

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50107460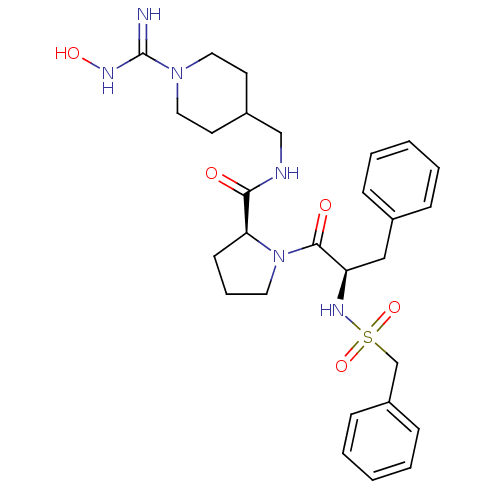 ((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Competitive kinetic for thrombin inhibition Ki was determined | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50280255 (7-Amino-3-{[(S)-1-((R)-2-amino-3-phenyl-propionyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Overall Inhibitory constant of the compound against thrombin was determined | Bioorg Med Chem Lett 2: 1607-1612 (1992) Article DOI: 10.1016/S0960-894X(00)80440-0 BindingDB Entry DOI: 10.7270/Q2G44Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50123324 (7-Methoxy-6-oxazol-5-yl-2-phenyl-1H-quinolin-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb PRI Curated by ChEMBL | Assay Description Inhibitory activity of the compound against IMPDH II with respect to IMP and NAD | Bioorg Med Chem Lett 13: 543-6 (2003) BindingDB Entry DOI: 10.7270/Q2SJ1JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107463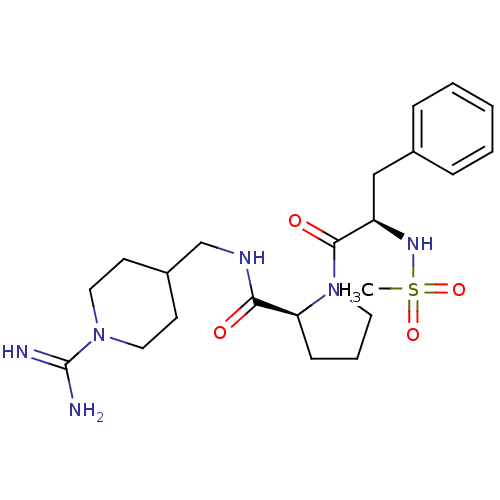 ((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Competitive kinetic for human alpha thrombin inhibition Ki was determined | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50039010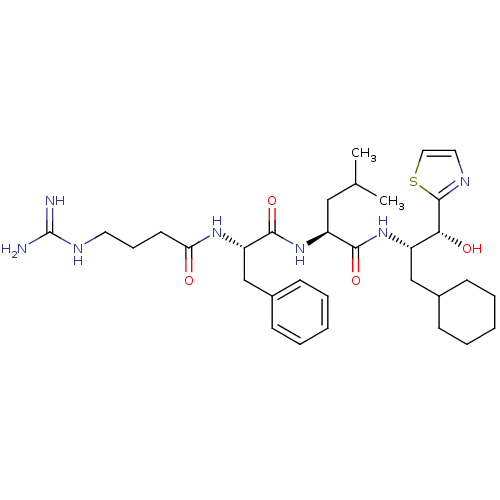 ((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-phenyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for thrombin was reported | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50287156 (2-Benzyl-1H-indole-5-carboxamidine | CHEMBL287401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Trypsin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Trypsin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Plasmin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM50151366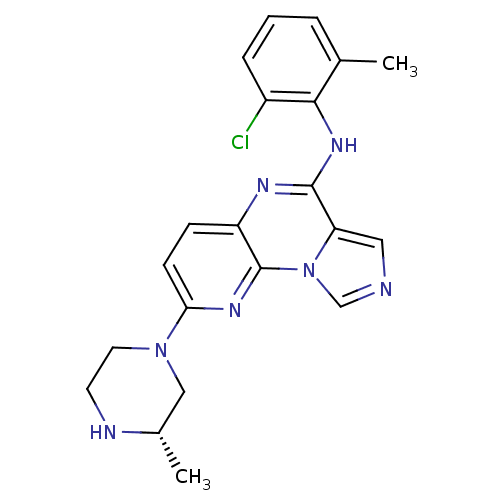 ((2-Chloro-6-methyl-phenyl)-[8-((S)-3-methyl-pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Lyn kinase | J Med Chem 47: 4517-29 (2004) Article DOI: 10.1021/jm030217e BindingDB Entry DOI: 10.7270/Q2R210VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit tissue-type plasminogen activator (t-PA) was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120101 ((2-Chloro-6-methyl-phenyl)-[8-(4-methyl-piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120116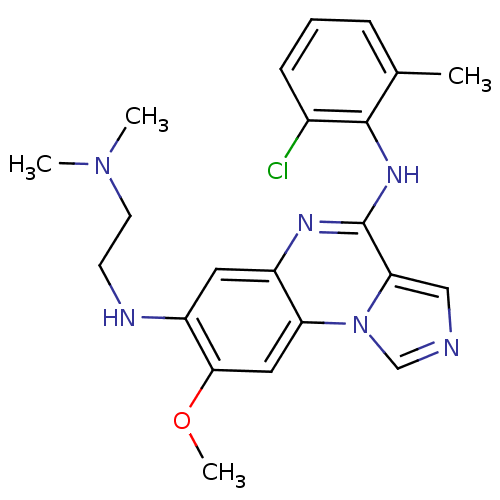 (CHEMBL108362 | N*4*-(2-Chloro-6-methyl-phenyl)-N*7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112911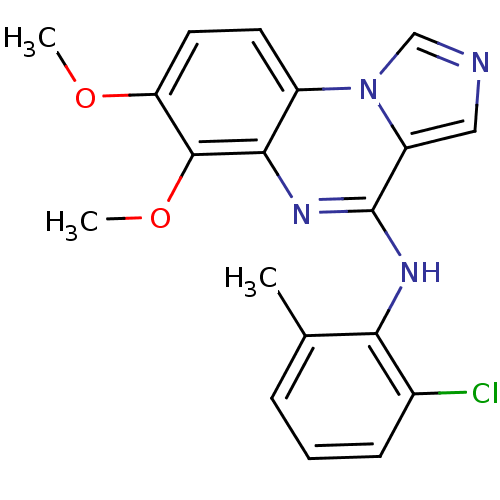 ((2-Chloro-6-methyl-phenyl)-(6,7-dimethoxy-imidazo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against recombinant p56 Lck tyrosine kinase expressed as a His-tagged protein in insect cells using a baculovirus expression syst... | Bioorg Med Chem Lett 12: 1361-4 (2002) BindingDB Entry DOI: 10.7270/Q2R78DJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107460 ((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120094 ((2-Chloro-6-methyl-phenyl)-(7,8-dimethoxy-imidazo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120087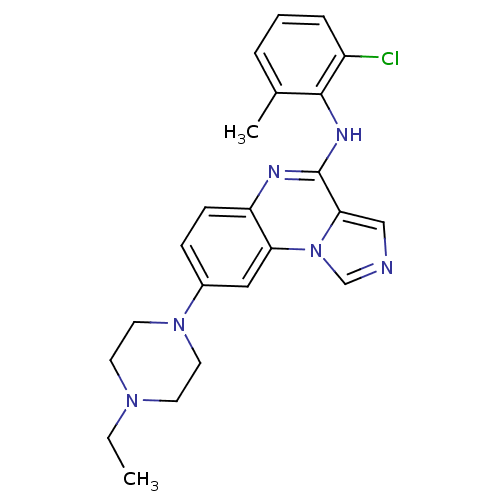 ((2-Chloro-6-methyl-phenyl)-[8-(4-ethyl-piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120090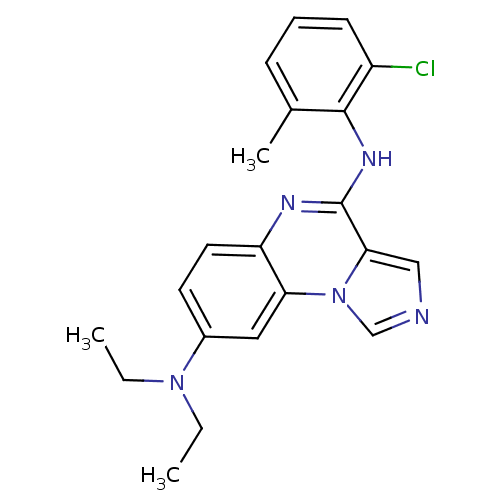 (CHEMBL108686 | N*4*-(2-Chloro-6-methyl-phenyl)-N*8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107460 ((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of thrombin was determined | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50380371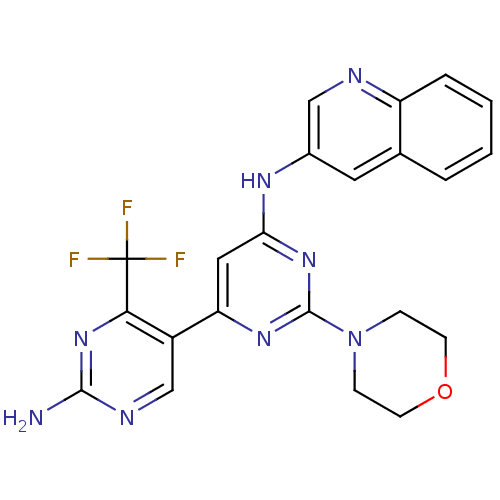 (CHEMBL2017970) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay | ACS Med Chem Lett 2: 774-779 (2011) Article DOI: 10.1021/ml200156t BindingDB Entry DOI: 10.7270/Q2N29XXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120125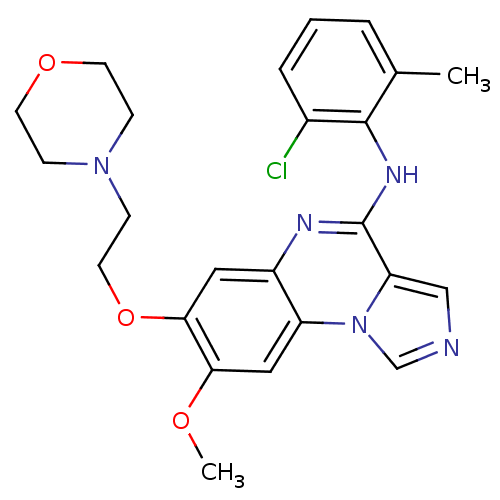 ((2-Chloro-6-methyl-phenyl)-[8-methoxy-7-(2-morphol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112947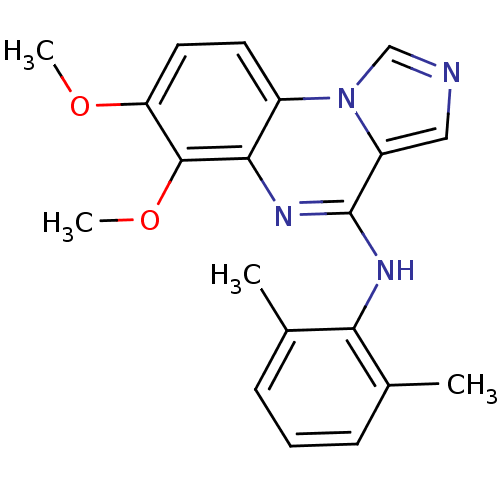 ((6,7-Dimethoxy-imidazo[1,5-a]quinoxalin-4-yl)-(2,6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against recombinant p56 Lck tyrosine kinase expressed as a His-tagged protein in insect cells using a baculovirus expression syst... | Bioorg Med Chem Lett 12: 1361-4 (2002) BindingDB Entry DOI: 10.7270/Q2R78DJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120097 ((7,8-Dimethoxy-imidazo[1,5-a]quinoxalin-4-yl)-(2,6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50380366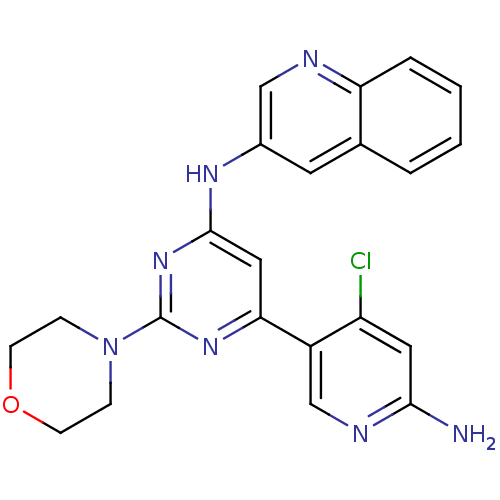 (CHEMBL2017965) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay | ACS Med Chem Lett 2: 774-779 (2011) Article DOI: 10.1021/ml200156t BindingDB Entry DOI: 10.7270/Q2N29XXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50380369 (CHEMBL2017968) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay | ACS Med Chem Lett 2: 774-779 (2011) Article DOI: 10.1021/ml200156t BindingDB Entry DOI: 10.7270/Q2N29XXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fgr (Homo sapiens (Human)) | BDBM50151366 ((2-Chloro-6-methyl-phenyl)-[8-((S)-3-methyl-pipera...