 Found 2081 hits with Last Name = 'james' and Initial = 'i'
Found 2081 hits with Last Name = 'james' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19770
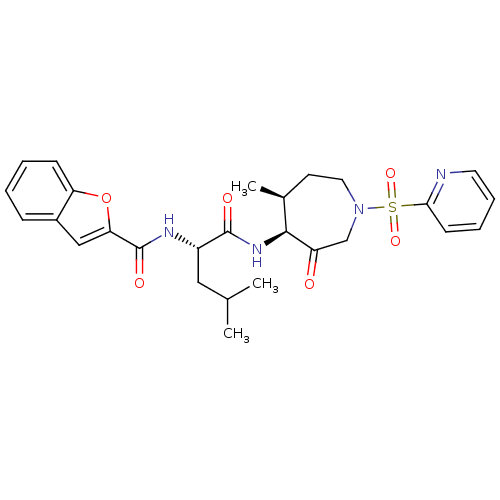
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1[C@@H](C)CCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-20(29-27(34)23-15-19-8-4-5-9-22(19)37-23)26(33)30-25-18(3)11-13-31(16-21(25)32)38(35,36)24-10-6-7-12-28-24/h4-10,12,15,17-18,20,25H,11,13-14,16H2,1-3H3,(H,29,34)(H,30,33)/t18-,20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Procathepsin L
(Homo sapiens (Human)) | BDBM19770

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1[C@@H](C)CCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-20(29-27(34)23-15-19-8-4-5-9-22(19)37-23)26(33)30-25-18(3)11-13-31(16-21(25)32)38(35,36)24-10-6-7-12-28-24/h4-10,12,15,17-18,20,25H,11,13-14,16H2,1-3H3,(H,29,34)(H,30,33)/t18-,20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0410 | -58.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin L2
(Homo sapiens (Human)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0680 | -57.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50366620
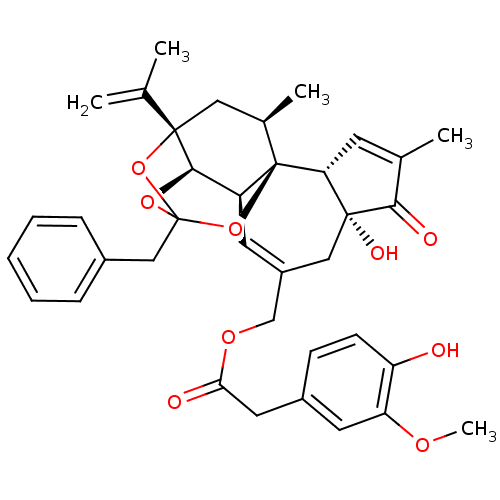
(RESINIFERATOXIN)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. |
J Med Chem 39: 2939-52 (1996)
Article DOI: 10.1021/jm960139d
BindingDB Entry DOI: 10.7270/Q2CV4JD5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50366620

(RESINIFERATOXIN)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. |
J Med Chem 39: 2939-52 (1996)
Article DOI: 10.1021/jm960139d
BindingDB Entry DOI: 10.7270/Q2CV4JD5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19775
