

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM50418932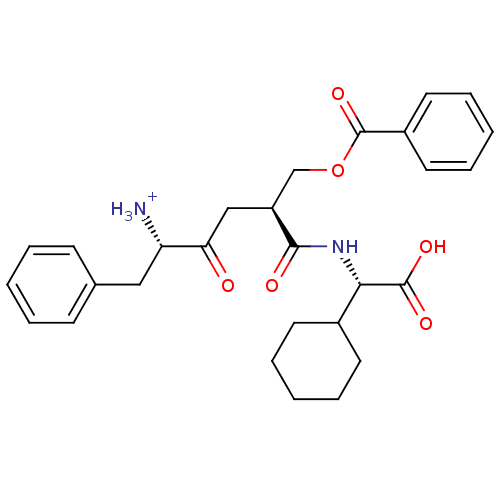 (CHEMBL1807354) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.68E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting | Bioorg Med Chem Lett 21: 4597-601 (2011) Article DOI: 10.1016/j.bmcl.2011.05.108 BindingDB Entry DOI: 10.7270/Q2VM4DHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM50418933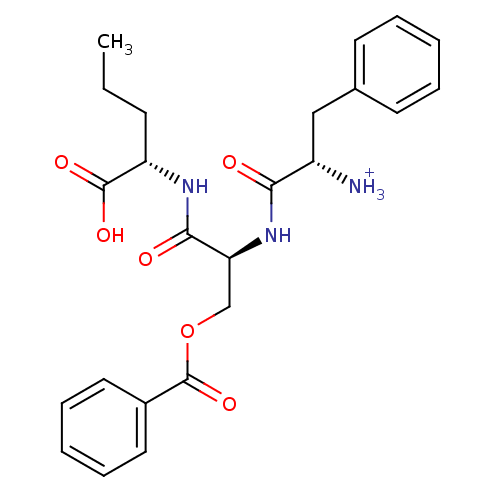 (CHEMBL1807356) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.23E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting | Bioorg Med Chem Lett 21: 4597-601 (2011) Article DOI: 10.1016/j.bmcl.2011.05.108 BindingDB Entry DOI: 10.7270/Q2VM4DHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM50418934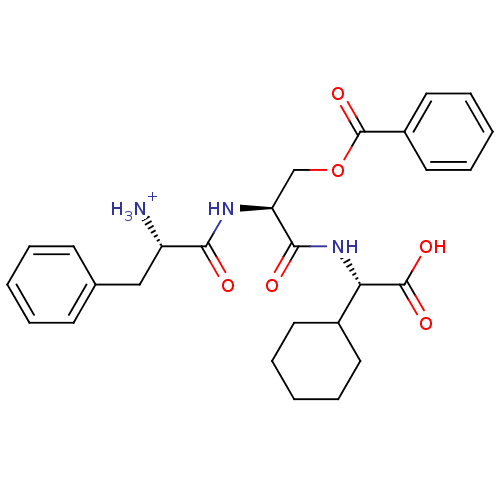 (CHEMBL1807357) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.45E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting | Bioorg Med Chem Lett 21: 4597-601 (2011) Article DOI: 10.1016/j.bmcl.2011.05.108 BindingDB Entry DOI: 10.7270/Q2VM4DHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM50418935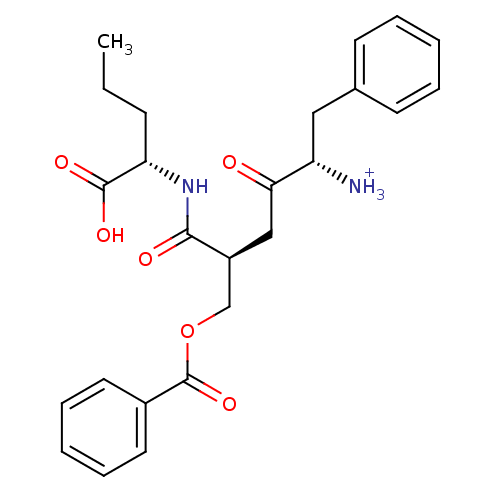 (CHEMBL1807353) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting | Bioorg Med Chem Lett 21: 4597-601 (2011) Article DOI: 10.1016/j.bmcl.2011.05.108 BindingDB Entry DOI: 10.7270/Q2VM4DHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM50418931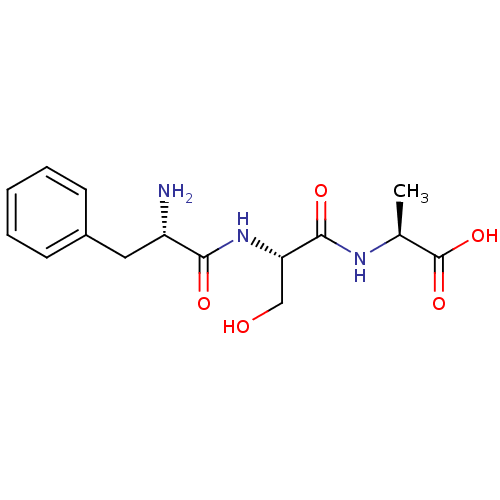 (CHEMBL1807351) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.57E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting | Bioorg Med Chem Lett 21: 4597-601 (2011) Article DOI: 10.1016/j.bmcl.2011.05.108 BindingDB Entry DOI: 10.7270/Q2VM4DHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM50418936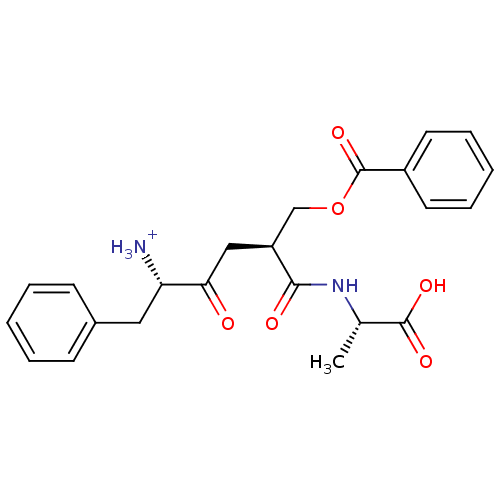 (CHEMBL1807352) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.24E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity to PEPT1 in human Caco2 cells assessed as inhibition of [14C]Gly-Sar apical uptake after 5 mins by liquid scintillation counting | Bioorg Med Chem Lett 21: 4597-601 (2011) Article DOI: 10.1016/j.bmcl.2011.05.108 BindingDB Entry DOI: 10.7270/Q2VM4DHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103118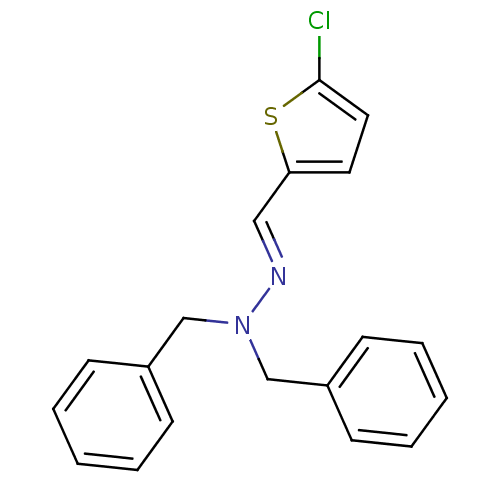 (CHEMBL66059 | N,N-Dibenzyl-N'-[1-(5-chloro-thiophe...