

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50093599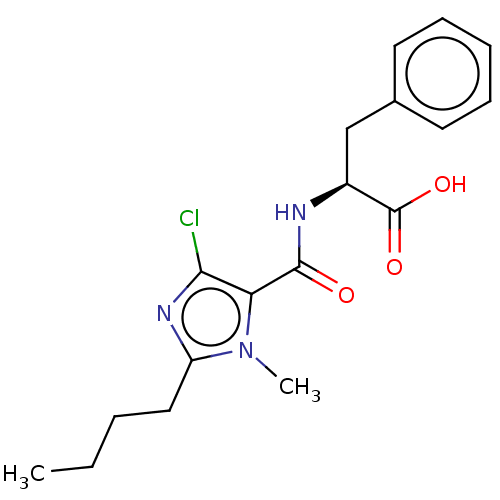 (CHEMBL3585803) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method | Bioorg Med Chem 23: 3526-33 (2015) Article DOI: 10.1016/j.bmc.2015.04.024 BindingDB Entry DOI: 10.7270/Q2DB83M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021153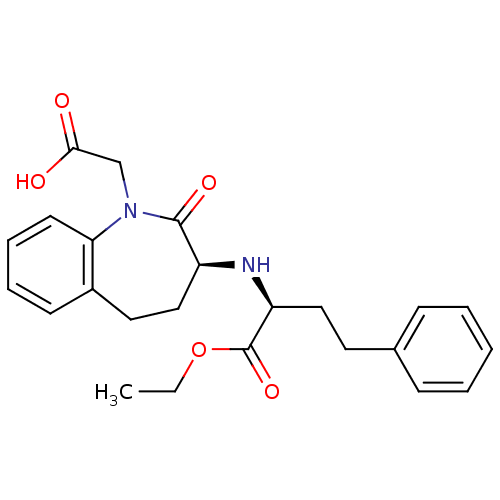 (1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method | Bioorg Med Chem 23: 3526-33 (2015) Article DOI: 10.1016/j.bmc.2015.04.024 BindingDB Entry DOI: 10.7270/Q2DB83M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50368166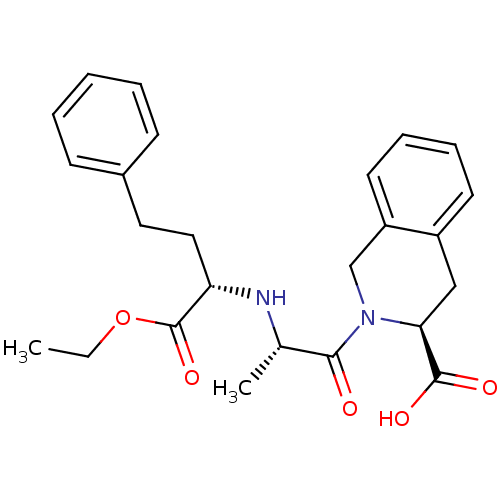 (Accupril | QUINAPRIL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method | Bioorg Med Chem 23: 3526-33 (2015) Article DOI: 10.1016/j.bmc.2015.04.024 BindingDB Entry DOI: 10.7270/Q2DB83M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50017129 ((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method | Bioorg Med Chem 23: 3526-33 (2015) Article DOI: 10.1016/j.bmc.2015.04.024 BindingDB Entry DOI: 10.7270/Q2DB83M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021153 (1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins | Bioorg Med Chem 21: 4485-93 (2013) Article DOI: 10.1016/j.bmc.2013.05.031 BindingDB Entry DOI: 10.7270/Q2P55PXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50084681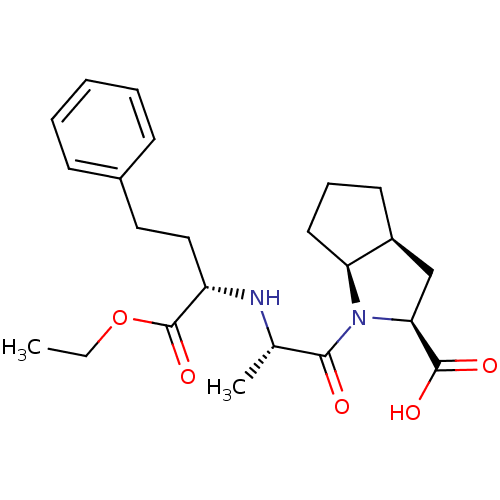 (1-[2-(1-Ethoxycarbonyl-3-phenyl-propylamino)-propi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins | Bioorg Med Chem 21: 4485-93 (2013) Article DOI: 10.1016/j.bmc.2013.05.031 BindingDB Entry DOI: 10.7270/Q2P55PXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50084681 (1-[2-(1-Ethoxycarbonyl-3-phenyl-propylamino)-propi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method | Bioorg Med Chem 23: 3526-33 (2015) Article DOI: 10.1016/j.bmc.2015.04.024 BindingDB Entry DOI: 10.7270/Q2DB83M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367879 (LISINOPRIL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins | Bioorg Med Chem 21: 4485-93 (2013) Article DOI: 10.1016/j.bmc.2013.05.031 