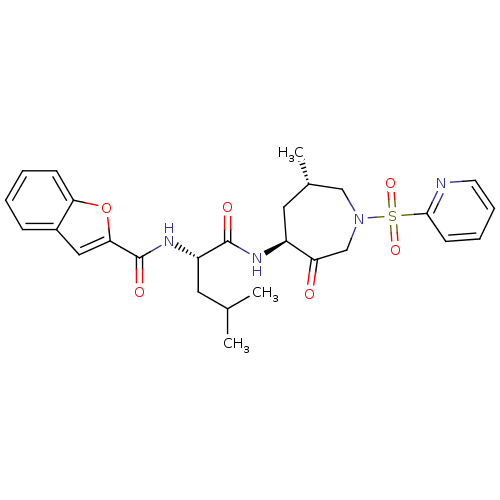
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1C[C@H](C)CN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)12-21(30-27(34)24-14-19-8-4-5-9-23(19)37-24)26(33)29-20-13-18(3)15-31(16-22(20)32)38(35,36)25-10-6-7-11-28-25/h4-11,14,17-18,20-21H,12-13,15-16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50052327

(6-hydroxy-15-isopropenyl-4,13,17-trimethyl-5-oxo-(...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)c2ccccc2)ccc1O |t:10,27,THB:35:15:12:17.19.18,16:15:12:17.19.18| Show InChI InChI=1S/C36H38O9/c1-20(2)34-17-22(4)35-26(32(34)43-36(44-34,45-35)25-9-7-6-8-10-25)14-24(18-33(40)29(35)13-21(3)31(33)39)19-42-30(38)16-23-11-12-27(37)28(15-23)41-5/h6-15,22,26,29,32,37,40H,1,16-19H2,2-5H3/t22-,26+,29-,32?,33-,34+,35?,36?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. |
J Med Chem 39: 2939-52 (1996)
Article DOI: 10.1021/jm960139d
BindingDB Entry DOI: 10.7270/Q2CV4JD5 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50084650
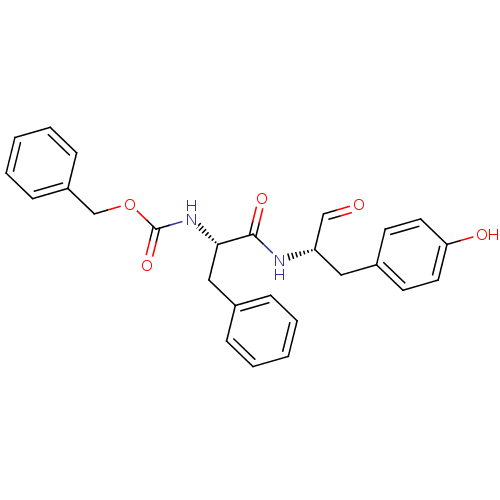
(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002402
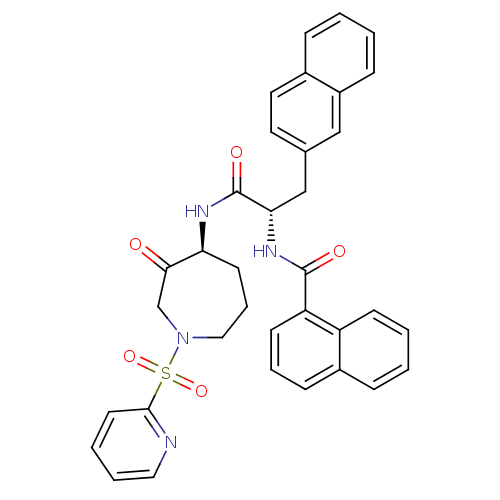
(CHEMBL194643)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cccc2ccccc12 Show InChI InChI=1S/C35H32N4O5S/c40-32-23-39(45(43,44)33-16-5-6-19-36-33)20-8-15-30(32)37-35(42)31(22-24-17-18-25-9-1-2-11-27(25)21-24)38-34(41)29-14-7-12-26-10-3-4-13-28(26)29/h1-7,9-14,16-19,21,30-31H,8,15,20,22-23H2,(H,37,42)(H,38,41)/t30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50084650

(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50002399
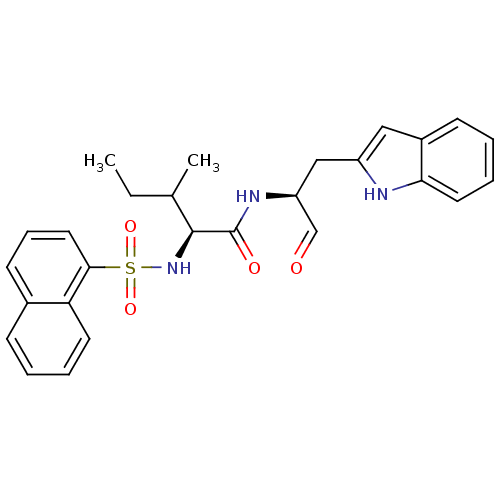
