

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50178975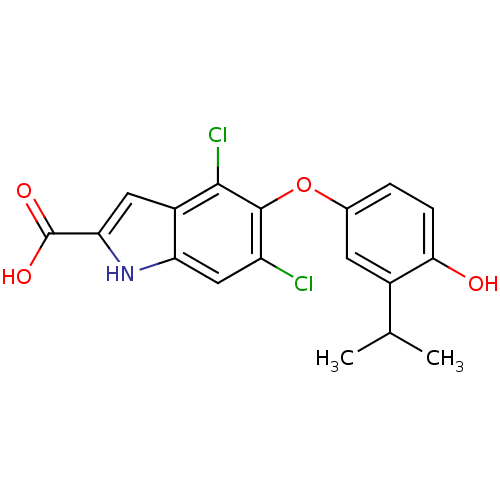 (4,6-dichloro-5-(4-hydroxy-3-isopropylphenoxy)-1H-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Binding affinity to TRbeta1 | J Med Chem 55: 5649-75 (2012) Article DOI: 10.1021/jm2004706 BindingDB Entry DOI: 10.7270/Q2DZ09FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Feline coronavirus (strain FIPV WSU-79/1146) (FCoV...) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (PEDV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18869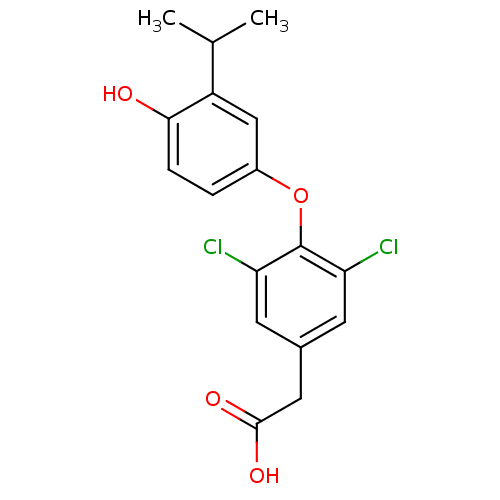 (2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Binding affinity to TRbeta1 | J Med Chem 55: 5649-75 (2012) Article DOI: 10.1021/jm2004706 BindingDB Entry DOI: 10.7270/Q2DZ09FJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (HCoV-OC43) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-NL63) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-HKU1) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (MHV-A59) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-229E) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50196446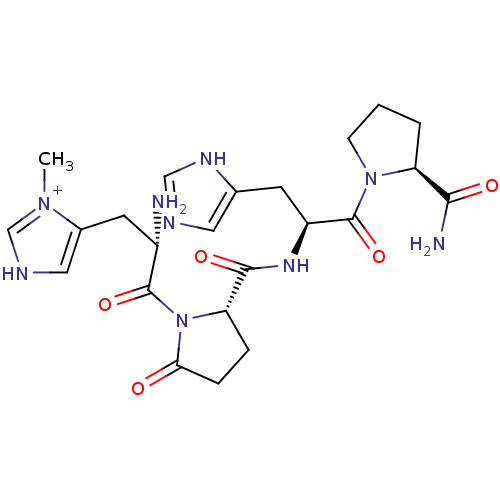 (CHEMBL224730 | [N tau(1)-Me-His]-TRH) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR1 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50173826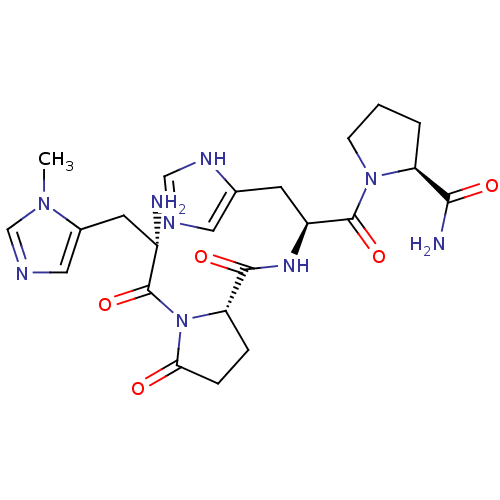 (1-[(S)-2-Amino-3-(3-methyl-3H-imidazol-4-yl)-propi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory constant against thyrotropin releasing hormone receptor 1 expressed in HEK 293EM cells upon incubation at 37 degee C for 1 hr at