

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244370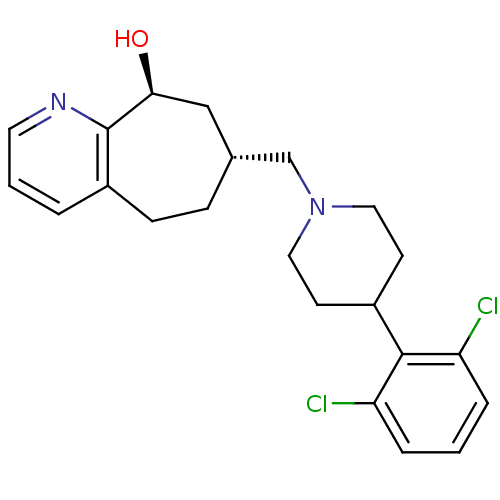 ((7R,9S)-7-((4-(2,6-dichlorophenyl)piperidin-1-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244371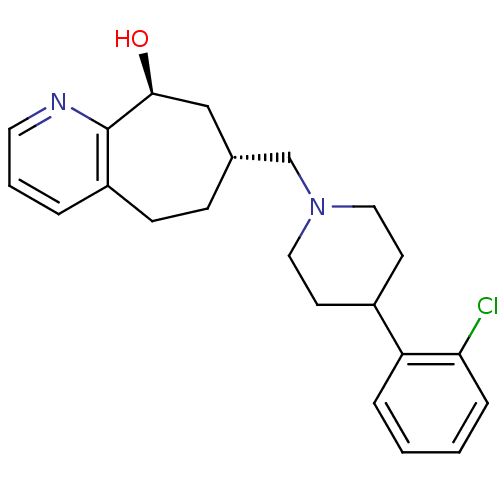 ((7R,9S)-7-((4-(2-chlorophenyl)piperidin-1-yl)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123720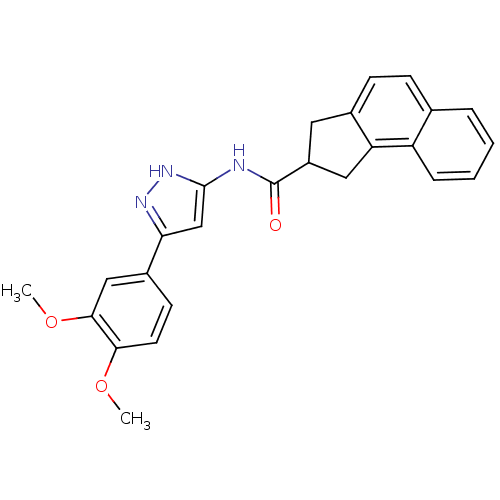 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123724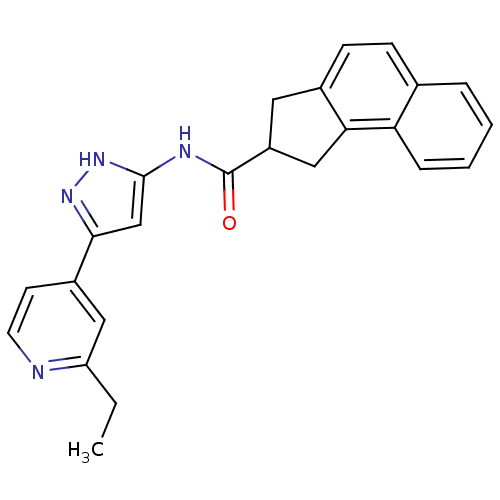 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243726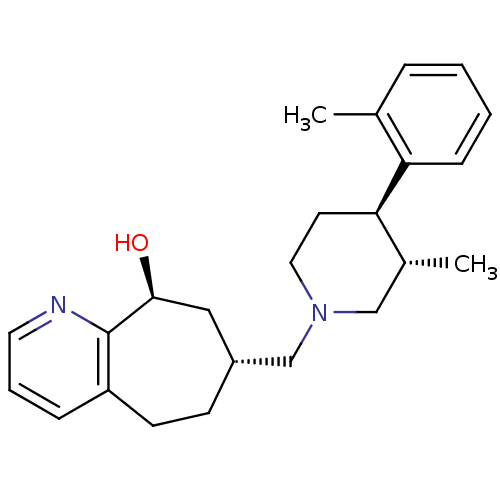 ((7R,9S)-7-(((3S,4R)-3-methyl-4-o-tolylpiperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243729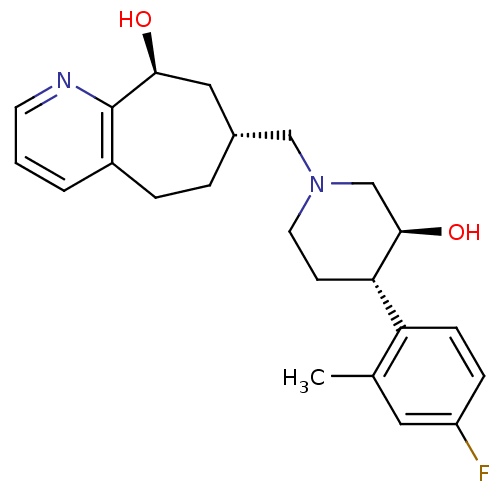 ((7R,9S)-7-(((3S,4S)-4-(4-fluoro-2-methylphenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243727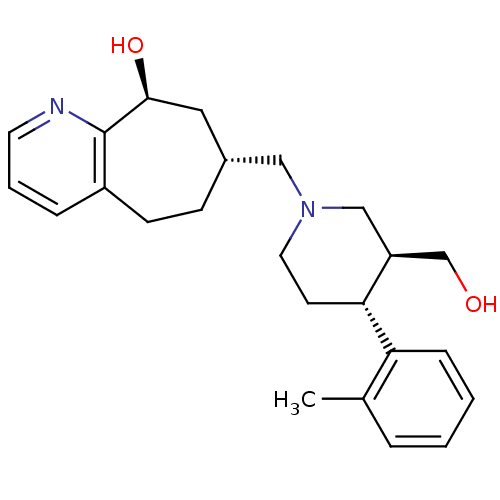 ((7R,9S)-7-(((3S,4R)-3-(hydroxymethyl)-4-o-tolylpip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243730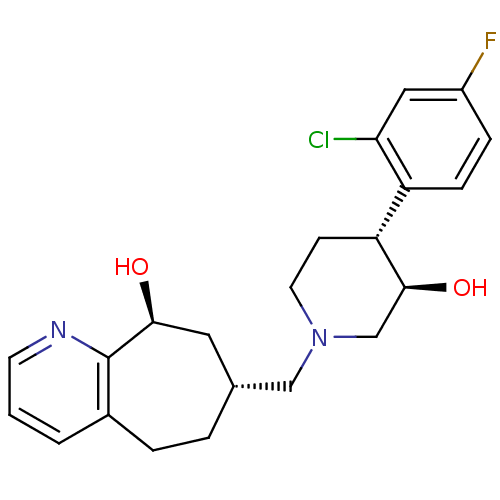 ((7R,9S)-7-(((3R,4R)-4-(2-chloro-4-fluorophenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244297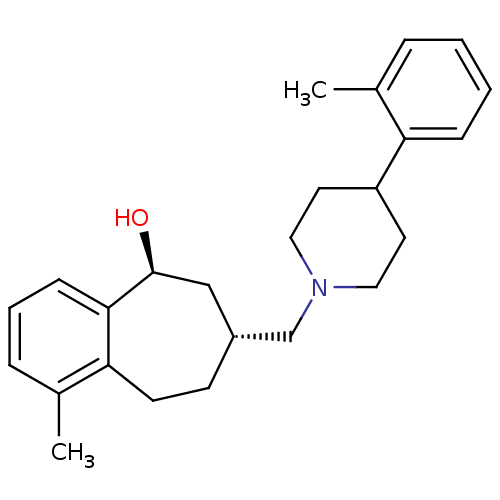 (CHEMBL513585 | cis-1-methyl-7-((4-o-tolylpiperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123724 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123724 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50073047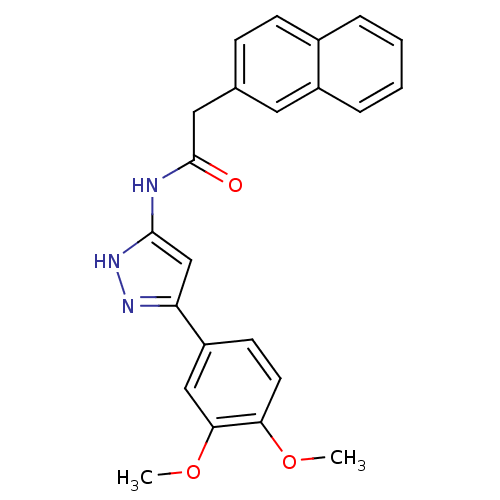 (CHEMBL291666 | N-[5-(3,4-Dimethoxy-phenyl)-1H-pyra...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243728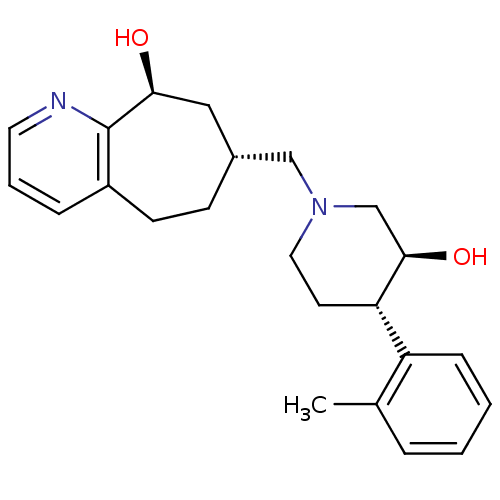 ((7R,9S)-7-(((3S,4S)-3-hydroxy-4-o-tolylpiperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123729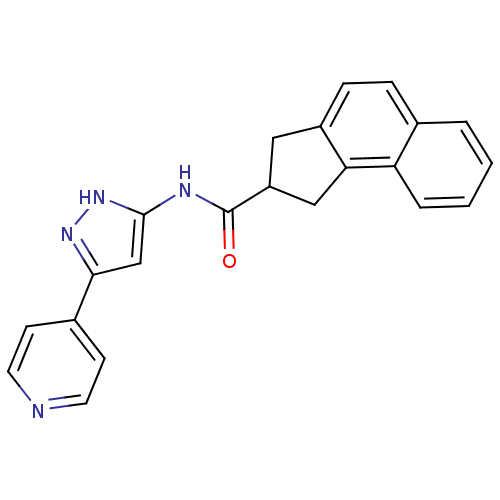 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244336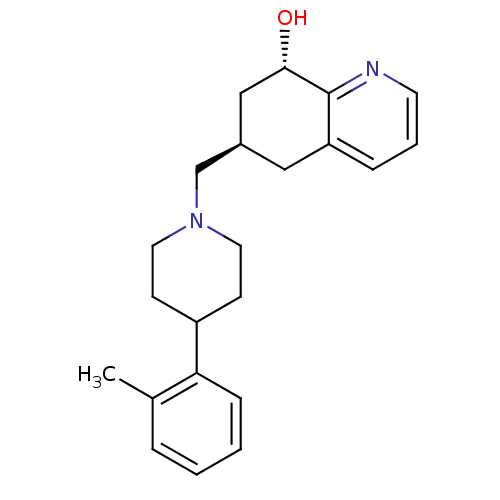 ((6R,8S)-6-((4-o-tolylpiperidin-1-yl)methyl)-5,6,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243887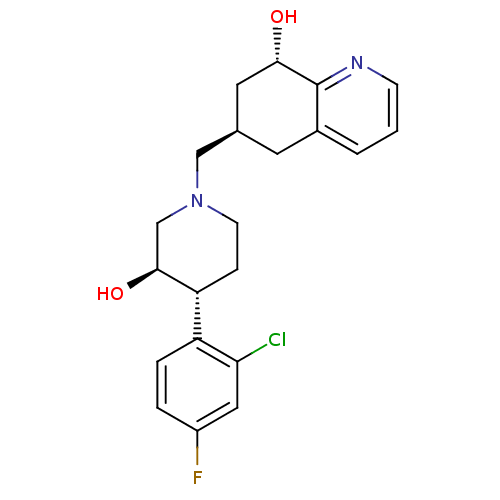 ((-)-(3R,4R)-4-(2-Chloro-4-fluorophenyl)-3-hydroxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50409778 (CHEMBL2110162) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244334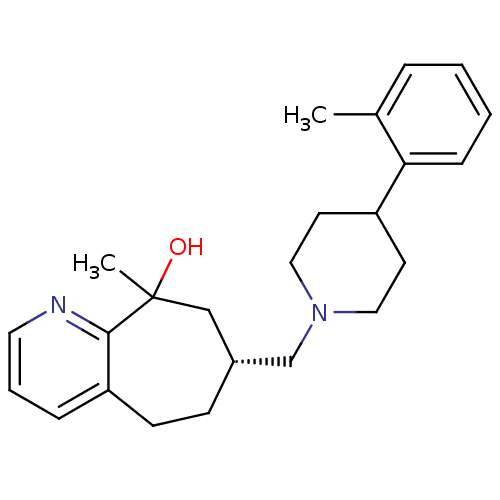 ((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244334 ((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244295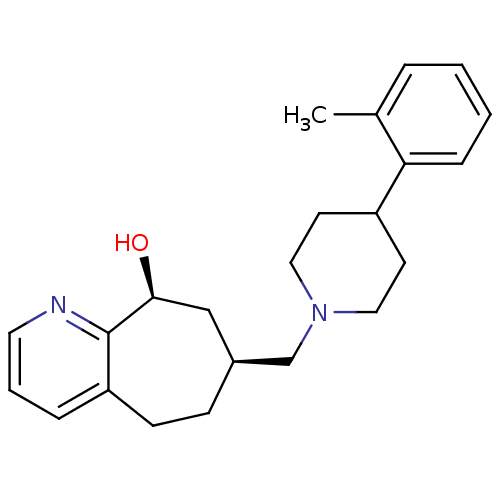 ((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244295 ((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244372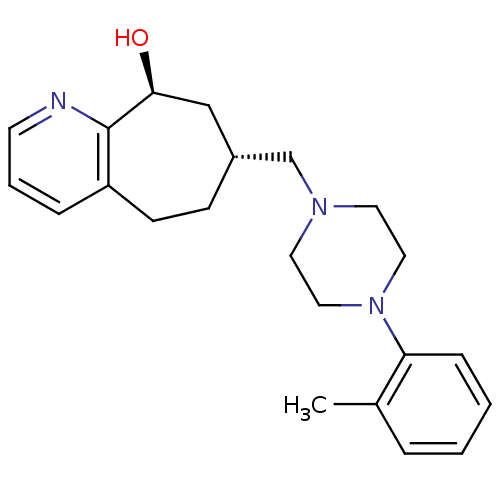 ((7R,9S)-7-((4-o-tolylpiperazin-1-yl)methyl)-6,7,8,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243886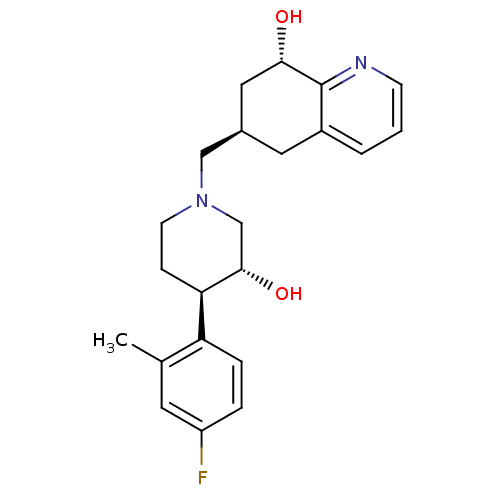 ((6R,8S)-6-(((3R,4R)-4-(4-fluoro-2-methylphenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244335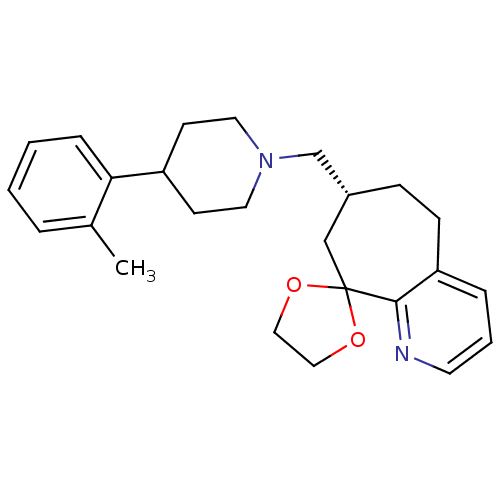 ((3R)-3-{[4-(2-methylphenyl)piperidin-1-yl]methyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244373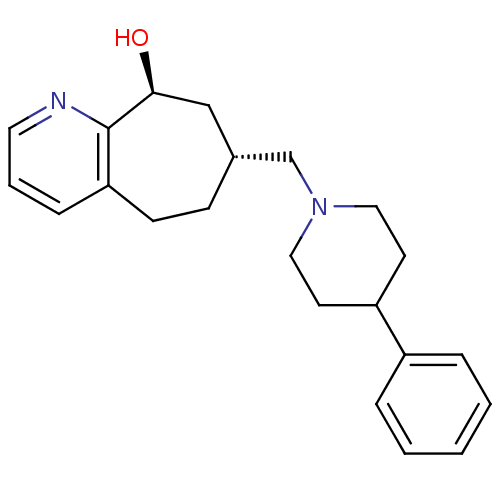 ((7R,9S)-7-((4-phenylpiperidin-1-yl)methyl)-6,7,8,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123735 (CHEMBL160443 | Indan-2-carboxylic acid [5-(3,4-dim...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244333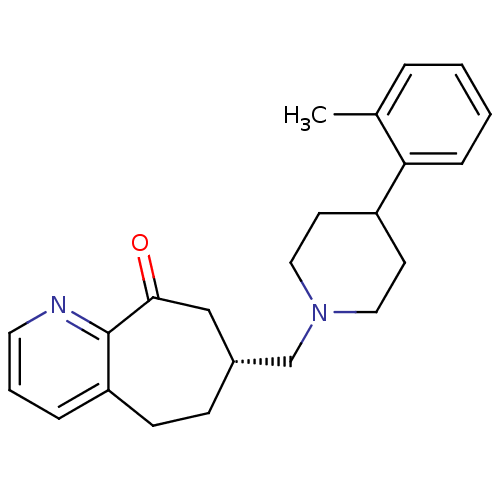 ((R)-7-((4-o-tolylpiperidin-1-yl)methyl)-5,6,7,8-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243797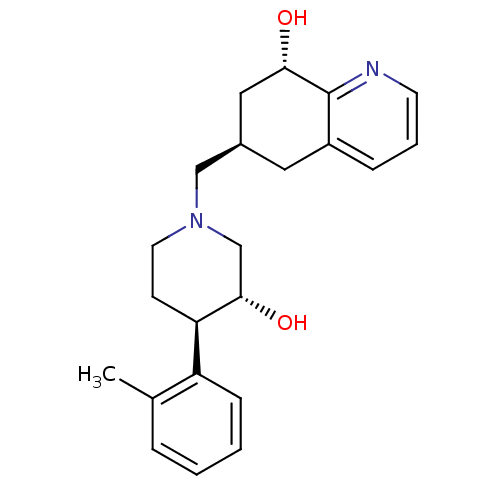 ((6R,8S)-6-(((3R,4R)-3-hydroxy-4-o-tolylpiperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244374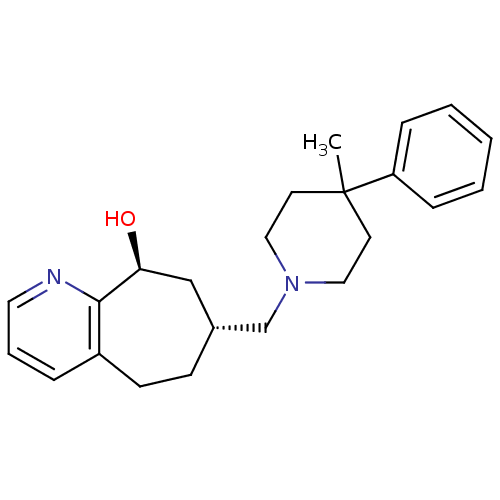 ((7R,9S)-7-((4-methyl-4-phenylpiperidin-1-yl)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123717 (CHEMBL435036 | Indan-2-carboxylic acid [5-(4-chlor...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123726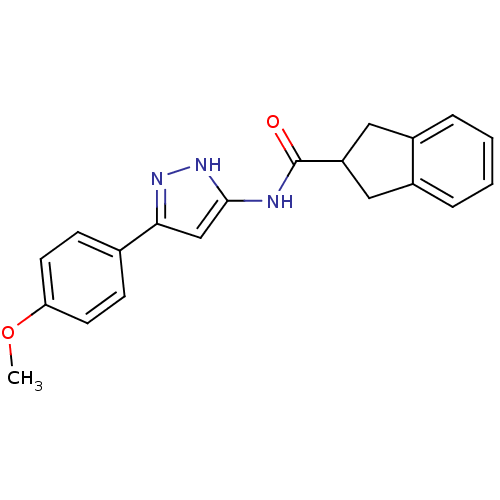 (CHEMBL159070 | Indan-2-carboxylic acid [5-(4-metho...