 Found 94 hits with Last Name = 'jain' and Initial = 'g'
Found 94 hits with Last Name = 'jain' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50084621
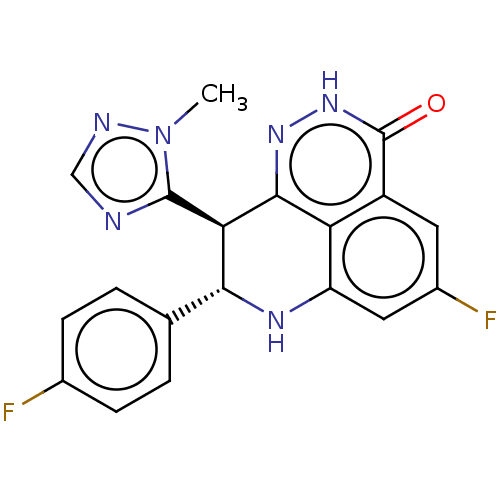
(BMN 673 | Talazoparib)Show SMILES Cn1ncnc1[C@@H]1[C@H](Nc2cc(F)cc3c2c1n[nH]c3=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,24H,1H3,(H,26,28)/t15-,16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 (unknown origin) after 1 min in presence of NAD by top count analysis |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50084621

(BMN 673 | Talazoparib)Show SMILES Cn1ncnc1[C@@H]1[C@H](Nc2cc(F)cc3c2c1n[nH]c3=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,24H,1H3,(H,26,28)/t15-,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) after 1 min in presence of NAD by top count analysis |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length PARP1 after 4 mins by [32P]NAD+ incorporation assay |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50316226
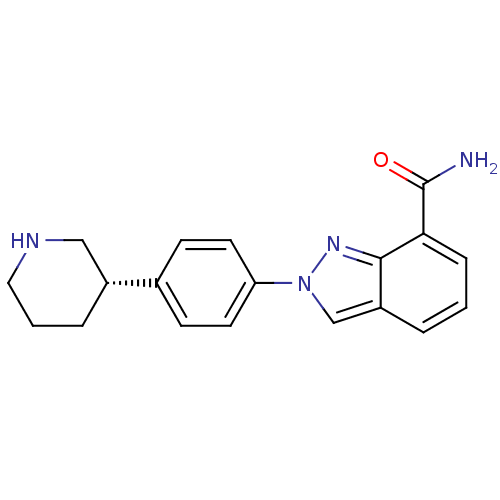
((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50316226

((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 (unknown origin) |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50068774
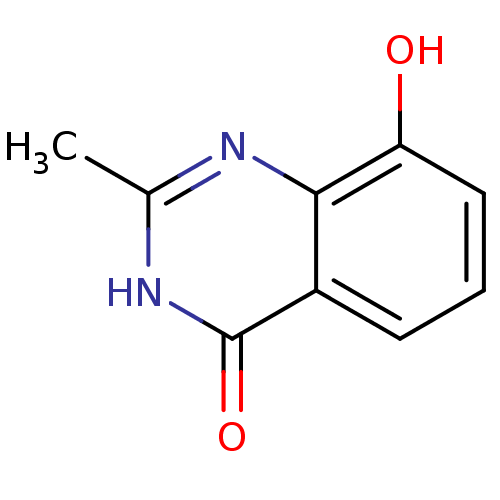
(8-HYDROXY-2-METHYL-3-HYDRO-QUINAZOLIN-4-ONE | 8-Hy...)Show InChI InChI=1S/C9H8N2O2/c1-5-10-8-6(9(13)11-5)3-2-4-7(8)12/h2-4,12H,1H3,(H,10,11,13) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27506
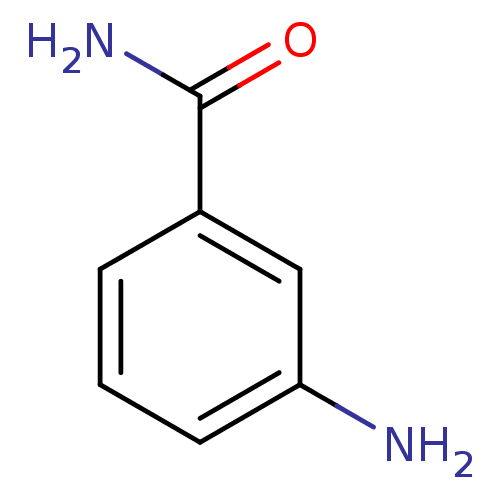
(3-AB | 3-aminobenzamide | CHEMBL81977)Show InChI InChI=1S/C7H8N2O/c8-6-3-1-2-5(4-6)7(9)10/h1-4H,8H2,(H2,9,10) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM206061
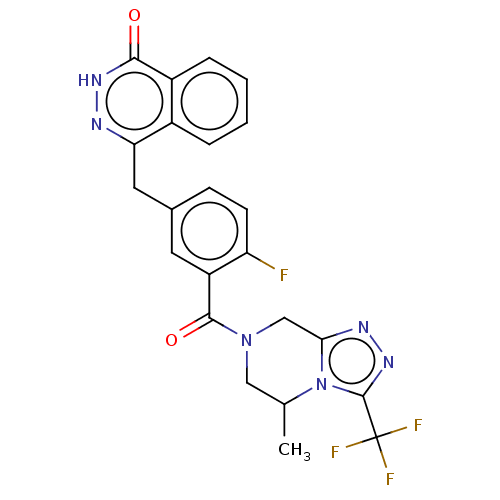
(US9255106, S3)Show SMILES CC1CN(Cc2nnc(n12)C(F)(F)F)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP2 using histone as substrate after 1 hr in presence of NAD+ by ELISA |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50084621