(CHEMBL197958)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1cc2ccccc2[nH]1)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-11-19-9-4-6-12-23(19)25)27(32)29-22(17-31)16-21-15-20-10-5-7-13-24(20)28-21/h4-15,17-18,22,26,28,30H,3,16H2,1-2H3,(H,29,32)/t18?,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002374
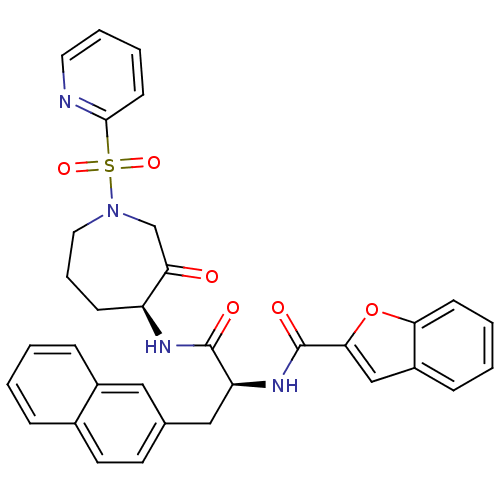
(CHEMBL194861)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C33H30N4O6S/c38-28-21-37(44(41,42)31-13-5-6-16-34-31)17-7-11-26(28)35-32(39)27(19-22-14-15-23-8-1-2-9-24(23)18-22)36-33(40)30-20-25-10-3-4-12-29(25)43-30/h1-6,8-10,12-16,18,20,26-27H,7,11,17,19,21H2,(H,35,39)(H,36,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288192
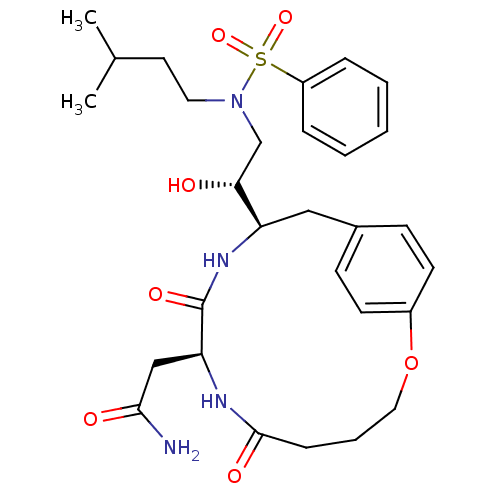
(CHEMBL83186 | N-[2-Hydroxy-2-((S)-8-isopropyl-6,9-...)Show SMILES CC(C)CCN(C[C@@H](O)[C@H]1Cc2ccc(OCCCC(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C29H40N4O7S/c1-20(2)14-15-33(41(38,39)23-7-4-3-5-8-23)19-26(34)24-17-21-10-12-22(13-11-21)40-16-6-9-28(36)31-25(18-27(30)35)29(37)32-24/h3-5,7-8,10-13,20,24-26,34H,6,9,14-19H2,1-2H3,(H2,30,35)(H,31,36)(H,32,37)/t24-,25+,26-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 6: 2531-2536 (1996)
Article DOI: 10.1016/0960-894X(96)00468-4
BindingDB Entry DOI: 10.7270/Q2S1830C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19775

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1C[C@H](C)CN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)12-21(30-27(34)24-14-19-8-4-5-9-23(19)37-24)26(33)29-20-13-18(3)15-31(16-22(20)32)38(35,36)25-10-6-7-11-28-25/h4-11,14,17-18,20-21H,12-13,15-16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | -52.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50084650

