 Found 3904 hits with Last Name = 'james' and Initial = 'j'
Found 3904 hits with Last Name = 'james' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50393247
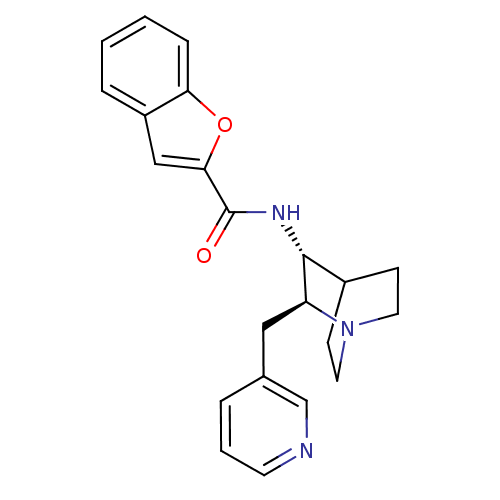
(CHEMBL1258006)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1cc2ccccc2o1 |r,wU:3.2,wD:10.12,TLB:11:10:6.5:8.9,THB:2:3:6.5:8.9,(5.71,-17.16,;5.73,-15.62,;4.4,-14.83,;3.06,-15.59,;1.59,-14.95,;.24,-15.55,;-.04,-16.95,;1.33,-16.32,;1.07,-14.41,;1.52,-13.3,;2.87,-16.97,;3.63,-18.31,;2.84,-19.64,;1.3,-19.62,;.52,-20.94,;1.28,-22.29,;2.83,-22.3,;3.61,-20.97,;7.07,-14.87,;7.24,-13.35,;8.76,-13.04,;9.54,-11.72,;11.07,-11.74,;11.83,-13.08,;11.04,-14.41,;9.51,-14.39,;8.47,-15.52,)| Show InChI InChI=1S/C22H23N3O2/c26-22(20-13-17-5-1-2-6-19(17)27-20)24-21-16-7-10-25(11-8-16)18(21)12-15-4-3-9-23-14-15/h1-6,9,13-14,16,18,21H,7-8,10-12H2,(H,24,26)/t18-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllycaconitine form alpha7 nAchR in rat hippocampal membranes |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399780
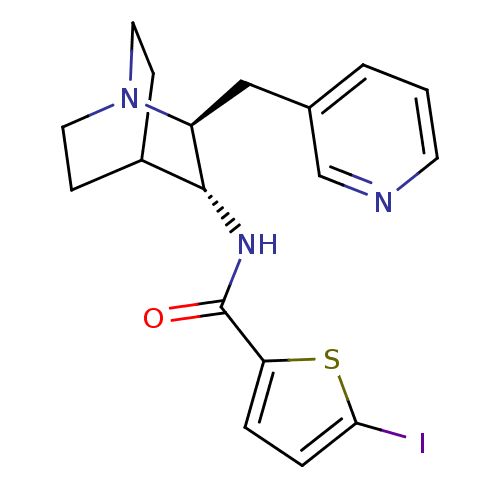
(CHEMBL2179874)Show SMILES Ic1ccc(s1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:9.9,wD:16.19,(34.89,-18.91,;33.35,-18.9,;32.45,-17.65,;30.99,-18.12,;30.98,-19.66,;32.44,-20.14,;29.83,-20.69,;29.83,-22.23,;28.5,-19.92,;27.16,-20.68,;25.83,-19.9,;24.51,-20.68,;24.51,-22.22,;25.83,-22.98,;25.05,-21.65,;26.43,-21.15,;27.16,-22.22,;28.5,-22.99,;28.49,-24.53,;27.16,-25.3,;27.15,-26.84,;28.49,-27.61,;29.83,-26.83,;29.82,-25.3,)| Show InChI InChI=1S/C18H20IN3OS/c19-16-4-3-15(24-16)18(23)21-17-13-5-8-22(9-6-13)14(17)10-12-2-1-7-20-11-12/h1-4,7,11,13-14,17H,5-6,8-10H2,(H,21,23)/t14-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399779