(BMN 673 | Talazoparib)Show SMILES Cn1ncnc1[C@@H]1[C@H](Nc2cc(F)cc3c2c1n[nH]c3=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,24H,1H3,(H,26,28)/t15-,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) after 1 min in presence of NAD by top count analysis |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 2
(Mus musculus (Mouse)) | BDBM50536937

(CHEMBL4554200)Show SMILES CC(C)n1cc2CN(Cc2n1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C24H22FN5O2/c1-14(2)30-12-16-11-29(13-22(16)28-30)24(32)19-9-15(7-8-20(19)25)10-21-17-5-3-4-6-18(17)23(31)27-26-21/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PARP2 by chemiluminescence assay |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM206061

(US9255106, S3)Show SMILES CC1CN(Cc2nnc(n12)C(F)(F)F)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) by NAD+ based assay |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50536939

(CHEMBL4569417)Show SMILES CC(C)(C)[Si](C)(C)OCCCN1CCN(CC1=O)C(=O)c1cc(Cn2c3ccccc3c(=O)[nH]c2=O)ccc1F Show InChI InChI=1S/C29H37FN4O5Si/c1-29(2,3)40(4,5)39-16-8-13-32-14-15-33(19-25(32)35)27(37)22-17-20(11-12-23(22)30)18-34-24-10-7-6-9-21(24)26(36)31-28(34)38/h6-7,9-12,17H,8,13-16,18-19H2,1-5H3,(H,31,36,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) using histone as substrate after 1 hr by ELISA |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM206061

(US9255106, S3)Show SMILES CC1CN(Cc2nnc(n12)C(F)(F)F)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of NAD+ by ELISA |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50536936
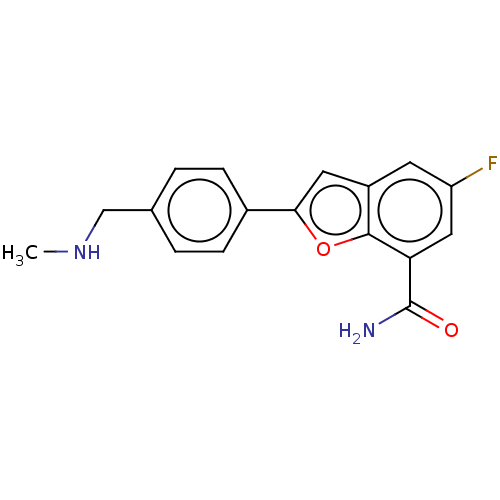
(CHEMBL4584534)Show InChI InChI=1S/C17H15FN2O2/c1-20-9-10-2-4-11(5-3-10)15-7-12-6-13(18)8-14(17(19)21)16(12)22-15/h2-8,20H,9H2,1H3,(H2,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 (unknown origin) by biotinylated NAD+-based luminescence assay |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50316226

((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 (unknown origin) |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50536936

(CHEMBL4584534)Show InChI InChI=1S/C17H15FN2O2/c1-20-9-10-2-4-11(5-3-10)15-7-12-6-13(18)8-14(17(19)21)16(12)22-15/h2-8,20H,9H2,1H3,(H2,19,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of NAD+ by ELISA |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50316226