BindingDB Entry DOI: 10.7270/Q2P55PXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50438379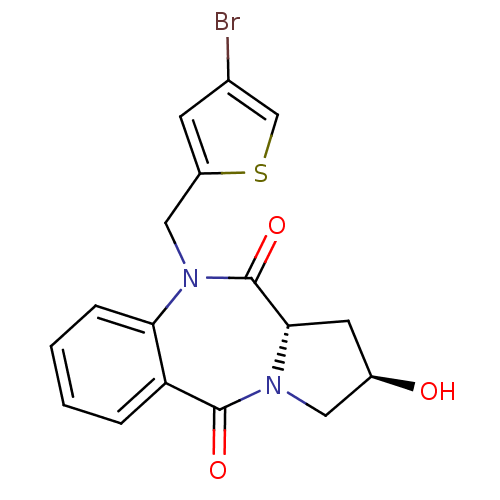 (CHEMBL2413588) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 272 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins | Bioorg Med Chem 21: 4485-93 (2013) Article DOI: 10.1016/j.bmc.2013.05.031 BindingDB Entry DOI: 10.7270/Q2P55PXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367879 (LISINOPRIL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 318 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method | Bioorg Med Chem 23: 3526-33 (2015) Article DOI: 10.1016/j.bmc.2015.04.024 BindingDB Entry DOI: 10.7270/Q2DB83M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50438380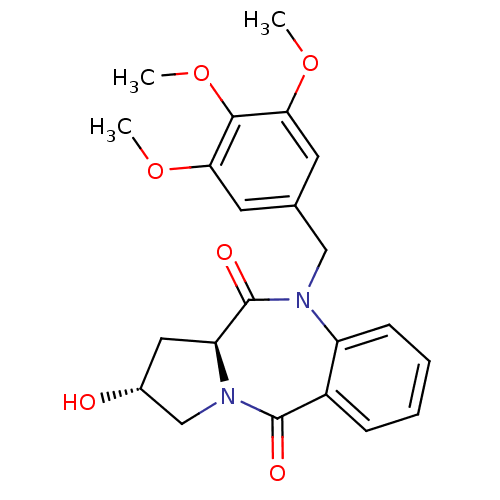 (CHEMBL2413608) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 376 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins | Bioorg Med Chem 21: 4485-93 (2013) Article DOI: 10.1016/j.bmc.2013.05.031 BindingDB Entry DOI: 10.7270/Q2P55PXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50438382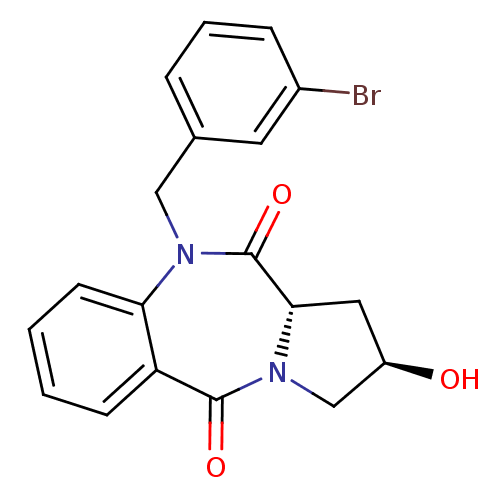 (CHEMBL2413596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 418 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins | Bioorg Med Chem 21: 4485-93 (2013) Article DOI: 10.1016/j.bmc.2013.05.031 BindingDB Entry DOI: 10.7270/Q2P55PXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50093606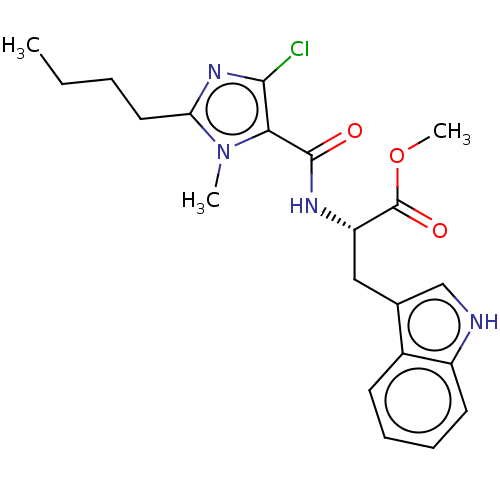 (CHEMBL3585794) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 531 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method | Bioorg Med Chem 23: 3526-33 (2015) Article DOI: 10.1016/j.bmc.2015.04.024 BindingDB Entry DOI: 10.7270/Q2DB83M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021416 (CHEMBL3289921) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 607 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as inhibition of hippuryl-histidyl-leucine substrate hydrolysis pretreated for 10 mins followed by addition of... | Eur J Med Chem 83: 344-54 (2014) Article DOI: 10.1016/j.ejmech.2014.06.035 BindingDB Entry DOI: 