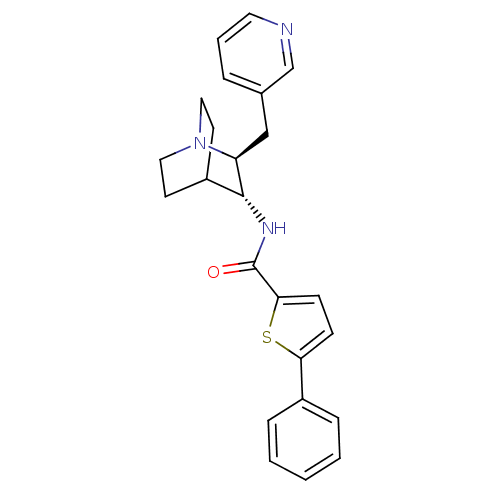
(CHEMBL2179875)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1ccc(s1)-c1ccccc1 |r,wU:3.2,wD:10.12,(40.04,-22.15,;40.04,-20.61,;38.71,-19.84,;37.37,-20.6,;36.04,-19.83,;34.72,-20.6,;34.72,-22.14,;36.04,-22.91,;35.26,-21.57,;36.64,-21.07,;37.37,-22.14,;38.71,-22.92,;38.7,-24.46,;37.37,-25.22,;37.36,-26.76,;38.7,-27.53,;40.04,-26.76,;40.03,-25.22,;41.19,-19.58,;41.2,-18.04,;42.66,-17.58,;43.56,-18.82,;42.65,-20.06,;45.1,-18.83,;45.86,-20.17,;47.4,-20.18,;48.18,-18.85,;47.4,-17.51,;45.87,-17.51,)| Show InChI InChI=1S/C24H25N3OS/c28-24(22-9-8-21(29-22)18-6-2-1-3-7-18)26-23-19-10-13-27(14-11-19)20(23)15-17-5-4-12-25-16-17/h1-9,12,16,19-20,23H,10-11,13-15H2,(H,26,28)/t20-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399777
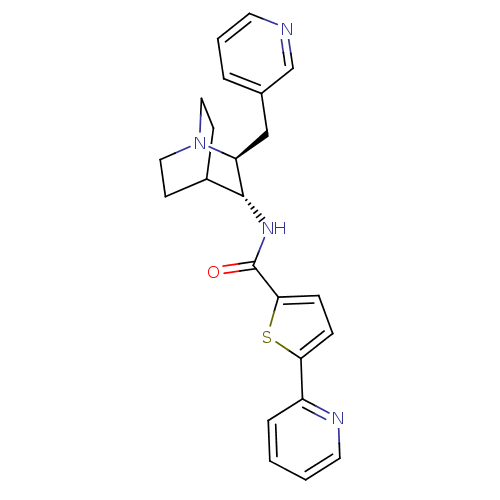
(CHEMBL2179877)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1ccc(s1)-c1ccccn1 |r,wU:3.2,wD:10.12,(17.82,-36.07,;17.82,-34.53,;16.49,-33.76,;15.15,-34.53,;13.82,-33.75,;12.49,-34.53,;12.49,-36.07,;13.82,-36.83,;13.04,-35.49,;14.42,-34.99,;15.15,-36.07,;16.48,-36.84,;16.48,-38.38,;15.15,-39.14,;15.14,-40.68,;16.48,-41.46,;17.82,-40.68,;17.81,-39.14,;18.97,-33.5,;18.98,-31.97,;20.44,-31.5,;21.34,-32.75,;20.43,-33.98,;22.88,-32.76,;23.65,-31.43,;25.18,-31.43,;25.95,-32.77,;25.18,-34.1,;23.64,-34.1,)| Show InChI InChI=1S/C23H24N4OS/c28-23(21-7-6-20(29-21)18-5-1-2-11-25-18)26-22-17-8-12-27(13-9-17)19(22)14-16-4-3-10-24-15-16/h1-7,10-11,15,17,19,22H,8-9,12-14H2,(H,26,28)/t19-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393247