pH 7.4 us... | J Med Chem 48: 6162-5 (2005) Article DOI: 10.1021/jm0505462 BindingDB Entry DOI: 10.7270/Q2PK0FQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50196451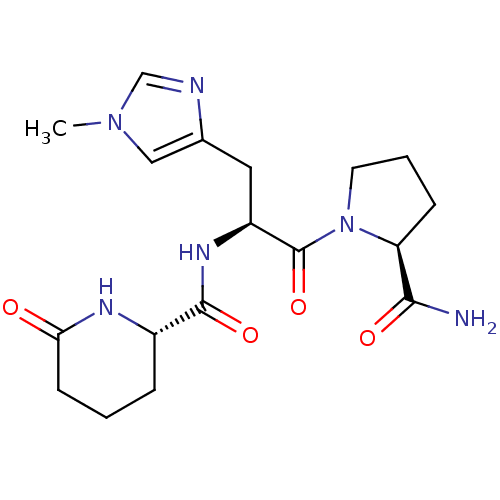 ((2S)-1-{(2S)-3-(1-methyl-1H-4-imidazolyl)-2-[(2S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR1 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (IBV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18869 (2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Binding affinity to TRalpha1 | J Med Chem 55: 5649-75 (2012) Article DOI: 10.1021/jm2004706 BindingDB Entry DOI: 10.7270/Q2DZ09FJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50072394 (1-[3-(1H-4-imidazolyl)-2-(5-oxotetrahydro-1H-2-pyr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR2 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50072394 (1-[3-(1H-4-imidazolyl)-2-(5-oxotetrahydro-1H-2-pyr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory constant against thyrotropin releasing hormone receptor 2 expressed in HEK 293EM cells upon incubation at 37 degee C for 1 hr at pH 7.4 us... | J Med Chem 48: 6162-5 (2005) Article DOI: 10.1021/jm0505462 BindingDB Entry DOI: 10.7270/Q2PK0FQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50196457 ((2S)-1-{(2S)-3-(1-ethyl-1H-4-imidazolyl)-2-[(2S)-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR1 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077282 (CHEMBL2311098 | H-Cpa-cyclo[DCys-Pal-DTrp-Lys-Tle-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077280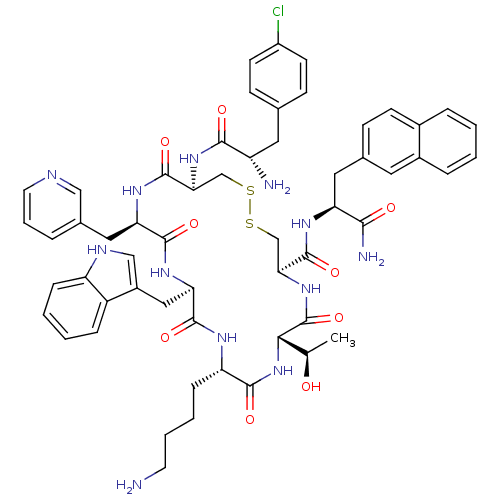 (CHEMBL408347 | H-Cpa-cyclo[DCys-Pal-DTrp-Lys-Thr-C...