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123727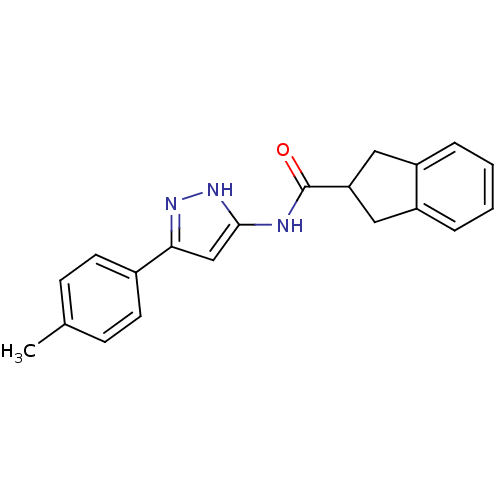 (CHEMBL158759 | Indan-2-carboxylic acid (5-p-tolyl-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123728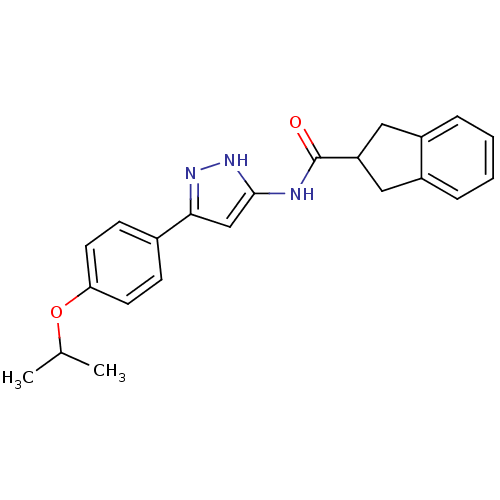 (CHEMBL160722 | Indan-2-carboxylic acid [5-(4-isopr...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123734 (CHEMBL158940 | Indan-2-carboxylic acid [5-(3-chlor...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM50336510 (7,8-dihydroxy-1-methoxy-3-methyl-10-oxo-1,10-dihyd...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta-lactamase IMP-1 assessed as hydrolysis of nitrocefin by UV spectrophotometric analysis | Antimicrob Agents Chemother 54: 3625-9 (2010) Article DOI: 10.1128/AAC.01397-09 BindingDB Entry DOI: 10.7270/Q29C6XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM50336511 ((4R,5S,6S)-3-(benzo[b]thiophen-2-ylthio)-6-((R)-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta-lactamase IMP-1 assessed as hydrolysis of nitrocefin by UV spectrophotometric analysis | Antimicrob