(CHEMBL1258006)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1cc2ccccc2o1 |r,wU:3.2,wD:10.12,TLB:11:10:6.5:8.9,THB:2:3:6.5:8.9,(5.71,-17.16,;5.73,-15.62,;4.4,-14.83,;3.06,-15.59,;1.59,-14.95,;.24,-15.55,;-.04,-16.95,;1.33,-16.32,;1.07,-14.41,;1.52,-13.3,;2.87,-16.97,;3.63,-18.31,;2.84,-19.64,;1.3,-19.62,;.52,-20.94,;1.28,-22.29,;2.83,-22.3,;3.61,-20.97,;7.07,-14.87,;7.24,-13.35,;8.76,-13.04,;9.54,-11.72,;11.07,-11.74,;11.83,-13.08,;11.04,-14.41,;9.51,-14.39,;8.47,-15.52,)| Show InChI InChI=1S/C22H23N3O2/c26-22(20-13-17-5-1-2-6-19(17)27-20)24-21-16-7-10-25(11-8-16)18(21)12-15-4-3-9-23-14-15/h1-6,9,13-14,16,18,21H,7-8,10-12H2,(H,24,26)/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399781
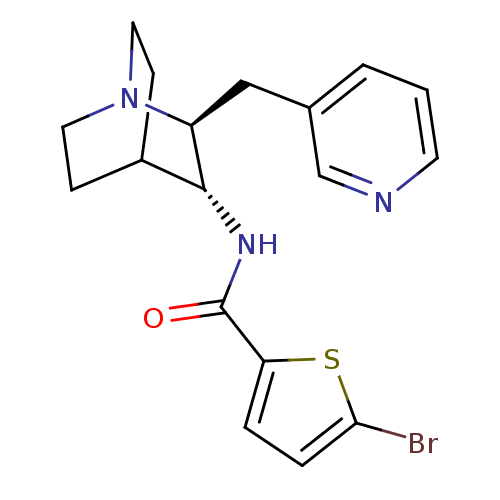
(CHEMBL2179873)Show SMILES Brc1ccc(s1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:9.9,wD:16.19,(21.88,-19.63,;20.34,-19.62,;19.44,-18.37,;17.98,-18.84,;17.97,-20.37,;19.43,-20.86,;16.82,-21.41,;16.81,-22.95,;15.48,-20.63,;14.15,-21.4,;12.82,-20.62,;11.49,-21.4,;11.49,-22.94,;12.82,-23.7,;12.03,-22.36,;13.41,-21.86,;14.15,-22.94,;15.48,-23.71,;15.48,-25.25,;14.14,-26.01,;14.14,-27.55,;15.47,-28.33,;16.81,-27.55,;16.81,-26.01,)| Show InChI InChI=1S/C18H20BrN3OS/c19-16-4-3-15(24-16)18(23)21-17-13-5-8-22(9-6-13)14(17)10-12-2-1-7-20-11-12/h1-4,7,11,13-14,17H,5-6,8-10H2,(H,21,23)/t14-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399776
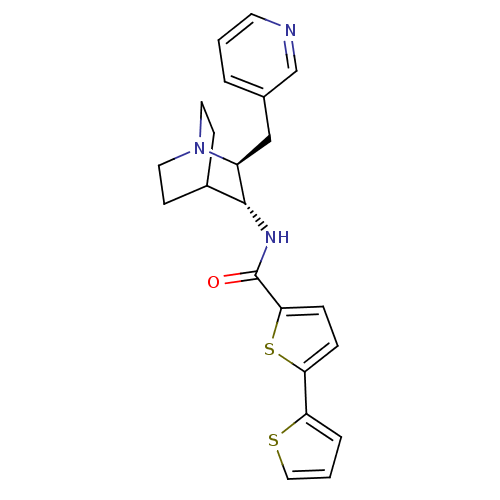
(CHEMBL2179878)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1ccc(s1)-c1cccs1 |r,wU:3.2,wD:10.12,(34.31,-36.29,;34.32,-34.75,;32.99,-33.98,;31.65,-34.74,;30.32,-33.97,;28.99,-34.74,;28.99,-36.28,;30.32,-37.05,;29.54,-35.71,;30.91,-35.21,;31.65,-36.28,;32.98,-37.06,;32.98,-38.6,;31.64,-39.36,;31.64,-40.9,;32.97,-41.67,;34.31,-40.9,;34.31,-39.36,;35.47,-33.72,;35.48,-32.19,;36.94,-31.72,;37.84,-32.96,;36.93,-34.2,;39.38,-32.97,;40.29,-31.73,;41.75,-32.21,;41.74,-33.75,;40.27,-34.22,)| Show