(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002399

(CHEMBL197958)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1cc2ccccc2[nH]1)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-11-19-9-4-6-12-23(19)25)27(32)29-22(17-31)16-21-15-20-10-5-7-13-24(20)28-21/h4-15,17-18,22,26,28,30H,3,16H2,1-2H3,(H,29,32)/t18?,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50002366
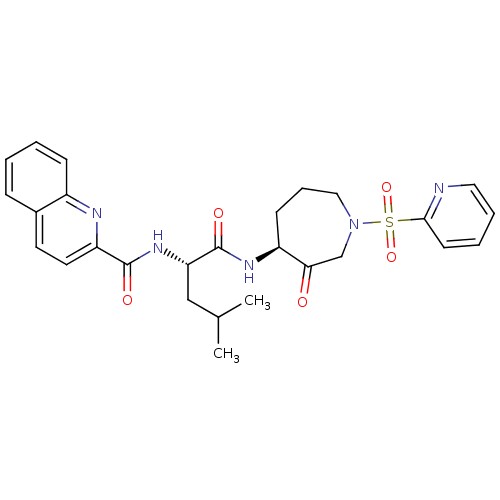
(CHEMBL433985)Show SMILES CC(C)C[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C27H31N5O5S/c1-18(2)16-23(31-26(34)22-13-12-19-8-3-4-9-20(19)29-22)27(35)30-21-10-7-15-32(17-24(21)33)38(36,37)25-11-5-6-14-28-25/h3-6,8-9,11-14,18,21,23H,7,10,15-17H2,1-2H3,(H,30,35)(H,31,34)/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083761
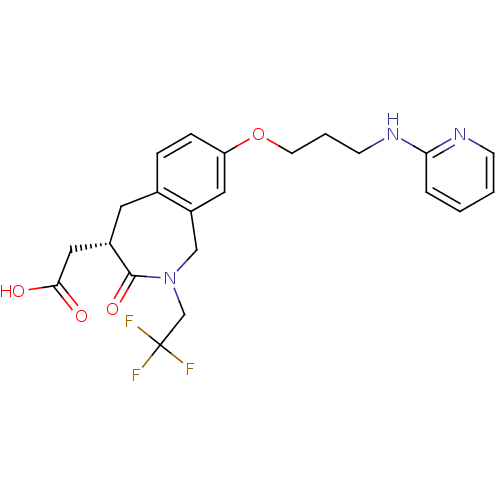
(CHEMBL87429 | [3-Oxo-8-[3-(pyridin-2-ylamino)-prop...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2CN(CC(F)(F)F)C1=O Show InChI InChI=1S/C22H24F3N3O4/c23-22(24,25)14-28-13-17-11-18(32-9-3-8-27-19-4-1-2-7-26-19)6-5-15(17)10-16(21(28)31)12-20(29)30/h1-2,4-7,11,16H,3,8-10,12-14H2,(H,26,27)(H,29,30)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50052320

(13-benzyl-6-hydroxy-15-isopropenyl-4,17-dimethyl-5...)Show SMILES COc1ccc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc1OC |t:11,36,THB:17:16:13:25.27.26,24:16:13:25.27.26| Show InChI InChI=1S/C38H42O9/c1-22(2)36-18-24(4)38-28(34(36)45-37(46-36,47-38)20-25-10-8-7-9-11-25)15-27(19-35(41)31(38)14-23(3)33(35)40)21-44-32(39)17-26-12-13-29(42-5)30(16-26)43-6/h7-16,24,28,31,34,41H,1,17-21H2,2-6H3/t24-,28+,31-,34?,35-,36+,37?,38?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. |