((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length PARP1 after 4 mins by [32P]NAD+ incorporation assay |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50536937

(CHEMBL4554200)Show SMILES CC(C)n1cc2CN(Cc2n1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C24H22FN5O2/c1-14(2)30-12-16-11-29(13-22(16)28-30)24(32)19-9-15(7-8-20(19)25)10-21-17-5-3-4-6-18(17)23(31)27-26-21/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 by chemiluminescence assay |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50536942

(CHEMBL4569989)Show SMILES NC(=O)c1cccc2[nH]c(CN3CCC(CC3)C(=O)Nc3cc4ccccc4cn3)nc12 Show InChI InChI=1S/C24H24N6O2/c25-23(31)18-6-3-7-19-22(18)28-21(27-19)14-30-10-8-15(9-11-30)24(32)29-20-12-16-4-1-2-5-17(16)13-26-20/h1-7,12-13,15H,8-11,14H2,(H2,25,31)(H,27,28)(H,26,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50536939

(CHEMBL4569417)Show SMILES CC(C)(C)[Si](C)(C)OCCCN1CCN(CC1=O)C(=O)c1cc(Cn2c3ccccc3c(=O)[nH]c2=O)ccc1F Show InChI InChI=1S/C29H37FN4O5Si/c1-29(2,3)40(4,5)39-16-8-13-32-14-15-33(19-25(32)35)27(37)22-17-20(11-12-23(22)30)18-34-24-10-7-6-9-21(24)26(36)31-28(34)38/h6-7,9-12,17H,8,13-16,18-19H2,1-5H3,(H,31,36,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 (unknown origin) using histone as substrate after 1 hr by ELISA |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50536935

(CHEMBL4533690)Show SMILES Fc1ccc(Cn2c3ccccc3c(=O)[nH]c2=O)cc1C(=O)NC1CCN(CC2CC2)C1 Show InChI InChI=1S/C24H25FN4O3/c25-20-8-7-16(13-29-21-4-2-1-3-18(21)22(30)27-24(29)32)11-19(20)23(31)26-17-9-10-28(14-17)12-15-5-6-15/h1-4,7-8,11,15,17H,5-6,9-10,12-14H2,(H,26,31)(H,27,30,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 by ELISA |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242265
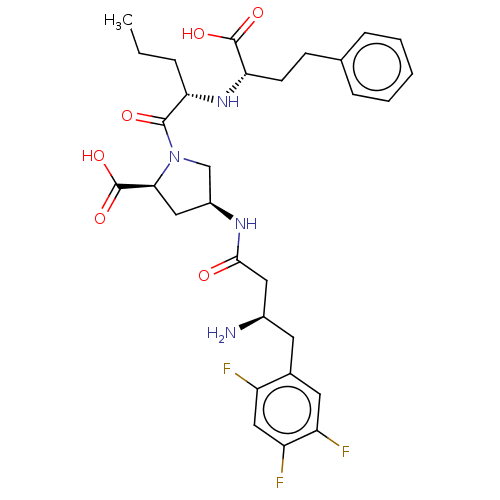
(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50242266
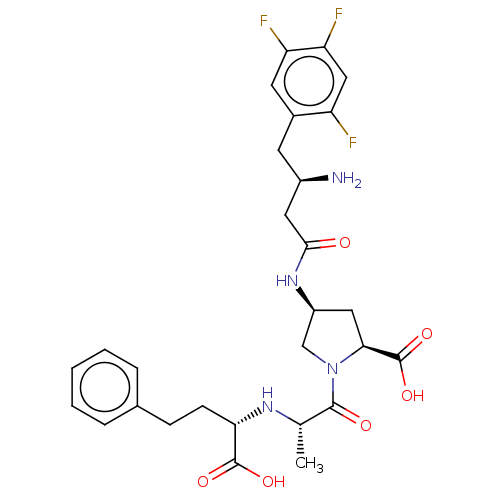
(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242265