InChI InChI=1S/C22H23N3OS2/c26-22(20-6-5-19(28-20)18-4-2-12-27-18)24-21-16-7-10-25(11-8-16)17(21)13-15-3-1-9-23-14-15/h1-6,9,12,14,16-17,21H,7-8,10-11,13H2,(H,24,26)/t17-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399767
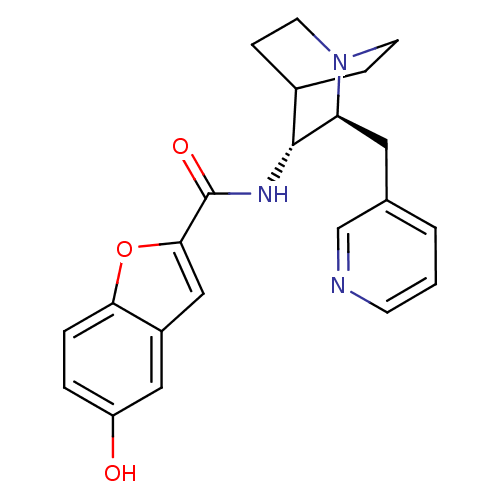
(CHEMBL2180253)Show SMILES Oc1ccc2oc(cc2c1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:13.14,wD:20.24,(39.86,-14.21,;39.23,-15.62,;40.13,-16.87,;39.49,-18.26,;37.97,-18.41,;37.07,-19.65,;35.61,-19.17,;35.61,-17.63,;37.08,-17.16,;37.71,-15.77,;34.45,-20.2,;34.45,-21.74,;33.12,-19.43,;31.78,-20.19,;30.45,-19.41,;29.12,-20.19,;29.12,-21.74,;30.45,-22.5,;29.66,-21.16,;31.04,-20.66,;31.78,-21.74,;33.12,-22.51,;33.11,-24.05,;31.77,-24.82,;31.77,-26.36,;33.11,-27.13,;34.45,-26.36,;34.45,-24.82,)| Show InChI InChI=1S/C22H23N3O3/c26-17-3-4-19-16(11-17)12-20(28-19)22(27)24-21-15-5-8-25(9-6-15)18(21)10-14-2-1-7-23-13-14/h1-4,7,11-13,15,18,21,26H,5-6,8-10H2,(H,24,27)/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine human alpha4beta2 nAChR in SH-EP1 cell membranes |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399773

(CHEMBL2179881)Show SMILES COc1ccc2oc(cc2c1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:14.15,wD:21.25,(40.87,-44.06,;39.33,-44.22,;38.7,-45.63,;39.6,-46.88,;38.97,-48.27,;37.44,-48.42,;36.54,-49.66,;35.08,-49.18,;35.09,-47.64,;36.55,-47.17,;37.18,-45.78,;33.93,-50.21,;33.92,-51.75,;32.59,-49.44,;31.25,-50.2,;29.92,-49.42,;28.59,-50.2,;28.59,-51.75,;29.92,-52.51,;29.14,-51.17,;30.52,-50.67,;31.25,-51.75,;32.59,-52.52,;32.59,-54.06,;31.25,-54.83,;31.24,-56.37,;32.58,-57.15,;33.92,-56.37,;33.92,-54.83,)| Show