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50196451 ((2S)-1-{(2S)-3-(1-methyl-1H-4-imidazolyl)-2-[(2S)-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR2 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077269 (CHEMBL385746 | H-Cpa-cyclo[DCys-2Pal-DTrp-Lys-Thr-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077301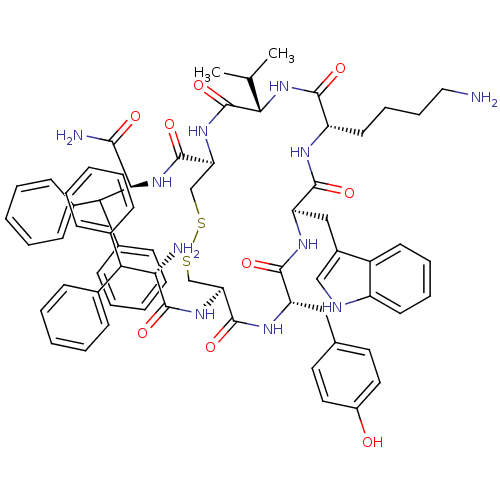 (10-(4-Amino-butyl)-19-(2-amino-3,3-diphenyl-propio...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077294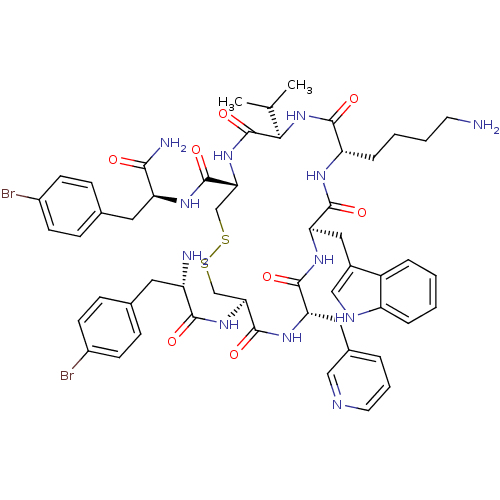 (CHEMBL437448 | H-Bpa-cyclo[DCys-Pal-DTrp-Lys-Val-C...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50077285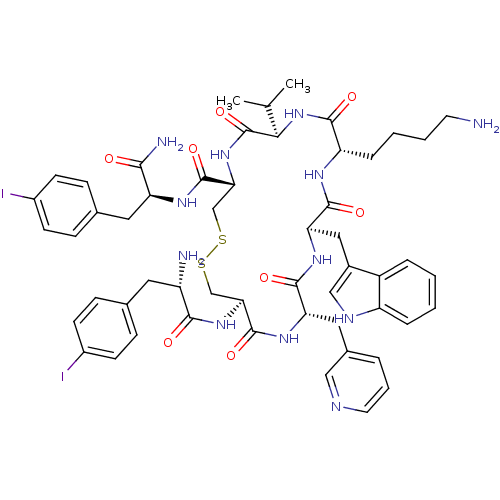 (10-(4-Amino-butyl)-19-[2-amino-3-(4-iodo-phenyl)-p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 3 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077288 (CHEMBL2372956 | H-Cpa-cyclo[DCys-Pal-DTrp-Lys-Tle-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50072394 (1-[3-(1H-4-imidazolyl)-2-(5-oxotetrahydro-1H-2-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR1 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50072394 (1-[3-(1H-4-imidazolyl)-2-(5-oxotetrahydro-1H-2-pyr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory constant against thyrotropin releasing hormone receptor 1 expressed in HEK 293EM cells upon incubation at 37 degee C for 1 hr at pH 7.4 us... | J Med Chem 48: 6162-5 (2005) Article DOI: 10.1021/jm0505462 BindingDB Entry DOI: 10.7270/Q2PK0FQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077305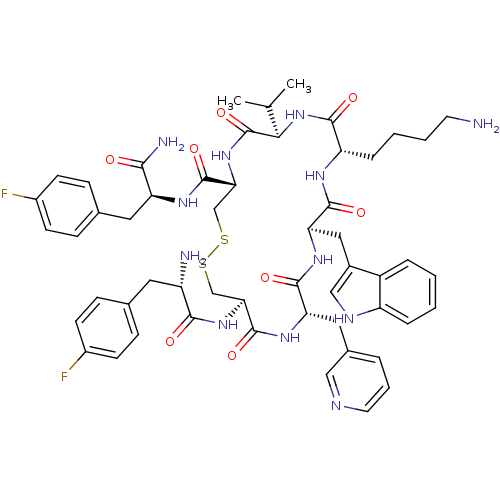 (CHEMBL407195 | H-Fpa-cyclo[DCys-Pal-DTrp-Lys-Val-C...