

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014156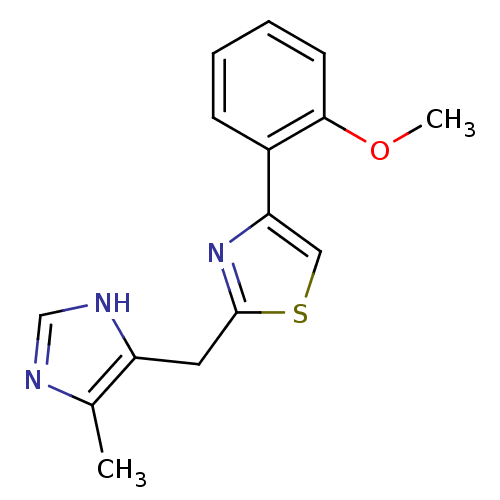 (4-(2-Methoxy-phenyl)-2-(5-methyl-1H-imidazol-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014164 (8-[2-(5-Methyl-1H-imidazol-4-ylmethyl)-thiazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Mus musculus (house mouse)) | BDBM50000492 ((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014159 (4-(2-Fluoro-phenyl)-2-(5-methyl-1H-imidazol-4-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50014407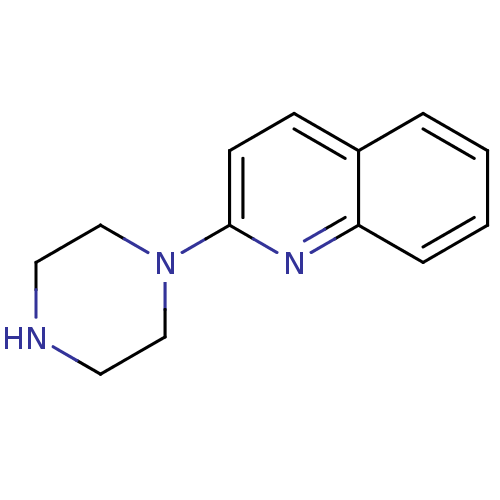 (2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50013043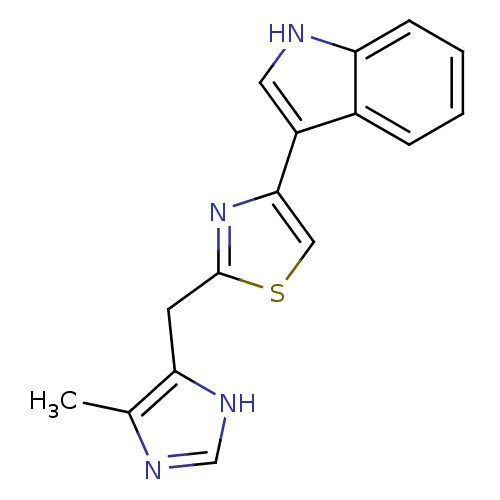 (3-[2-(5-Methyl-1H-imidazol-4-ylmethyl)-thiazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM85330 (CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM85330 (CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50222218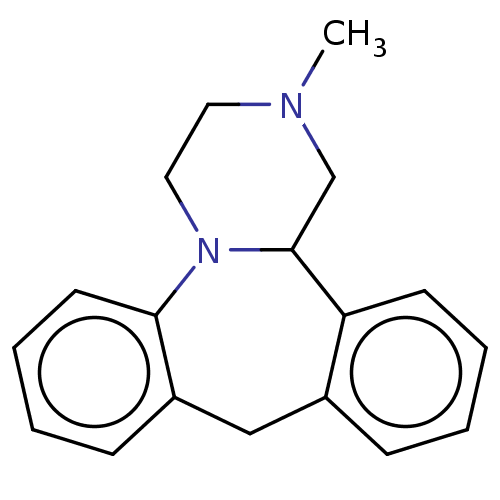 (CHEBI:51137 | Mianserin) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 361 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Muscarinic acetylcholine receptor was determined by using [3H]QNB as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1C