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103125 (CHEMBL69115 | N,N-Dibenzyl-N'-[1-(4-methoxy-phenyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103111 (CHEMBL69665 | N,N-Dibenzyl-N'-[1-(4-chloro-phenyl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103119 (CHEMBL302504 | N,N-Dibenzyl-N'-[1-(5-ethyl-furan-2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103107 (CHEMBL65842 | N,N-Dibenzyl-N'-[1-(3-methoxy-phenyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103128 (CHEMBL68984 | N,N-Dibenzyl-N'-[1-furan-2-yl-meth-(...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103106 (CHEMBL69480 | N,N-Dibenzyl-N'-[1-thiophen-2-yl-met...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103122 (CHEMBL69334 | N,N-Dibenzyl-N'-[1-phenyl-meth-(E)-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103110 (CHEMBL305258 | N,N-Dibenzyl-N'-[1-(3,4-dichloro-ph...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103114 (CHEMBL69541 | N,N-Dibenzyl-N'-[1-cyclohexyl-meth-(...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103115 (4-(Dibenzyl-hydrazonomethyl)-phenol | CHEMBL70032) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103126 (CHEMBL68011 | N,N-Dibenzyl-N'-[1-pyridin-3-yl-meth...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50030630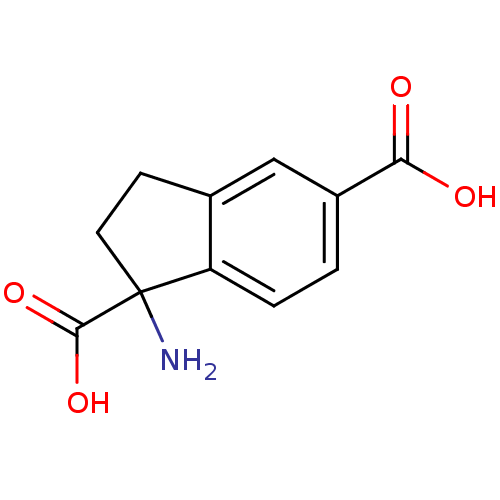 ((RS)-1-aminoindan-1,5-dicarboxylic acid | 1-Amino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Perugia Curated by ChEMBL | Assay Description Ability to inhibit mGluR1-alpha-mediated PI (phospho inosotol) hydrolysis was determined at BHK cells at 100 Micro M Concentration | J Med Chem 38: 3717-9 (1995) BindingDB Entry DOI: 10.7270/Q2V123TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103120 (CHEMBL69497 | N,N-Dibenzyl-N'-[1-pyridin-2-yl-meth...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103124 (CHEMBL67272 | [5-(Dibenzyl-hydrazonomethyl)-furan-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103112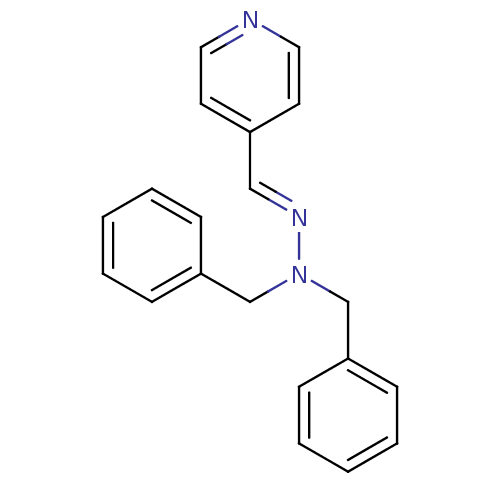 (CHEMBL71650 | N,N-Dibenzyl-N'-[1-pyridin-4-yl-meth...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103121 (CHEMBL419070 | N,N-Dibenzyl-N'-[1-phenyl-eth-(E)-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103109 (CHEMBL66774 | N,N-Dibenzyl-N'-[3,4-dihydro-2H-naph...