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077290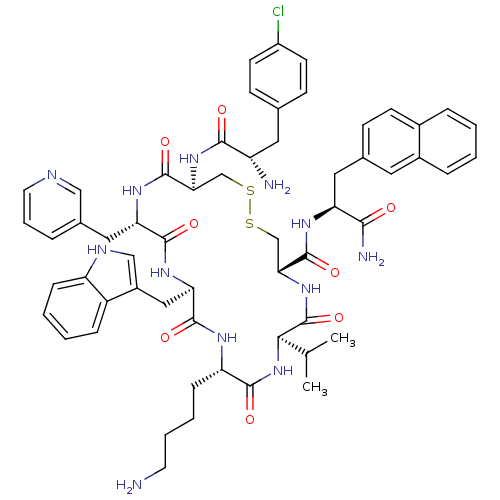 (CHEMBL437220 | H-Cpa-cyclo[DCys-Pal-DTrp-Lys-Val-C...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077279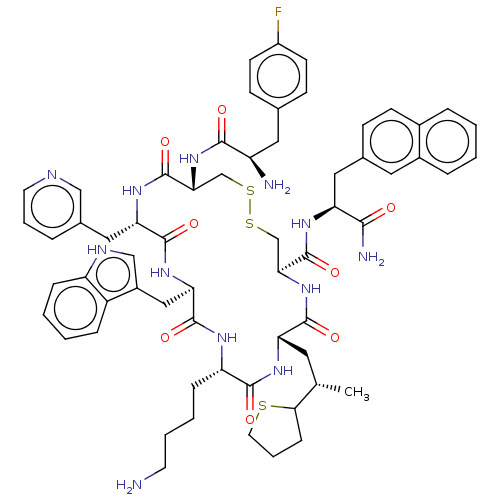 (CHEMBL2372964 | H-Fpa-cyclo[DCys-Pal-DTrp-Lys-Tle-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077278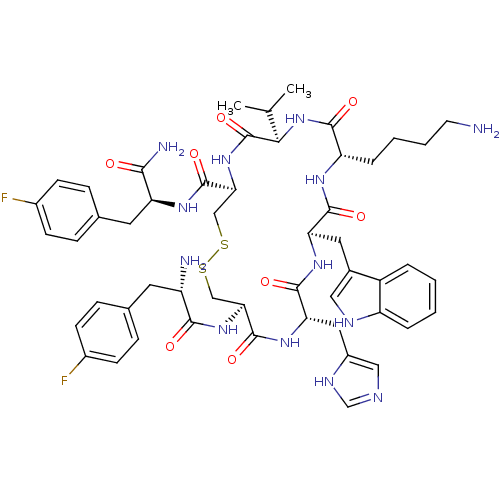 (CHEMBL407496 | H-Fpa-cyclo[DCys-His-DTrp-Lys-Val-C...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077285 (10-(4-Amino-butyl)-19-[2-amino-3-(4-iodo-phenyl)-p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077283 (CHEMBL439136 | H-Cpa-cyclo[DCys-Pal-DTrp-Lys-Val-C...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50196457 ((2S)-1-{(2S)-3-(1-ethyl-1H-4-imidazolyl)-2-[(2S)-6...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR2 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50077281 (CHEMBL438726 | H-Pfp-cyclo[DCys-D2Pal-DTrp-Lys-Val...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 1 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077297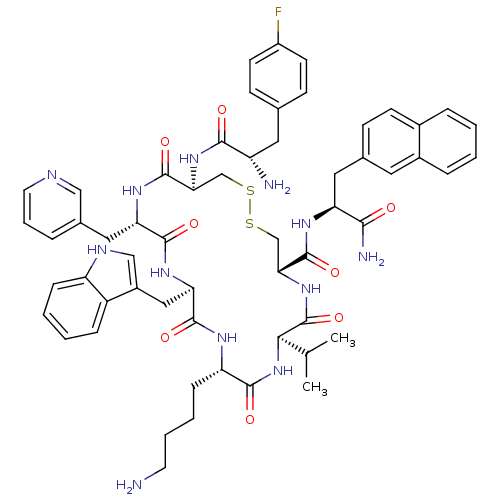 (CHEMBL415201 | H-Fpa-cyclo[DCys-Pal-DTrp-Lys-Val-C...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50455549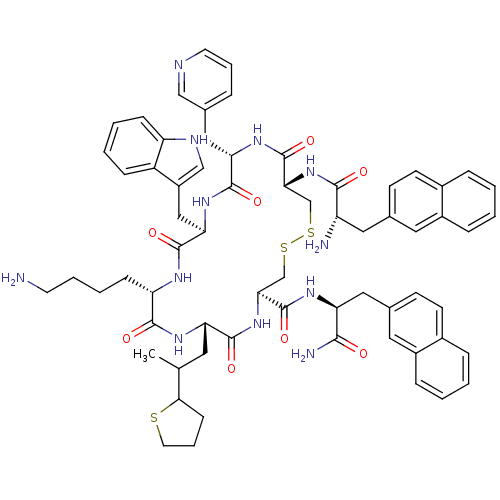 (CHEMBL2372960) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077293 (10-(4-Amino-butyl)-19-(2-amino-3-naphthalen-2-yl-p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50077295 (CHEMBL406491 | H-2Fpa-cyclo[DCys-His-DTrp-Lys-Val-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 1 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50077280 (CHEMBL408347 | H-Cpa-cyclo[DCys-Pal-DTrp-Lys-Thr-C...