(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50536935

(CHEMBL4533690)Show SMILES Fc1ccc(Cn2c3ccccc3c(=O)[nH]c2=O)cc1C(=O)NC1CCN(CC2CC2)C1 Show InChI InChI=1S/C24H25FN4O3/c25-20-8-7-16(13-29-21-4-2-1-3-18(21)22(30)27-24(29)32)11-19(20)23(31)26-17-9-10-28(14-17)12-15-5-6-15/h1-4,7-8,11,15,17H,5-6,9-10,12-14H2,(H,26,31)(H,27,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP2 by ELISA |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50536941
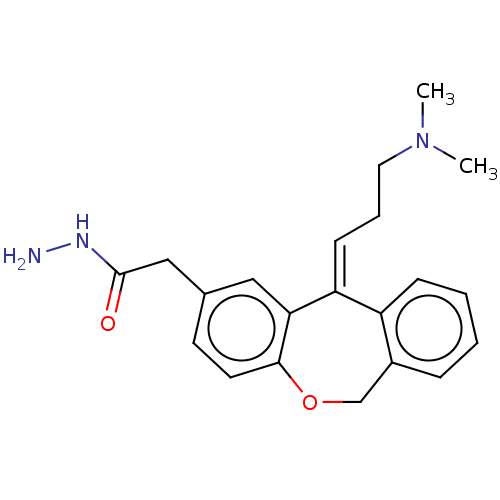
(CHEMBL4521985)Show InChI InChI=1S/C21H25N3O2/c1-24(2)11-5-8-18-17-7-4-3-6-16(17)14-26-20-10-9-15(12-19(18)20)13-21(25)23-22/h3-4,6-10,12H,5,11,13-14,22H2,1-2H3,(H,23,25)/b18-8+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50536938

(CHEMBL4521684)Show SMILES Clc1cccc(NC(=O)CCn2nc(-c3ccccc3)c3ccccc3c2=O)c1 Show InChI InChI=1S/C23H18ClN3O2/c24-17-9-6-10-18(15-17)25-21(28)13-14-27-23(29)20-12-5-4-11-19(20)22(26-27)16-7-2-1-3-8-16/h1-12,15H,13-14H2,(H,25,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) by colorimetric analysis |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242274
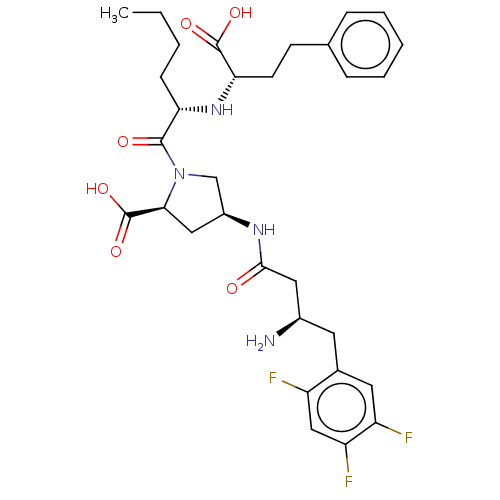
(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50536937

(CHEMBL4554200)Show SMILES CC(C)n1cc2CN(Cc2n1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C24H22FN5O2/c1-14(2)30-12-16-11-29(13-22(16)28-30)24(32)19-9-15(7-8-20(19)25)10-21-17-5-3-4-6-18(17)23(31)27-26-21/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TNKS1 by chemiluminescence assay |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242274

(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50536937

(CHEMBL4554200)Show SMILES CC(C)n1cc2CN(Cc2n1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C24H22FN5O2/c1-14(2)30-12-16-11-29(13-22(16)28-30)24(32)19-9-15(7-8-20(19)25)10-21-17-5-3-4-6-18(17)23(31)27-26-21/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TNKS2 by chemiluminescence assay |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27682
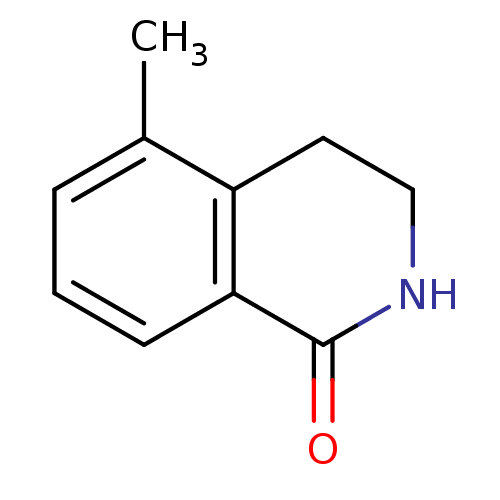
(5-methyl-1,2,3,4-tetrahydroisoquinolin-1-one | CHE...)Show InChI InChI=1S/C10H11NO/c1-7-3-2-4-9-8(7)5-6-11-10(9)12/h2-4H,5-6H2,1H3,(H,11,12) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) |
Eur J Med Chem 165: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.024
BindingDB Entry DOI: 10.7270/Q2Z03CPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 488 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data