Agents Chemother 54: 3625-9 (2010) Article DOI: 10.1128/AAC.01397-09 BindingDB Entry DOI: 10.7270/Q29C6XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM50121953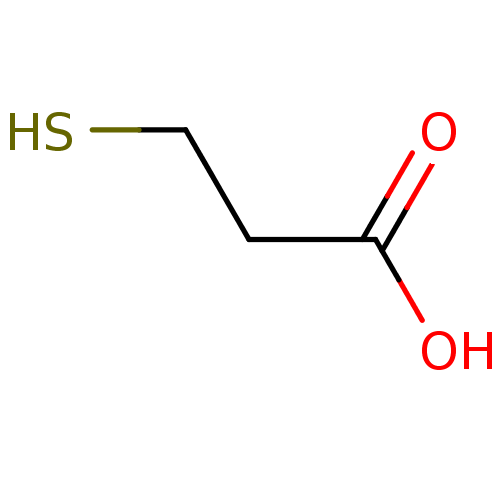 (2-mercaptoethanecarboxylic acid | 3-mercaptopropan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta-lactamase IMP-1 assessed as hydrolysis of nitrocefin by UV spectrophotometric analysis | Antimicrob Agents Chemother 54: 3625-9 (2010) Article DOI: 10.1128/AAC.01397-09 BindingDB Entry DOI: 10.7270/Q29C6XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM50336509 (CHEMBL116455 | Mercapto-acetic acid | Mercaptoacet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta-lactamase IMP-1 assessed as hydrolysis of nitrocefin by UV spectrophotometric analysis | Antimicrob Agents Chemother 54: 3625-9 (2010) Article DOI: 10.1128/AAC.01397-09 BindingDB Entry DOI: 10.7270/Q29C6XQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123732 (CHEMBL161398 | Indan-2-carboxylic acid (5-phenyl-1...