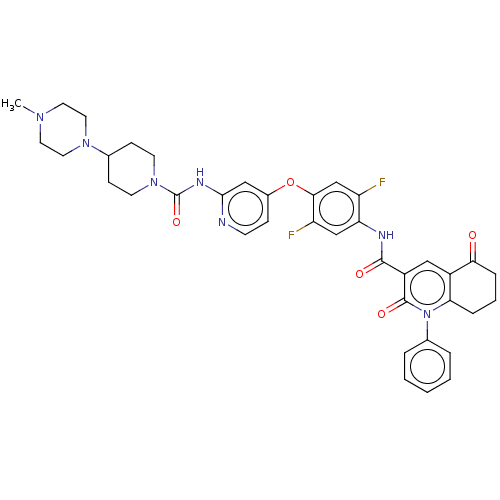
(CHEMBL4849679)Show SMILES CN1CCN(CC1)C1CCN(CC1)C(=O)Nc1cc(Oc2cc(F)c(NC(=O)c3cc4C(=O)CCCc4n(-c4ccccc4)c3=O)cc2F)ccn1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged Axl incubated for 1 hr by ADP-glo based luminometry analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116137
BindingDB Entry DOI: 10.7270/Q2PV6Q46 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434426
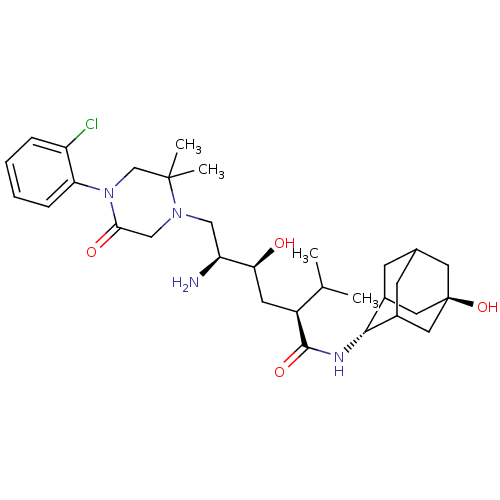
(CHEMBL2387566)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:5.5,3.3,29.30,wD:36.39,7.7,TLB:28:29:39.35.36:33.32.31,28:29:31:39.36.38,37:36:29:33.32.31,THB:35:34:31:39.36.38,35:36:29.34.33:31,38:36:29:33.32.31,38:32:29:39.35.36,(39.25,-40.47,;37.92,-41.24,;36.58,-40.47,;37.91,-42.78,;36.58,-43.55,;35.25,-42.78,;35.25,-41.24,;33.91,-43.55,;32.58,-42.77,;33.91,-45.09,;32.58,-45.86,;31.24,-45.09,;29.9,-45.86,;28.56,-45.09,;29.91,-47.4,;31.25,-48.16,;32.57,-47.39,;34.05,-47.79,;32.97,-48.88,;28.57,-48.17,;27.24,-47.41,;25.91,-48.18,;25.91,-49.72,;27.24,-50.5,;28.58,-49.72,;29.91,-50.49,;39.25,-43.55,;39.25,-45.09,;40.58,-42.79,;41.92,-43.55,;43.12,-42.28,;43.11,-40.79,;44.45,-40.31,;43.41,-41.55,;43.41,-43.13,;44.83,-43.7,;45.83,-42.42,;47.28,-42.94,;45.85,-40.89,;44.44,-42.77,)| Show InChI InChI=1S/C31H47ClN4O4/c1-18(2)22(29(39)34-28-20-9-19-10-21(28)14-31(40,12-19)13-20)11-26(37)24(33)15-35-16-27(38)36(17-30(35,3)4)25-8-6-5-7-23(25)32/h5-8,18-22,24,26,28,37,40H,9-17,33H2,1-4H3,(H,34,39)/t19?,20?,21?,22-,24-,26-,28-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063910
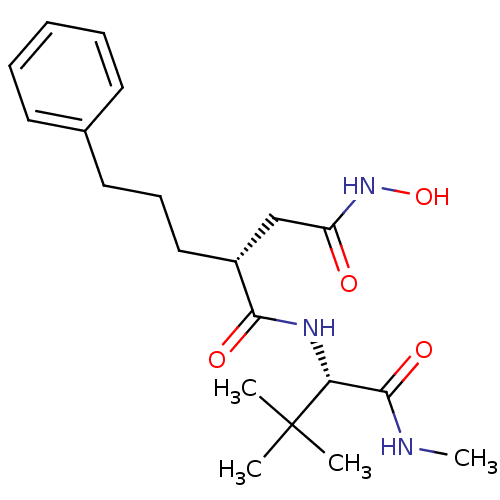
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CCCc1ccccc1)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C20H31N3O4/c1-20(2,3)17(19(26)21-4)22-18(25)15(13-16(24)23-27)12-8-11-14-9-6-5-7-10-14/h5-7,9-10,15,17,27H,8,11-13H2,1-4H3,(H,21,26)(H,22,25)(H,23,24)/t15-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50402219