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50030628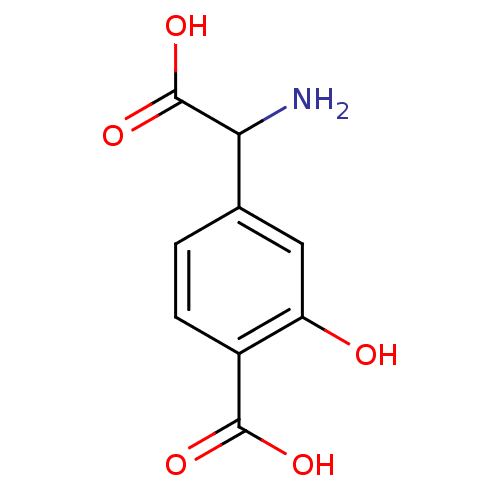 ((S)-4C3HPG | 4-(Amino-carboxy-methyl)-2-hydroxy-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Perugia Curated by ChEMBL | Assay Description Ability to inhibit mGluR1-alpha-mediated PI (phospho inosotol) hydrolysis was determined at BHK cells at 100 Micro M Concentration | J Med Chem 38: 3717-9 (1995) BindingDB Entry DOI: 10.7270/Q2V123TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50030628 ((S)-4C3HPG | 4-(Amino-carboxy-methyl)-2-hydroxy-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Perugia Curated by ChEMBL | Assay Description Ability to inhibit mGluR2-alpha induced cAMP formation was determined at BHK cells at 100 Micro M Concentration | J Med Chem 38: 3717-9 (1995) BindingDB Entry DOI: 10.7270/Q2V123TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103116 (CHEMBL68981 | N,N-Dibenzyl-N'-[6-methoxy-3,4-dihyd...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50030629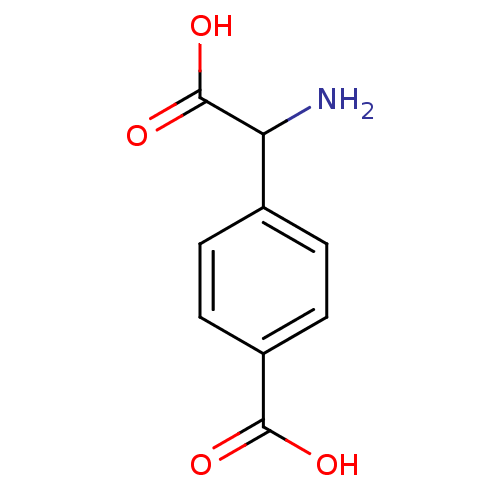 ((R,S)-4-Carboxy-3-hydroxyphenylglycine | (S)-4CPG ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Perugia Curated by ChEMBL | Assay Description Ability to inhibit mGluR1-alpha-mediated PI (phospho inosotol) hydrolysis was determined at BHK cells at 100 Micro M Concentration | J Med Chem 38: 3717-9 (1995) BindingDB Entry DOI: 10.7270/Q2V123TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103127 (CHEMBL69742 | N,N-Dibenzyl-N'-[1-(5-nitro-furan-2-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103123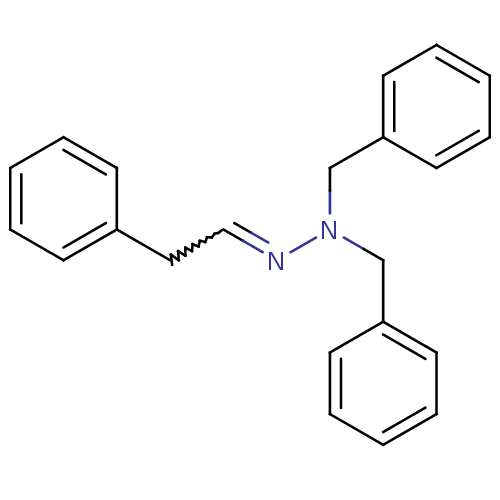 (CHEMBL68153 | N,N-Dibenzyl-N'-[2-phenyl-eth-(E)-yl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103117 (CHEMBL302496 | N,N-Dibenzyl-N'-[1-(3,5-dichloro-ph...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103108 (CHEMBL304503 | N,N-Dibenzyl-N'-[1-(2-methoxy-pheny...