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244296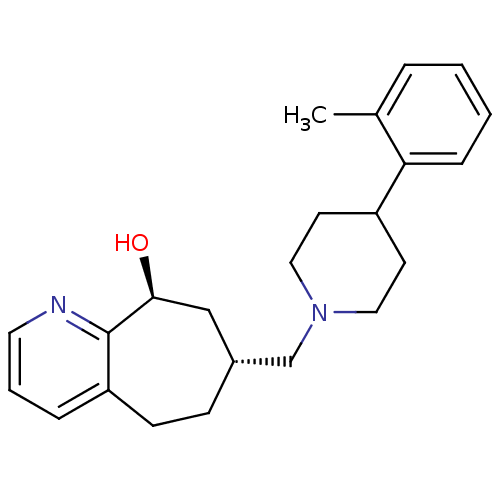 ((-)-(7R,9S)-7-{[4-(2-Methylphenyl)piperidin-1-yl]m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM50336508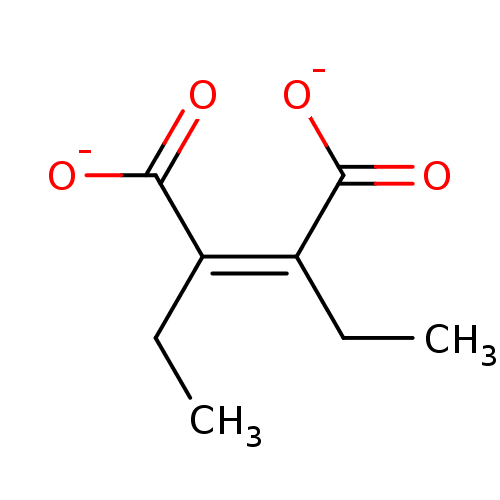 (CHEMBL1673299 | sodium 2,3-diethylmaleate) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta-lactamase IMP-1 assessed as hydrolysis of nitrocefin by UV spectrophotometric analysis | Antimicrob Agents Chemother 54: 3625-9 (2010) Article DOI: 10.1128/AAC.01397-09 BindingDB Entry DOI: 10.7270/Q29C6XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123730 (CHEMBL157588 | Indan-2-carboxylic acid [5-(2-chlor...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM50336508 (CHEMBL1673299 | sodium 2,3-diethylmaleate) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta-lactamase VIM-2 assessed as hydrolysis of nitrocefin by UV spectrophotometric analysis | Antimicrob Agents Chemother 54: 3625-9 (2010) Article DOI: 10.1128/AAC.01397-09 BindingDB Entry DOI: 10.7270/Q29C6XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141472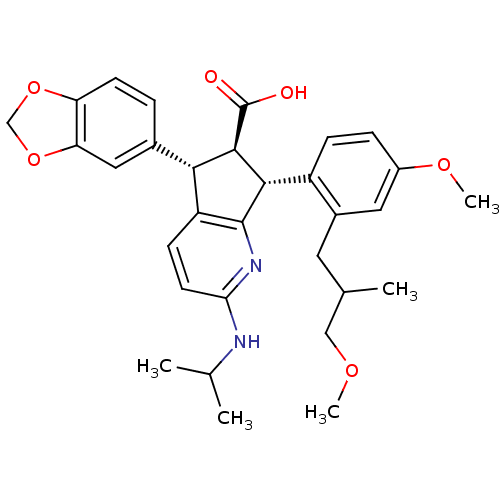 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-isopropylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141458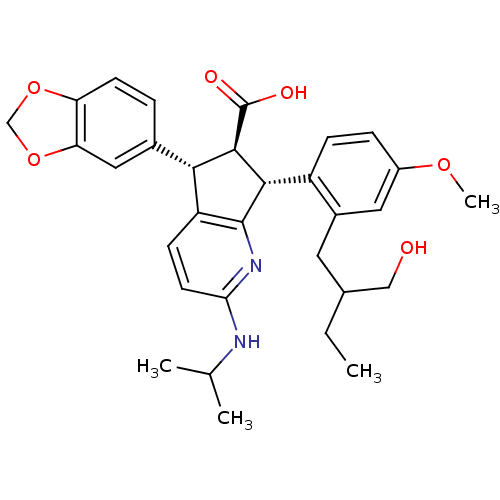 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141472 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-isopropylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141458 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141475 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141465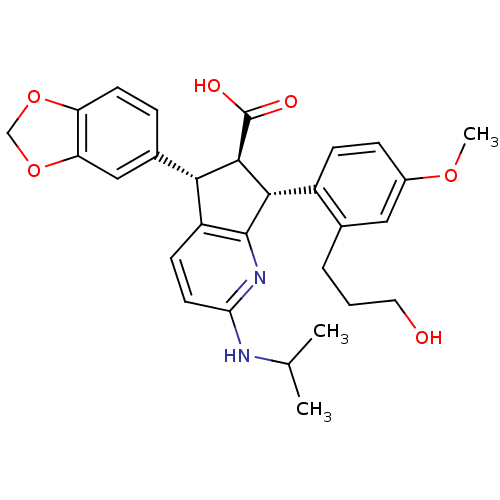 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(3-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141468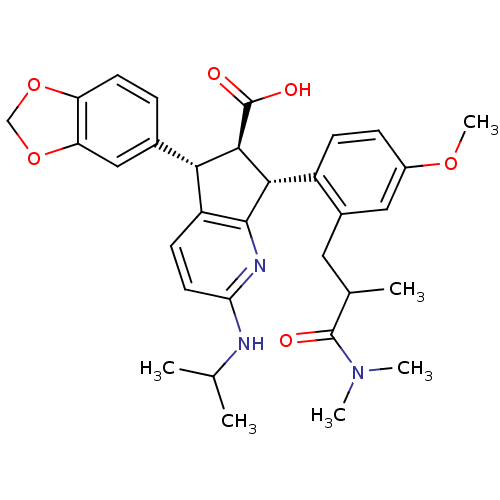 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-dimethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 510 total ) | Next | Last >> |