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 3 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells | J Med Chem 50: 5951-66 (2007) Article DOI: 10.1021/jm061490u BindingDB Entry DOI: 10.7270/Q2Q81CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50077285 (10-(4-Amino-butyl)-19-[2-amino-3-(4-iodo-phenyl)-p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 5 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50077283 (CHEMBL439136 | H-Cpa-cyclo[DCys-Pal-DTrp-Lys-Val-C...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 3 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50077308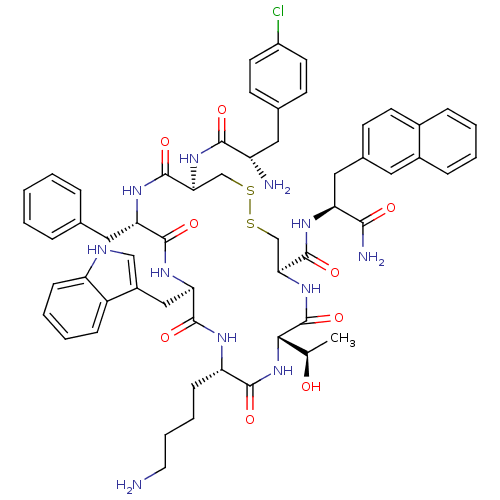 (10-(4-Amino-butyl)-19-[2-amino-3-(4-chloro-phenyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 2 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50077269 (CHEMBL385746 | H-Cpa-cyclo[DCys-2Pal-DTrp-Lys-Thr-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 3 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50077294 (CHEMBL437448 | H-Bpa-cyclo[DCys-Pal-DTrp-Lys-Val-C...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 3 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50077288 (CHEMBL2372956 | H-Cpa-cyclo[DCys-Pal-DTrp-Lys-Tle-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for binding affinity against human Somatostatin receptor type 3 | J Med Chem 42: 1863-71 (1999) Article DOI: 10.1021/jm9806289 BindingDB Entry DOI: 10.7270/Q2XW4J03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2501 total ) | Next | Last >> |