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103113 (CHEMBL69758 | [4-(Dibenzyl-hydrazonomethyl)-phenyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50030627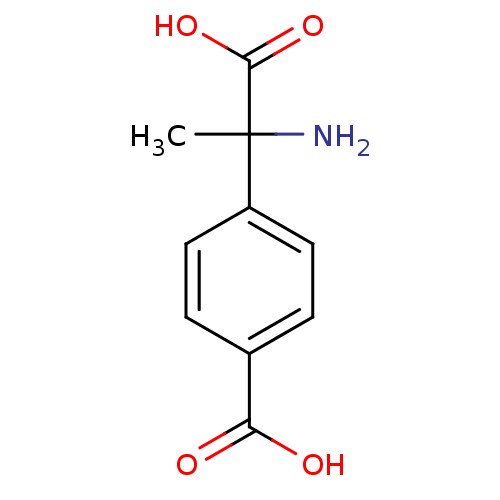 ((+-)-MCPG | (R,S)-alpha-Methyl-4-carboxyphenylglyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Perugia Curated by ChEMBL | Assay Description Ability to inhibit mGluR1-alpha-mediated PI (phospho inosotol) hydrolysis was determined at BHK cells at 100 Micro M Concentration | J Med Chem 38: 3717-9 (1995) BindingDB Entry DOI: 10.7270/Q2V123TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50030627 ((+-)-MCPG | (R,S)-alpha-Methyl-4-carboxyphenylglyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Perugia Curated by ChEMBL | Assay Description Ability to inhibit mGluR2-alpha induced cAMP formation was determined at BHK cells at 100 Micro M Concentration | J Med Chem 38: 3717-9 (1995) BindingDB Entry DOI: 10.7270/Q2V123TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50030629 ((R,S)-4-Carboxy-3-hydroxyphenylglycine | (S)-4CPG ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Perugia Curated by ChEMBL | Assay Description Ability to inhibit mGluR2-alpha induced cAMP formation was determined at BHK cells at 100 Micro M Concentration | J Med Chem 38: 3717-9 (1995) BindingDB Entry DOI: 10.7270/Q2V123TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50030627 ((+-)-MCPG | (R,S)-alpha-Methyl-4-carboxyphenylglyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Perugia Curated by ChEMBL | Assay Description Ability to inhibit mGluR4-alpha induced cAMP formation was determined at BHK cells at 100 Micro M Concentration | J Med Chem 38: 3717-9 (1995) BindingDB Entry DOI: 10.7270/Q2V123TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50030629 ((R,S)-4-Carboxy-3-hydroxyphenylglycine | (S)-4CPG ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Perugia Curated by ChEMBL | Assay Description Ability to inhibit mGluR4-alpha induced cAMP formation was determined at BHK cells at 100 Micro M Concentration | J Med Chem 38: 3717-9 (1995) BindingDB Entry DOI: 10.7270/Q2V123TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50030628 ((S)-4C3HPG | 4-(Amino-carboxy-methyl)-2-hydroxy-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Perugia Curated by ChEMBL | Assay Description Ability to inhibit mGluR4-alpha induced cAMP formation was determined at BHK cells at 100 Micro M Concentration | J Med Chem 38: 3717-9 (1995) BindingDB Entry DOI: 10.7270/Q2V123TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||