InChI InChI=1S/C23H25N3O3/c1-28-18-4-5-20-17(12-18)13-21(29-20)23(27)25-22-16-6-9-26(10-7-16)19(22)11-15-3-2-8-24-14-15/h2-5,8,12-14,16,19,22H,6-7,9-11H2,1H3,(H,25,27)/t19-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399770

(CHEMBL2180250)Show SMILES Cc1c(oc2ccccc12)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:13.14,wD:20.24,(33.38,-1.89,;34.63,-2.8,;34.62,-4.34,;36.08,-4.82,;36.98,-3.58,;38.5,-3.43,;39.14,-2.04,;38.24,-.79,;36.72,-.94,;36.09,-2.33,;33.47,-5.37,;33.46,-6.91,;32.13,-4.6,;30.79,-5.36,;29.46,-4.59,;28.13,-5.36,;28.13,-6.91,;29.46,-7.67,;28.68,-6.33,;30.06,-5.83,;30.79,-6.91,;32.13,-7.68,;32.13,-9.22,;30.79,-9.99,;30.79,-11.53,;32.12,-12.3,;33.46,-11.53,;33.46,-9.99,)| Show InChI InChI=1S/C23H25N3O2/c1-15-18-6-2-3-7-20(18)28-22(15)23(27)25-21-17-8-11-26(12-9-17)19(21)13-16-5-4-10-24-14-16/h2-7,10,14,17,19,21H,8-9,11-13H2,1H3,(H,25,27)/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399792
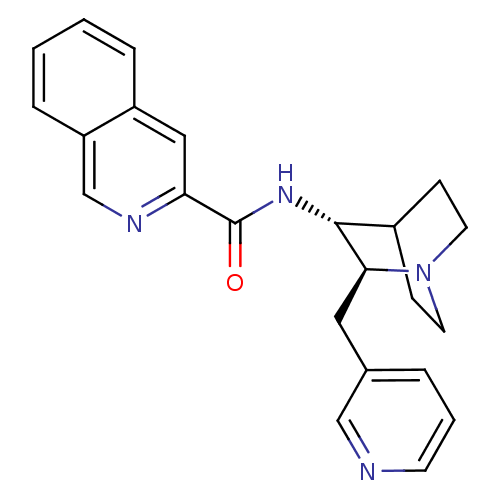
(CHEMBL2179862)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1cc2ccccc2cn1 |r,wU:3.2,wD:10.12,(41.77,-32.17,;41.78,-30.63,;40.44,-29.86,;39.11,-30.62,;37.78,-29.84,;36.45,-30.62,;36.45,-32.16,;37.78,-32.92,;36.99,-31.59,;38.37,-31.09,;39.11,-32.16,;40.44,-32.93,;40.44,-34.47,;39.1,-35.24,;39.1,-36.78,;40.43,-37.55,;41.77,-36.78,;41.77,-35.24,;43.11,-29.86,;43.11,-28.33,;44.44,-27.56,;44.44,-26.03,;45.77,-25.26,;47.11,-26.03,;47.1,-27.57,;45.77,-28.34,;45.77,-29.88,;44.44,-30.64,)| Show InChI InChI=1S/C23H24N4O/c28-23(20-13-18-5-1-2-6-19(18)15-25-20)26-22-17-7-10-27(11-8-17)21(22)12-16-4-3-9-24-14-16/h1-6,9,13-15,17,21-22H,7-8,10-12H2,(H,26,28)/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50079456