(CHEMBL2204732)Show SMILES CCOC[C@@H](CC(C)C)NC(=O)[C@@H]1CNC[C@@H](C1)N1CC(=O)N(CC1(C)C)c1ccccc1Cl |r| Show InChI InChI=1S/C26H41ClN4O3/c1-6-34-16-20(11-18(2)3)29-25(33)19-12-21(14-28-13-19)31-15-24(32)30(17-26(31,4)5)23-10-8-7-9-22(23)27/h7-10,18-21,28H,6,11-17H2,1-5H3,(H,29,33)/t19-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... |
Bioorg Med Chem Lett 22: 7677-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.103
BindingDB Entry DOI: 10.7270/Q2HD7WTK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434441

(CHEMBL2387551)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C27H36ClFN4O3/c1-17(2)20(26(36)31-19-11-9-18(29)10-12-19)13-24(34)22(30)14-32-15-25(35)33(16-27(32,3)4)23-8-6-5-7-21(23)28/h5-12,17,20,22,24,34H,13-16,30H2,1-4H3,(H,31,36)/t20-,22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Macaca fascicularis) | BDBM50402219

(CHEMBL2204732)Show SMILES CCOC[C@@H](CC(C)C)NC(=O)[C@@H]1CNC[C@@H](C1)N1CC(=O)N(CC1(C)C)c1ccccc1Cl |r| Show InChI InChI=1S/C26H41ClN4O3/c1-6-34-16-20(11-18(2)3)29-25(33)19-12-21(14-28-13-19)31-15-24(32)30(17-26(31,4)5)23-10-8-7-9-22(23)27/h7-10,18-21,28H,6,11-17H2,1-5H3,(H,29,33)/t19-,20+,21+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay |
Bioorg Med Chem Lett 22: 7677-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.103
BindingDB Entry DOI: 10.7270/Q2HD7WTK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434448

(CHEMBL2387544)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:5.5,3.3,29.30,wD:7.7,32.34,(38.68,-25.48,;37.35,-26.25,;36.01,-25.48,;37.34,-27.79,;36.01,-28.56,;34.68,-27.79,;34.68,-26.25,;33.34,-28.56,;32.01,-27.78,;33.34,-30.1,;32.01,-30.86,;30.67,-30.09,;29.33,-30.87,;27.99,-30.1,;29.34,-32.4,;30.68,-33.17,;32,-32.4,;33.48,-32.8,;32.4,-33.89,;28,-33.18,;26.67,-32.42,;25.34,-33.19,;25.34,-34.73,;26.67,-35.5,;28.01,-34.73,;29.34,-35.5,;38.68,-28.56,;38.68,-30.1,;40.01,-27.8,;41.34,-28.57,;42.67,-27.79,;44,-28.57,;44,-30.11,;45.33,-30.89,;42.66,-30.87,;41.33,-30.1,)| Show InChI InChI=1S/C27H43ClN4O4/c1-17(2)20(26(36)30-18-9-11-19(33)12-10-18)13-24(34)22(29)14-31-15-25(35)32(16-27(31,3)4)23-8-6-5-7-21(23)28/h5-8,17-20,22,24,33-34H,9-16,29H2,1-4H3,(H,30,36)/t18-,19-,20-,22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50434446
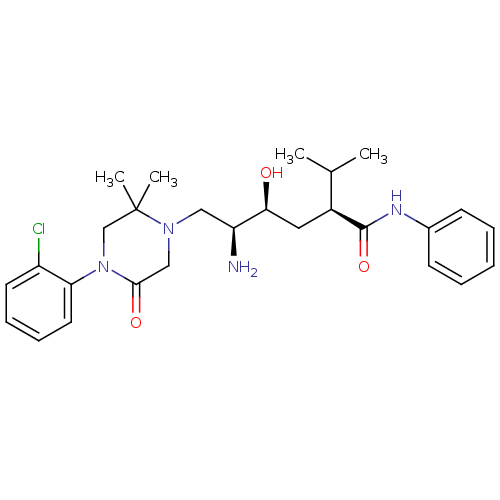
(CHEMBL2387546)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C27H37ClN4O3/c1-18(2)20(26(35)30-19-10-6-5-7-11-19)14-24(33)22(29)15-31-16-25(34)32(17-27(31,3)4)23-13-9-8-12-21(23)28/h5-13,18,20,22,24,33H,14-17,29H2,1-4H3,(H,30,35)/t20-,22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... |
Bioorg Med Chem 21: 3175-96 (2013)
Article DOI: 10.1016/j.bmc.2013.03.022
BindingDB Entry DOI: 10.7270/Q2GT5PJK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data