J Med Chem 39: 2939-52 (1996)
Article DOI: 10.1021/jm960139d
BindingDB Entry DOI: 10.7270/Q2CV4JD5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083763
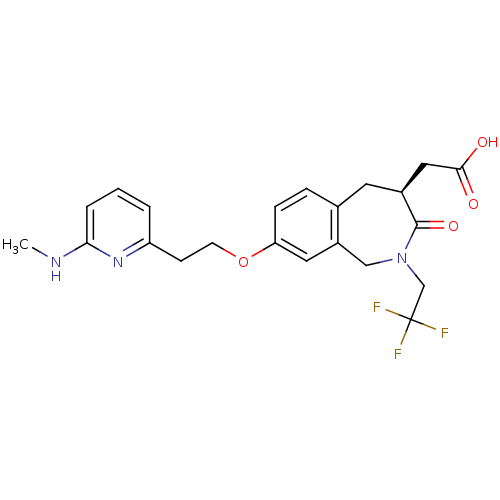
(CHEMBL86992 | [(S)-8-[2-(6-Methylamino-pyridin-2-y...)Show SMILES CNc1cccc(CCOc2ccc3C[C@@H](CC(O)=O)C(=O)N(CC(F)(F)F)Cc3c2)n1 Show InChI InChI=1S/C22H24F3N3O4/c1-26-19-4-2-3-17(27-19)7-8-32-18-6-5-14-9-15(11-20(29)30)21(31)28(12-16(14)10-18)13-22(23,24)25/h2-6,10,15H,7-9,11-13H2,1H3,(H,26,27)(H,29,30)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083764
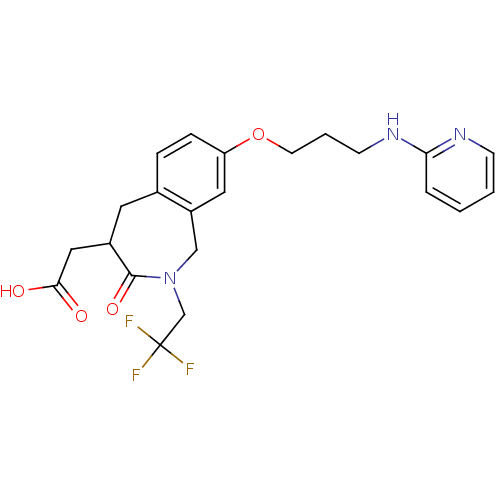
(CHEMBL421533 | [3-Oxo-8-[3-(pyridin-2-ylamino)-pro...)Show SMILES OC(=O)CC1Cc2ccc(OCCCNc3ccccn3)cc2CN(CC(F)(F)F)C1=O Show InChI InChI=1S/C22H24F3N3O4/c23-22(24,25)14-28-13-17-11-18(32-9-3-8-27-19-4-1-2-7-26-19)6-5-15(17)10-16(21(28)31)12-20(29)30/h1-2,4-7,11,16H,3,8-10,12-14H2,(H,26,27)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50078714
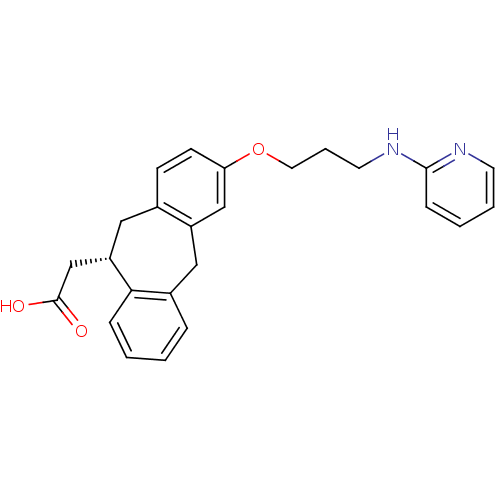
(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for non-peptide Vitronectin receptor (alpha V beta 3) |
Bioorg Med Chem Lett 9: 1807-12 (1999)
BindingDB Entry DOI: 10.7270/Q2Q23ZFC |
More data for this
Ligand-Target Pair | |
Cathepsin K
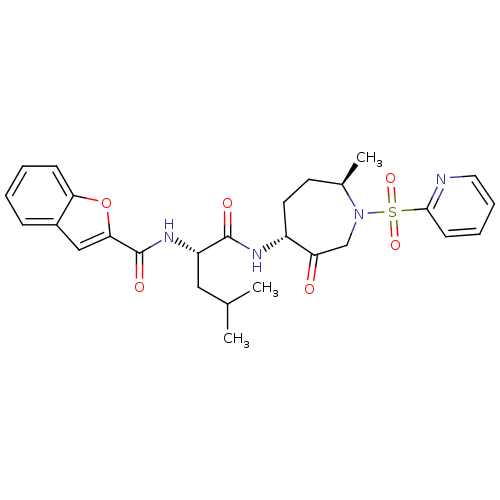
(Homo sapiens (Human)) | BDBM19780
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002400
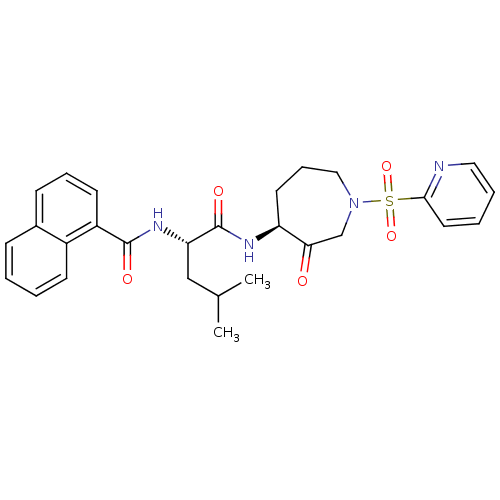