10.7270/Q2CZ38R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50093607 (CHEMBL3585792) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 647 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method | Bioorg Med Chem 23: 3526-33 (2015) Article DOI: 10.1016/j.bmc.2015.04.024 BindingDB Entry DOI: 10.7270/Q2DB83M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50093601 (CHEMBL3585802) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 657 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method | Bioorg Med Chem 23: 3526-33 (2015) Article DOI: 10.1016/j.bmc.2015.04.024 BindingDB Entry DOI: 10.7270/Q2DB83M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50438381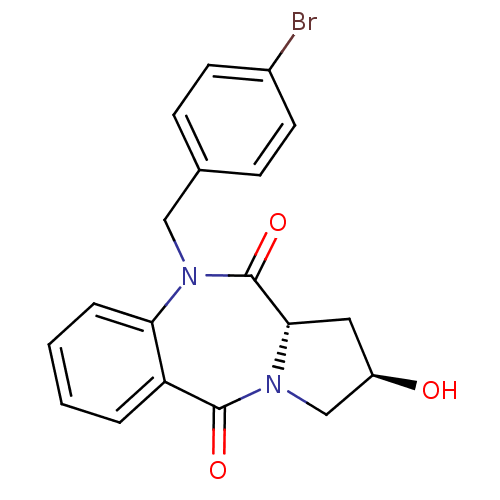 (CHEMBL2413597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 658 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins | Bioorg Med Chem 21: 4485-93 (2013) Article DOI: 10.1016/j.bmc.2013.05.031 BindingDB Entry DOI: 10.7270/Q2P55PXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021415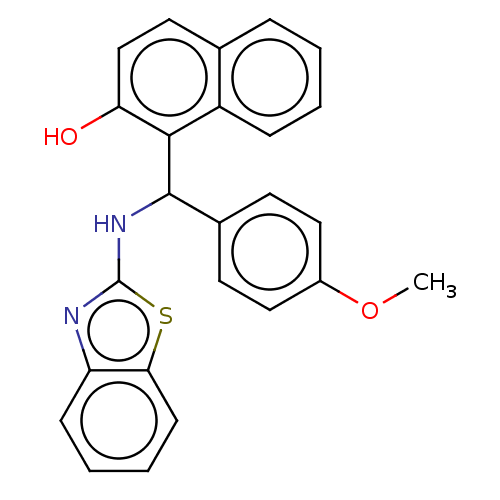 (CHEMBL3289900) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 837 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as inhibition of hippuryl-histidyl-leucine substrate hydrolysis pretreated for 10 mins followed by addition of... | Eur J Med Chem 83: 344-54 (2014) Article DOI: 10.1016/j.ejmech.2014.06.035 BindingDB Entry DOI: 10.7270/Q2CZ38R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50093604 (CHEMBL3585799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method | Bioorg Med Chem 23: 3526-33 (2015) Article DOI: 10.1016/j.bmc.2015.04.024 BindingDB Entry DOI: 10.7270/Q2DB83M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50042949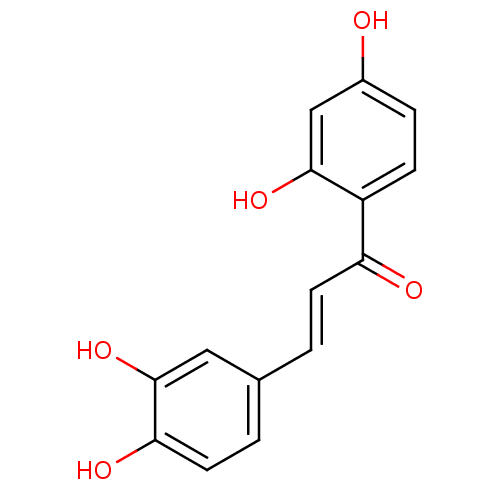 ((E)-1-(2,4-dihydroxyphenyl)-3-(3,4-dihydroxyphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as inhibition of hippuryl-histidyl-leucine substrate hydrolysis pretreated for 10 mins followed by addition of... | Eur J Med Chem 83: 344-54 (2014) Article DOI: 10.1016/j.ejmech.2014.06.035 BindingDB Entry DOI: 10.7270/Q2CZ38R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||