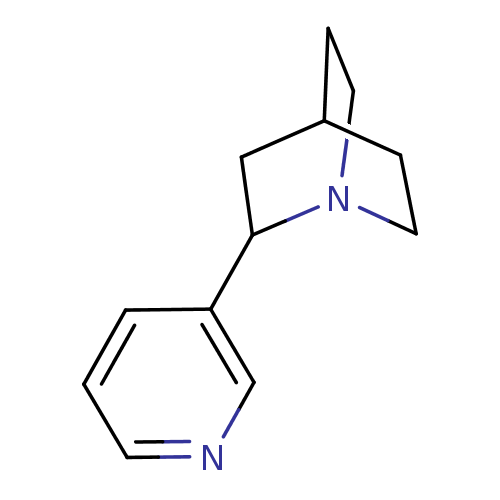
(2-Pyridin-3-yl-1-aza-bicyclo[2.2.2]octane | CHEMBL...)Show SMILES C1CN2CCC1CC2c1cccnc1 |(15.84,-6.24,;14.49,-7,;16.04,-8.15,;16.81,-6.8,;16.04,-5.47,;14.49,-5.47,;13.72,-6.8,;14.49,-8.15,;13.17,-8.93,;11.82,-8.15,;10.5,-8.95,;10.5,-10.47,;11.82,-11.27,;13.17,-10.47,)| Show InChI InChI=1S/C12H16N2/c1-2-11(9-13-5-1)12-8-10-3-6-14(12)7-4-10/h1-2,5,9-10,12H,3-4,6-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine human alpha4beta2 nAChR in SH-EP1 cell membranes |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393249
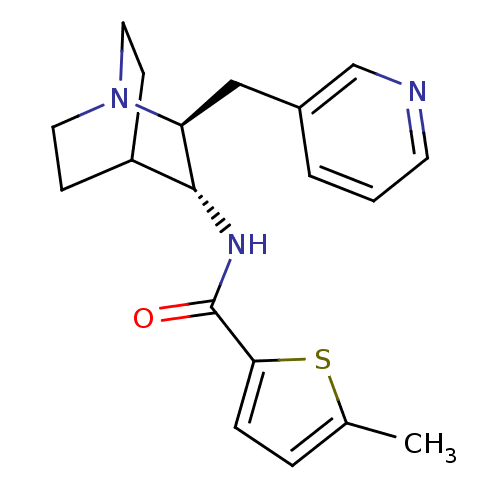
(CHEMBL2151441)Show SMILES Cc1ccc(s1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:9.9,wD:16.19,(5.74,.65,;4.2,.81,;3.44,2.15,;1.93,1.84,;1.77,.31,;3.17,-.33,;.43,-.46,;.43,-2,;-.9,.32,;-2.23,-.45,;-3.56,.33,;-4.89,-.44,;-4.89,-1.98,;-3.56,-2.75,;-4.3,-1.39,;-2.81,-1,;-2.23,-1.98,;-.9,-2.76,;-.9,-4.3,;-2.24,-5.06,;-2.24,-6.6,;-.91,-7.37,;.43,-6.6,;.43,-5.06,)| Show InChI InChI=1S/C19H23N3OS/c1-13-4-5-17(24-13)19(23)21-18-15-6-9-22(10-7-15)16(18)11-14-3-2-8-20-12-14/h2-5,8,12,15-16,18H,6-7,9-11H2,1H3,(H,21,23)/t16-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399775
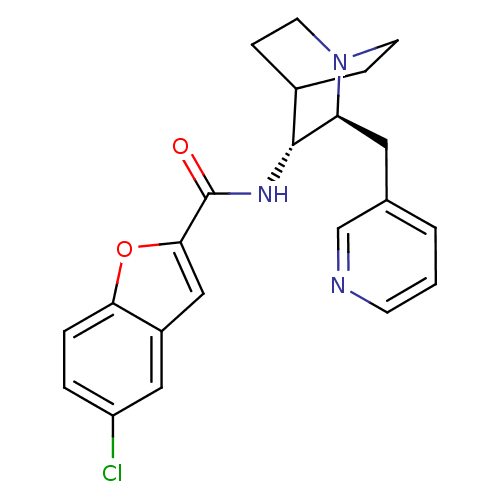
(CHEMBL2179879)Show SMILES Clc1ccc2oc(cc2c1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:13.14,wD:20.24,(12,-44.81,;11.37,-46.21,;12.27,-47.46,;11.63,-48.86,;10.11,-49.01,;9.21,-50.25,;7.75,-49.76,;7.75,-48.23,;9.22,-47.76,;9.85,-46.37,;6.59,-50.8,;6.59,-52.34,;5.26,-50.02,;3.92,-50.79,;2.59,-50.01,;1.26,-50.79,;1.26,-52.33,;2.59,-53.1,;1.8,-51.76,;3.18,-51.26,;3.92,-52.33,;5.25,-53.11,;5.25,-54.65,;3.91,-55.42,;3.91,-56.96,;5.25,-57.73,;6.59,-56.95,;6.58,-55.42,)| Show