(CHEMBL382286)Show SMILES CC(C)C[C@H](NC(=O)c1cccc2ccccc12)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C28H32N4O5S/c1-19(2)17-24(31-27(34)22-12-7-10-20-9-3-4-11-21(20)22)28(35)30-23-13-8-16-32(18-25(23)33)38(36,37)26-14-5-6-15-29-26/h3-7,9-12,14-15,19,23-24H,8,13,16-18H2,1-2H3,(H,30,35)(H,31,34)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19771
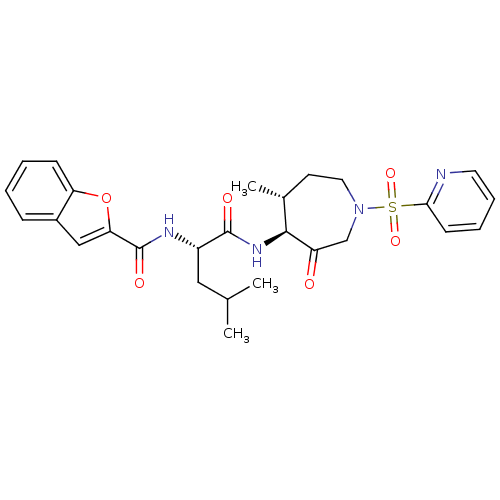
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1[C@H](C)CCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-20(29-27(34)23-15-19-8-4-5-9-22(19)37-23)26(33)30-25-18(3)11-13-31(16-21(25)32)38(35,36)24-10-6-7-12-28-24/h4-10,12,15,17-18,20,25H,11,13-14,16H2,1-3H3,(H,29,34)(H,30,33)/t18-,20+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | -49.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin S
(Homo sapiens (Human)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098580
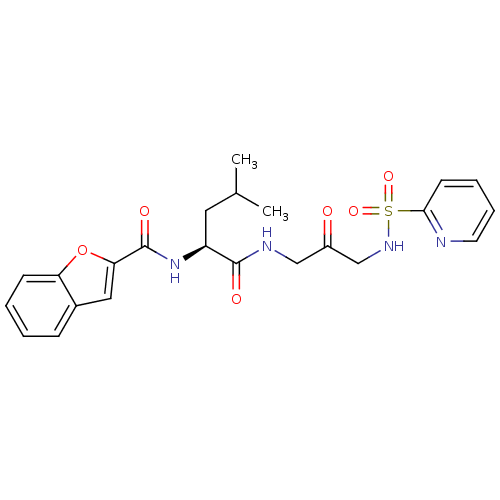
(Benzofuran-2-carboxylic acid {3-methyl-1-[2-oxo-3-...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)NCC(=O)CNS(=O)(=O)c1ccccn1 Show InChI InChI=1S/C23H26N4O6S/c1-15(2)11-18(27-23(30)20-12-16-7-3-4-8-19(16)33-20)22(29)25-13-17(28)14-26-34(31,32)21-9-5-6-10-24-21/h3-10,12,15,18,26H,11,13-14H2,1-2H3,(H,25,29)(H,27,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002367
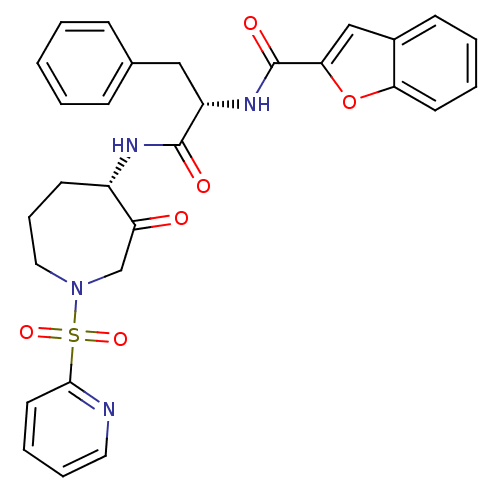
(CHEMBL407633)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccccc1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C29H28N4O6S/c34-24-19-33(40(37,38)27-14-6-7-15-30-27)16-8-12-22(24)31-28(35)23(17-20-9-2-1-3-10-20)32-29(36)26-18-21-11-4-5-13-25(21)39-26/h1-7,9-11,13-15,18,22-23H,8,12,16-17,19H2,(H,31,35)(H,32,36)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083762
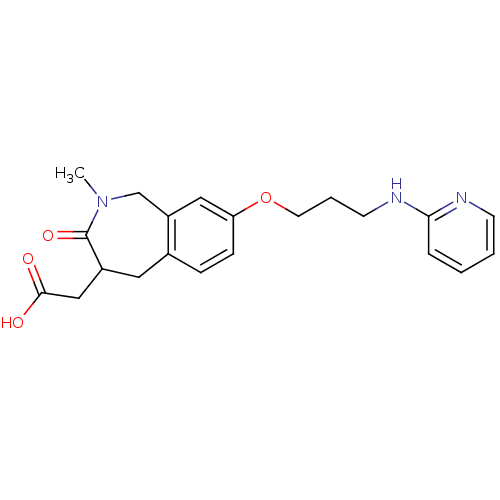