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fgr protein kinase | J Med Chem 47: 4517-29 (2004) Article DOI: 10.1021/jm030217e BindingDB Entry DOI: 10.7270/Q2R210VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120127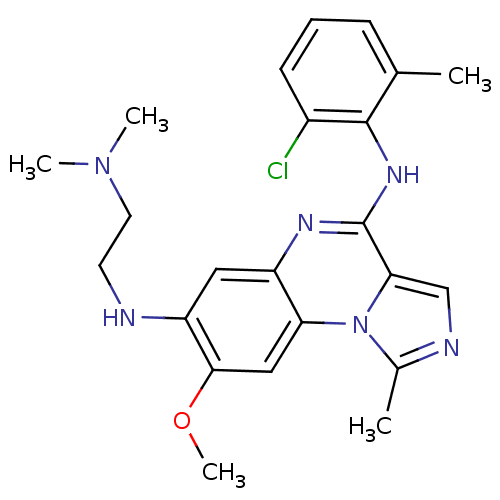 (CHEMBL106213 | N*4*-(2-Chloro-6-methyl-phenyl)-N*7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120109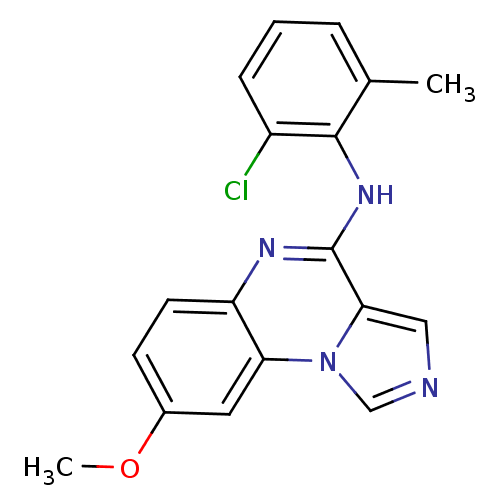 ((2-Chloro-6-methyl-phenyl)-(8-methoxy-imidazo[1,5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120128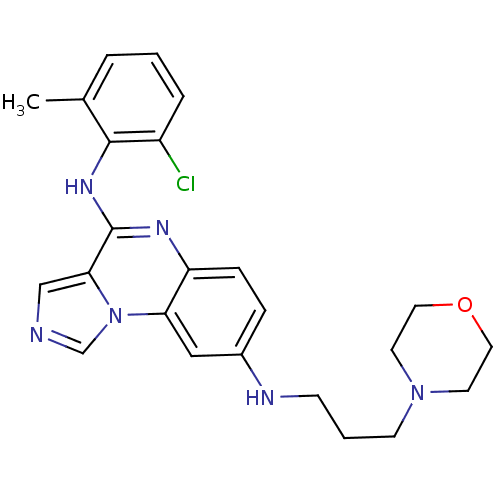 (CHEMBL325986 | N*4*-(2-Chloro-6-methyl-phenyl)-N*8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120093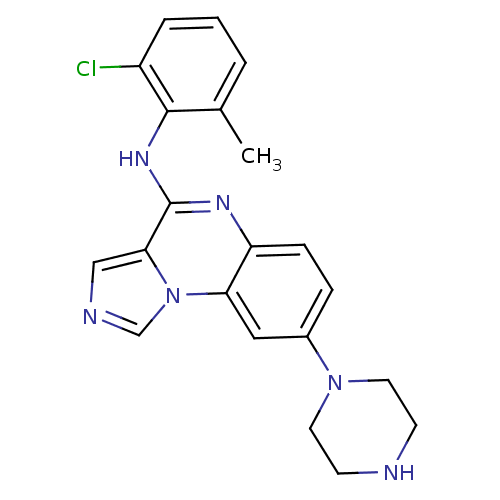 ((2-Chloro-6-methyl-phenyl)-(8-piperazin-1-yl-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1 (Homo sapiens (Human)) | BDBM50199036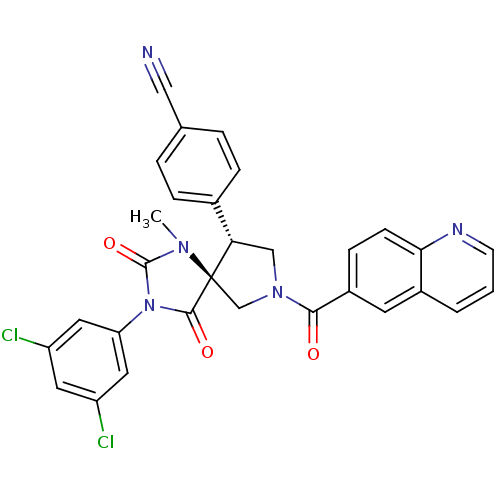 (4-[(5S,9R)-3-(3,5-dichloro-phenyl)-1-methyl-2,4-di...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep Curated by ChEMBL | Assay Description Inhibition of LFA1-mediated adhesion of T cell to HUVEC | J Med Chem 49: 6946-9 (2006) Article DOI: 10.1021/jm0610806 BindingDB Entry DOI: 10.7270/Q2SF2XC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120089 ((2-Chloro-6-methyl-phenyl)-[8-(3,5-dimethyl-pipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120130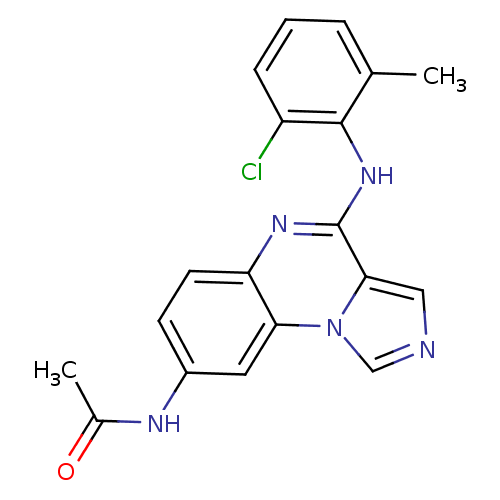 (CHEMBL110732 | N-(4-(2-chloro-6-methylphenylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120113 ((2-chloro-6-methyl-phenyl)-(7,9-dioxa-2,5,10b-tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120104 (4-(2-Chloro-6-methyl-phenylamino)-imidazo[1,5-a]qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50151366 ((2-Chloro-6-methyl-phenyl)-[8-((S)-3-methyl-pipera...