receptor was determined by using [3H]- mesulergine as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1B receptor was determined by using [3H]5-HT as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Rattus norvegicus) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Alpha-2 adrenergic receptor was determined by using [3H]p-aminoclonidine as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Beta adrenergic receptor was determined by using [3H]dihydroalprenolol as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Sigma opioid receptor was determined by using [3H](+)-3-(3-hydroxyphenyl)N-1-propyl-piperdine((+)-3-PPP) as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Alpha-1 adrenergic receptor was determined by using [3H]prazosin as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Mus musculus (Mouse)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Dopamine receptor D1 was determined by using [3H]SCH-23390 as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Dopamine receptor D2 was determined by using [3H]spiperone as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor mu 1 was determined by using [3H]naloxone as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1D receptor was determined by using [3H]5-HT as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (BOVINE) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 2 receptor was determined by using [3H]ketanserin as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1A receptor was determined by using [3H]8-OH-DPAT as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50014406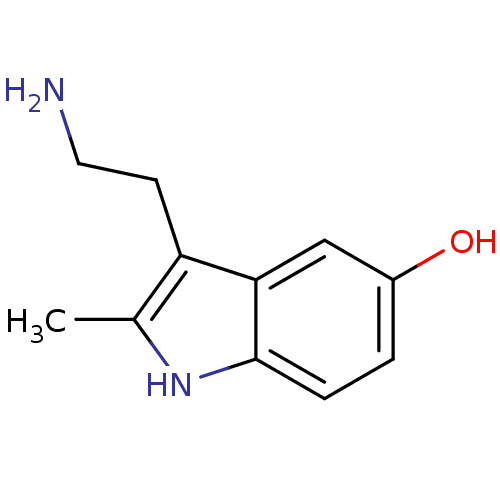 (2-Me 5-HT | 2-Methyl-5-hydroxytryptamine | 2-methy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032162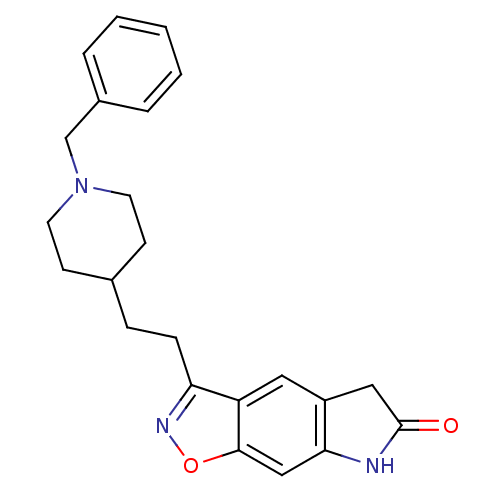 (3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,7-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032161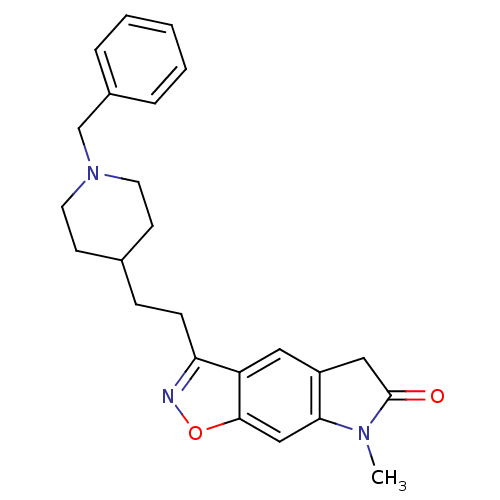 (3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-7-methyl-5,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032164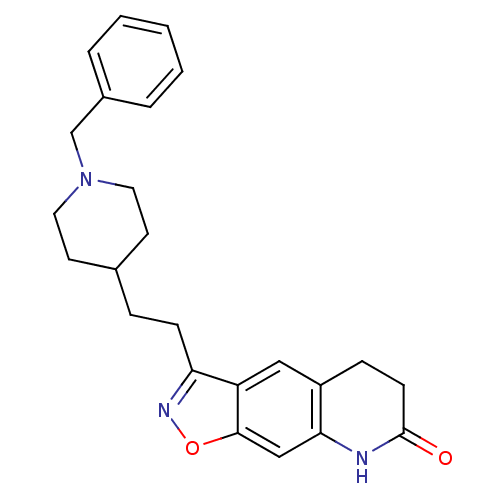 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dihydroiso...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015711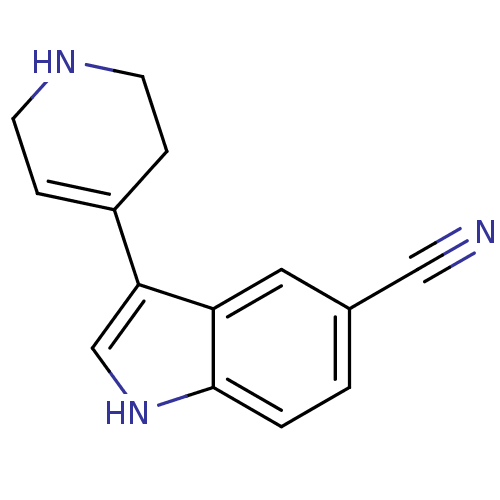 (3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1H-indole-5-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039721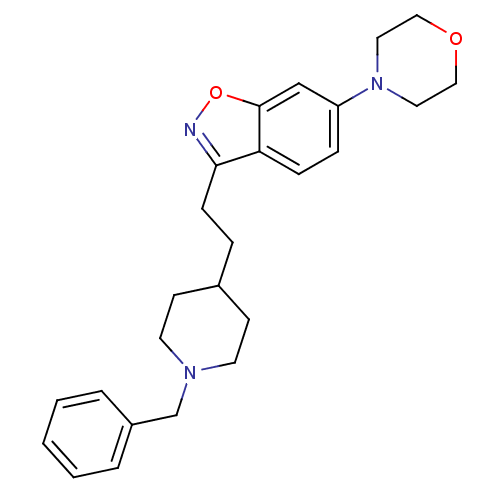 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-morpholinobe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032163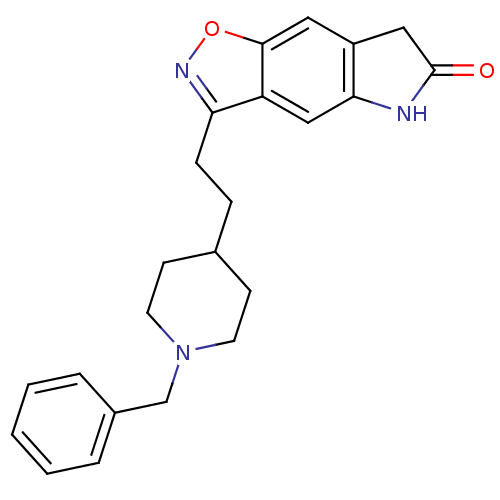 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-5H-isoxazolo[5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015714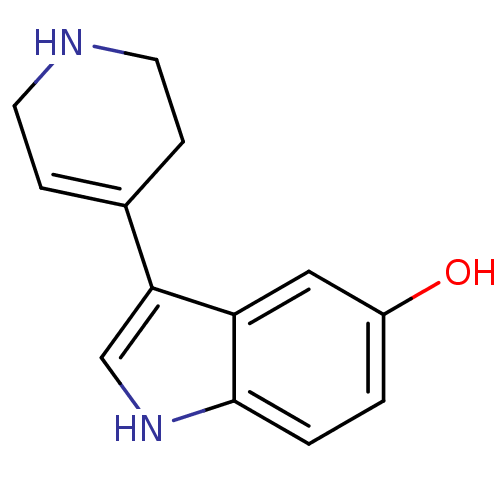 (3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1H-indol-5-ol ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015712 (5-Fluoro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM81498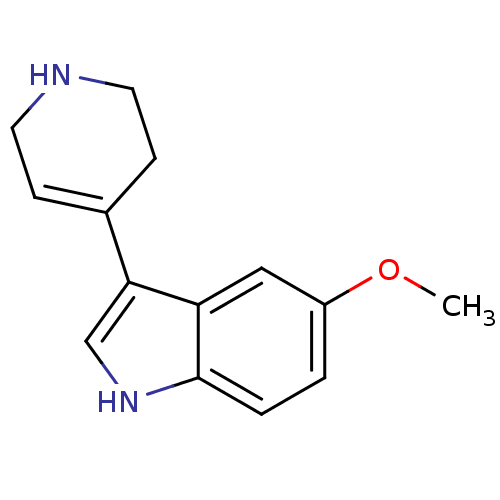 (5-Methoxy-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032165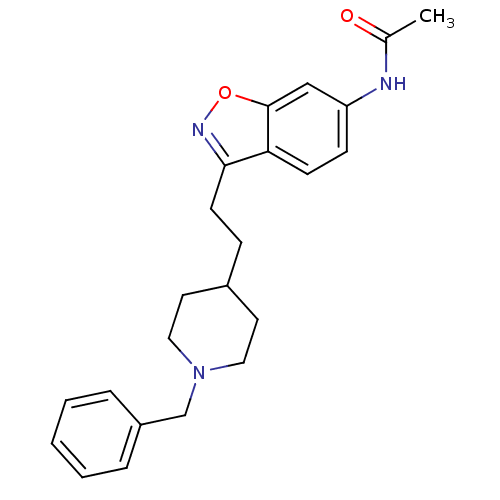 (CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032165 (CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor of bovine caudate using [3H]-5-HT as the radioligand | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015709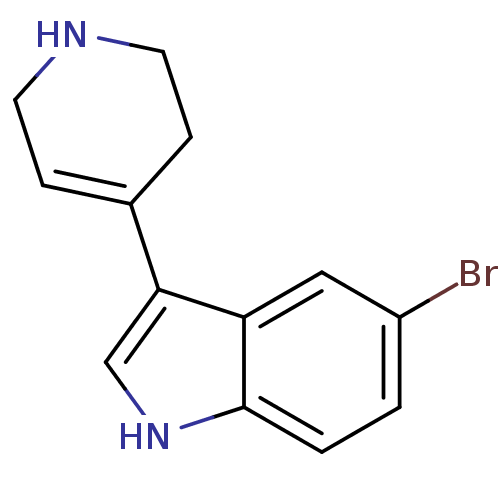 (5-Bromo-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015717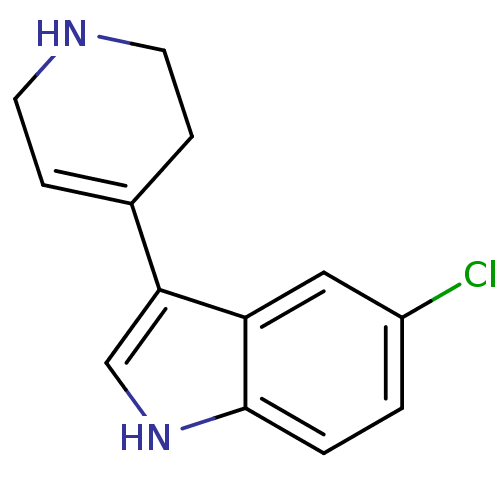 (5-Chloro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015718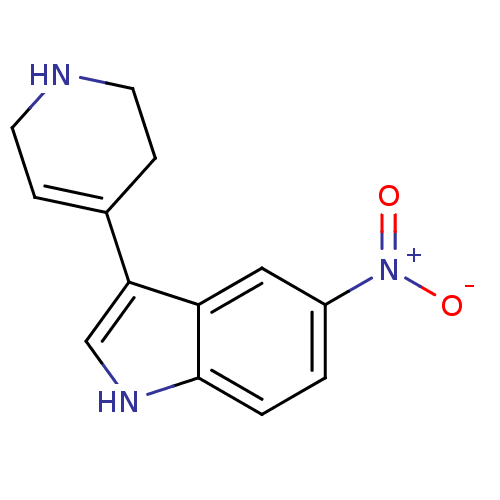 (5-Nitro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032160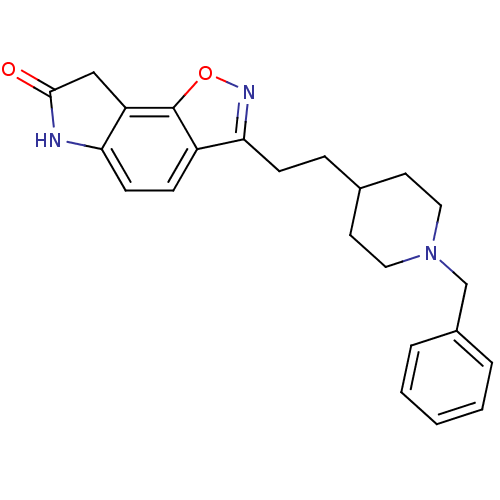 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-6H-isoxazolo[5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM31023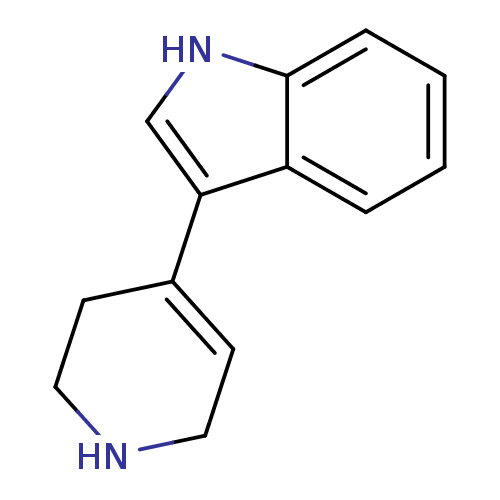 (3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT as the radioligand. | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50034001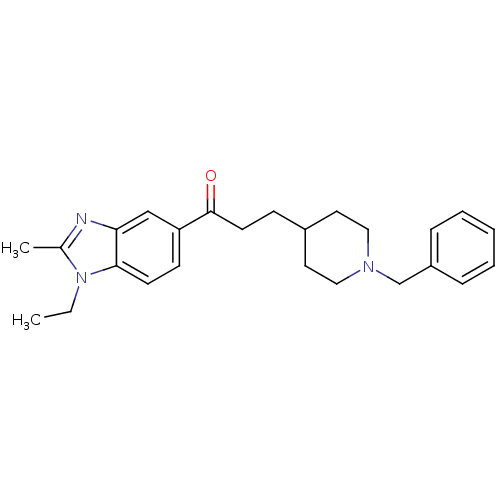 (3-(1-Benzyl-piperidin-4-yl)-1-(1-ethyl-2-methyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro | J Med Chem 38: 1084-9 (1995) BindingDB Entry DOI: 10.7270/Q21N81S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT as the radioligand. | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039729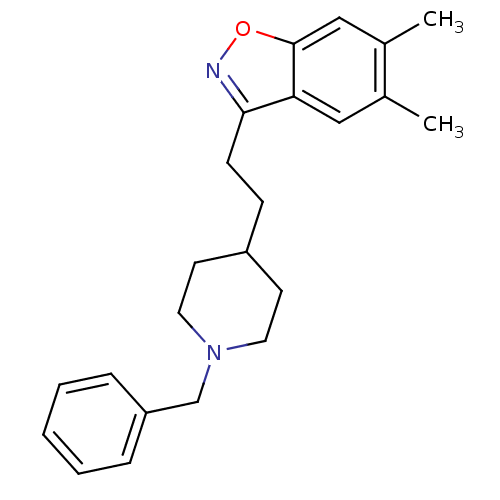 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dimethylbe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Butyrylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of the Butyrylcholinesterase from horse serum | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 145 total ) | Next | Last >> |