(CHEMBL314022 | {2-Methyl-3-oxo-8-[3-(pyridin-2-yla...)Show InChI InChI=1S/C21H25N3O4/c1-24-14-17-12-18(28-10-4-9-23-19-5-2-3-8-22-19)7-6-15(17)11-16(21(24)27)13-20(25)26/h2-3,5-8,12,16H,4,9-11,13-14H2,1H3,(H,22,23)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50059133
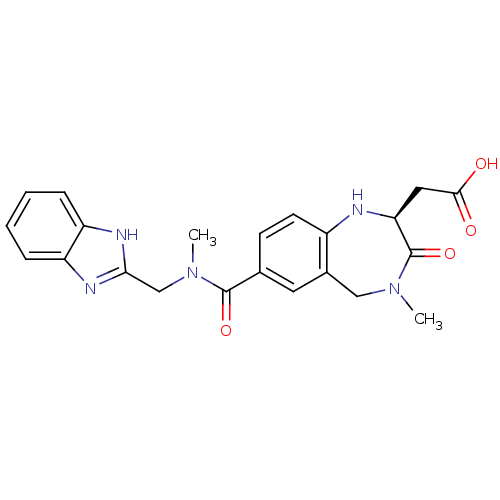
(CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...)Show SMILES CN(Cc1nc2ccccc2[nH]1)C(=O)c1ccc2N[C@@H](CC(O)=O)C(=O)N(C)Cc2c1 Show InChI InChI=1S/C22H23N5O4/c1-26-11-14-9-13(7-8-15(14)23-18(22(26)31)10-20(28)29)21(30)27(2)12-19-24-16-5-3-4-6-17(16)25-19/h3-9,18,23H,10-12H2,1-2H3,(H,24,25)(H,28,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098584
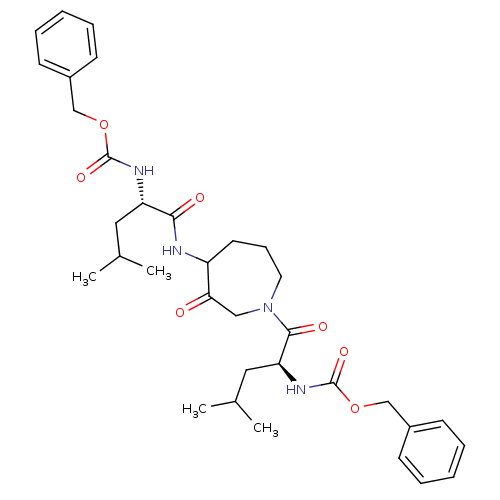
(CHEMBL31947 | {1-[4-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27?,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50002399

(CHEMBL197958)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1cc2ccccc2[nH]1)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-11-19-9-4-6-12-23(19)25)27(32)29-22(17-31)16-21-15-20-10-5-7-13-24(20)28-21/h4-15,17-18,22,26,28,30H,3,16H2,1-2H3,(H,29,32)/t18?,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50059133

(CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...)Show SMILES CN(Cc1nc2ccccc2[nH]1)C(=O)c1ccc2N[C@@H](CC(O)=O)C(=O)N(C)Cc2c1 Show InChI InChI=1S/C22H23N5O4/c1-26-11-14-9-13(7-8-15(14)23-18(22(26)31)10-20(28)29)21(30)27(2)12-19-24-16-5-3-4-6-17(16)25-19/h3-9,18,23H,10-12H2,1-2H3,(H,24,25)(H,28,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of HEK 293 cell adhesion to vitronectin by alpha V beta 3 |
Bioorg Med Chem Lett 9: 1807-12 (1999)