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src kinase | J Med Chem 47: 4517-29 (2004) Article DOI: 10.1021/jm030217e BindingDB Entry DOI: 10.7270/Q2R210VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120111 ((2-Chloro-6-methyl-phenyl)-(8-morpholin-4-yl-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50380367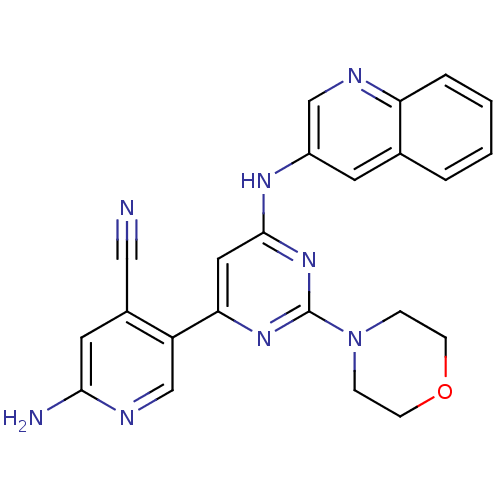 (CHEMBL2017966) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay | ACS Med Chem Lett 2: 774-779 (2011) Article DOI: 10.1021/ml200156t BindingDB Entry DOI: 10.7270/Q2N29XXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112938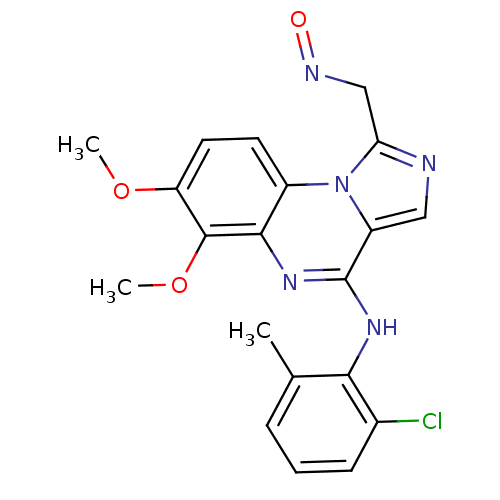 ((E)-4-(2-chloro-6-methylphenylamino)-6,7-dimethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against recombinant p56 Lck tyrosine kinase expressed as a His-tagged protein in insect cells using a baculovirus expression syst... | Bioorg Med Chem Lett 12: 1361-4 (2002) BindingDB Entry DOI: 10.7270/Q2R78DJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Coagulation factor X was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM50151366 ((2-Chloro-6-methyl-phenyl)-[8-((S)-3-methyl-pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Yes kinase | J Med Chem 47: 4517-29 (2004) Article DOI: 10.1021/jm030217e BindingDB Entry DOI: 10.7270/Q2R210VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120108 (CHEMBL326476 | N*4*-(2-Chloro-6-methyl-phenyl)-N*8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120115 (CHEMBL321862 | N*4*-(2-Chloro-6-fluoro-phenyl)-N*7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50123321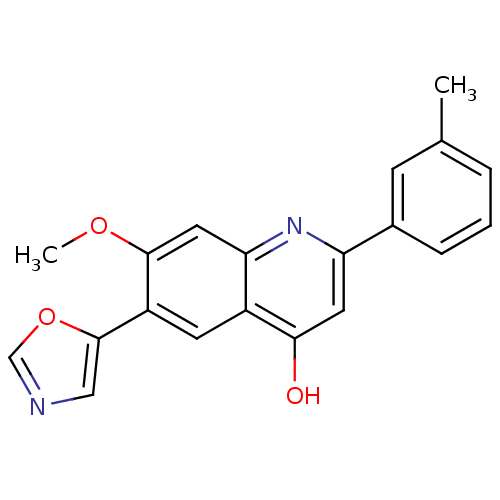 (7-Methoxy-6-oxazol-5-yl-2-m-tolyl-1H-quinolin-4-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb PRI Curated by ChEMBL | Assay Description Inhibition of human inosine monophosphate dehydrogenase IMPDH II | Bioorg Med Chem Lett 13: 543-6 (2003) BindingDB Entry DOI: 10.7270/Q2SJ1JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50123333 (2-(4-Bromo-phenyl)-7-methoxy-6-oxazol-5-yl-1H-quin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb PRI Curated by ChEMBL | Assay Description Inhibition of human inosine monophosphate dehydrogenase IMPDH II | Bioorg Med Chem Lett 13: 543-6 (2003) BindingDB Entry DOI: 10.7270/Q2SJ1JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50123358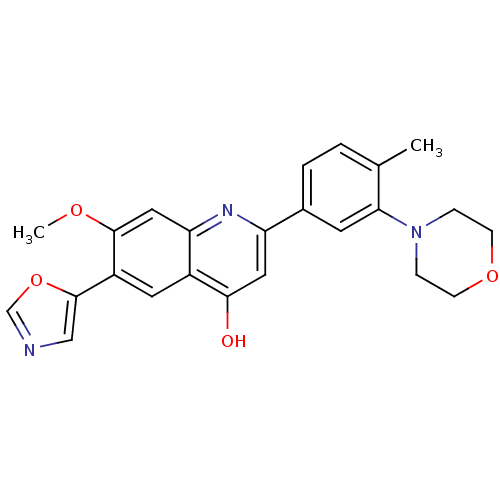 (7-Methoxy-2-(4-methyl-3-morpholin-4-yl-phenyl)-6-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb PRI Curated by ChEMBL | Assay Description Inhibitory activity against inosine monophosphate dehydrogenase IMPDH II | Bioorg Med Chem Lett 13: 547-51 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50123355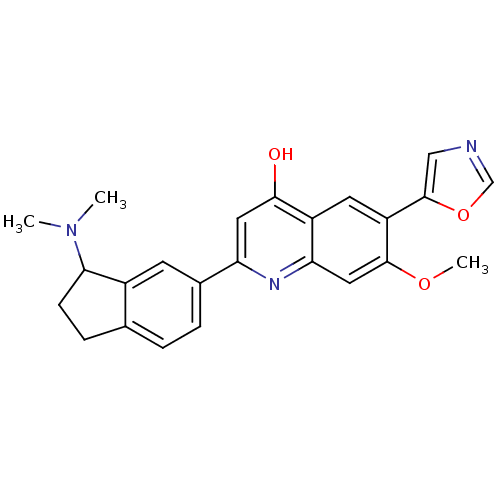 (2-(3-Dimethylamino-indan-5-yl)-7-methoxy-6-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb PRI Curated by ChEMBL | Assay Description Inhibitory activity against inosine monophosphate dehydrogenase IMPDH II | Bioorg Med Chem Lett 13: 547-51 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 678 total ) | Next | Last >> |