BindingDB Entry DOI: 10.7270/Q2Q23ZFC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.20 | -48.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098579
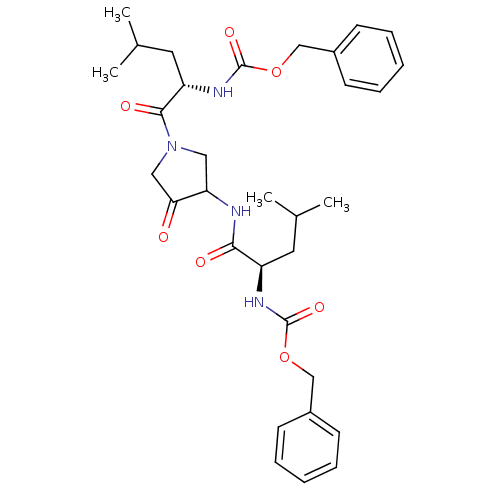
(CHEMBL29483 | {1-[3-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26+,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50126595
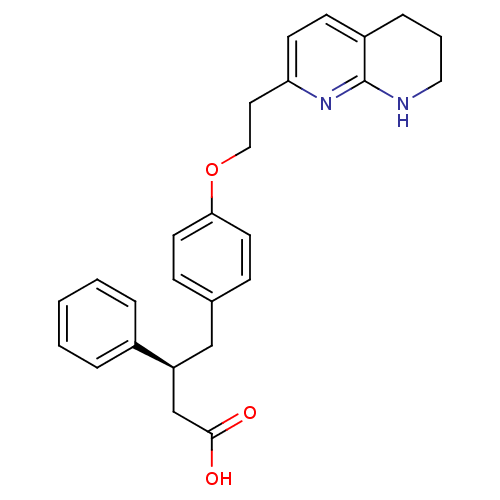
(3-Phenyl-4-{4-[2-(5,6,7,8-tetrahydro-[1,8]naphthyr...)Show SMILES OC(=O)C[C@H](Cc1ccc(OCCc2ccc3CCCNc3n2)cc1)c1ccccc1 Show InChI InChI=1S/C26H28N2O3/c29-25(30)18-22(20-5-2-1-3-6-20)17-19-8-12-24(13-9-19)31-16-14-23-11-10-21-7-4-15-27-26(21)28-23/h1-3,5-6,8-13,22H,4,7,14-18H2,(H,27,28)(H,29,30)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphav/beta 3 vitronectin receptor in HEK cells |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19779
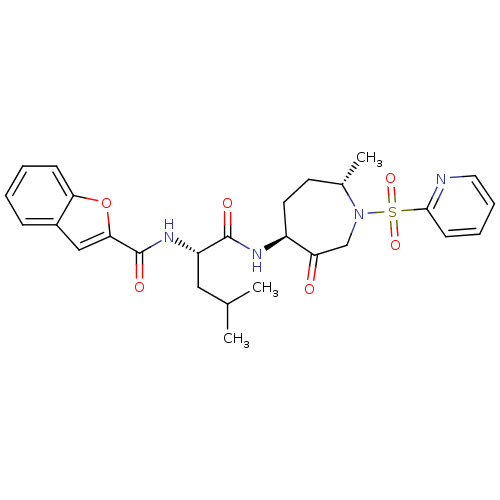
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | -48.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50126595

(3-Phenyl-4-{4-[2-(5,6,7,8-tetrahydro-[1,8]naphthyr...)Show SMILES OC(=O)C[C@H](Cc1ccc(OCCc2ccc3CCCNc3n2)cc1)c1ccccc1 Show InChI InChI=1S/C26H28N2O3/c29-25(30)18-22(20-5-2-1-3-6-20)17-19-8-12-24(13-9-19)31-16-14-23-11-10-21-7-4-15-27-26(21)28-23/h1-3,5-6,8-13,22H,4,7,14-18H2,(H,27,28)(H,29,30)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaV-beta5 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50078714

(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaV-beta3 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098575
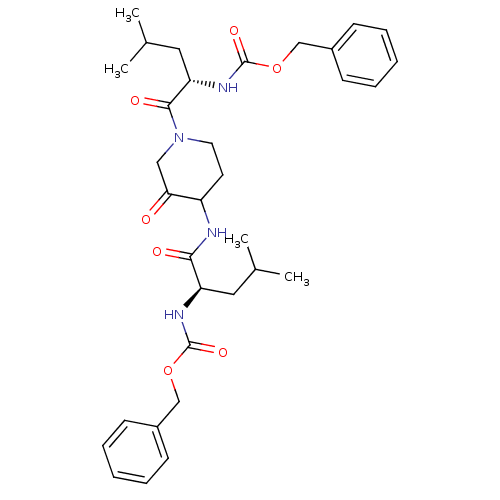
(CHEMBL281086 | {1-[4-(2-Benzyloxycarbonylamino-4-m...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data