InChI InChI=1S/C22H22ClN3O2/c23-17-3-4-19-16(11-17)12-20(28-19)22(27)25-21-15-5-8-26(9-6-15)18(21)10-14-2-1-7-24-13-14/h1-4,7,11-13,15,18,21H,5-6,8-10H2,(H,25,27)/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399783
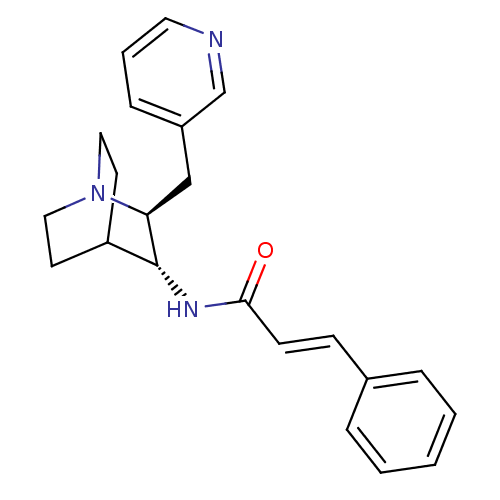
(CHEMBL2179871)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)\C=C\c1ccccc1 |r,wU:3.2,wD:10.12,(18.34,-5.97,;18.35,-4.43,;17.02,-3.65,;15.68,-4.42,;14.35,-3.64,;13.02,-4.42,;13.02,-5.96,;14.35,-6.72,;13.57,-5.38,;14.94,-4.88,;15.68,-5.96,;17.01,-6.73,;17.01,-8.27,;15.67,-9.04,;15.67,-10.57,;17.01,-11.35,;18.34,-10.57,;18.34,-9.03,;19.68,-3.66,;21.02,-4.43,;22.35,-3.67,;23.68,-4.45,;25.01,-3.68,;25.02,-2.14,;23.68,-1.37,;22.35,-2.14,)| Show InChI InChI=1S/C22H25N3O/c26-21(9-8-17-5-2-1-3-6-17)24-22-19-10-13-25(14-11-19)20(22)15-18-7-4-12-23-16-18/h1-9,12,16,19-20,22H,10-11,13-15H2,(H,24,26)/b9-8+/t20-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399782
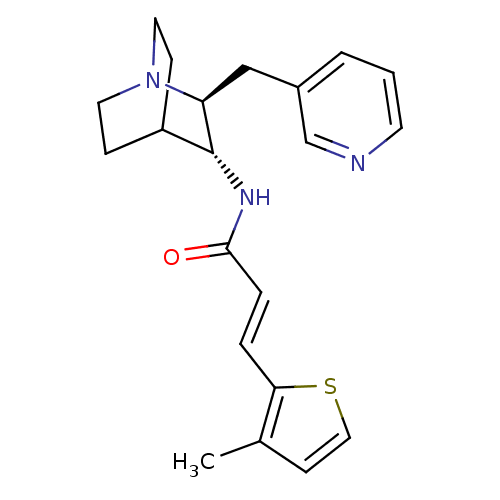
(CHEMBL2179872)Show SMILES Cc1ccsc1\C=C\C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:11.11,wD:18.21,(35.12,-1.47,;36.37,-2.37,;37.83,-1.89,;38.74,-3.13,;37.84,-4.38,;36.38,-3.91,;35.04,-4.67,;33.71,-3.9,;32.37,-4.67,;32.37,-6.21,;31.04,-3.89,;29.7,-4.66,;28.38,-3.88,;27.05,-4.66,;27.05,-6.2,;28.38,-6.96,;27.59,-5.62,;28.97,-5.13,;29.7,-6.2,;31.04,-6.97,;31.04,-8.51,;29.7,-9.28,;29.7,-10.81,;31.03,-11.59,;32.37,-10.81,;32.36,-9.28,)| Show InChI InChI=1S/C21H25N3OS/c1-15-8-12-26-19(15)4-5-20(25)23-21-17-6-10-24(11-7-17)18(21)13-16-3-2-9-22-14-16/h2-5,8-9,12,14,17-18,21H,6-7,10-11,13H2,1H3,(H,23,25)/b5-4+/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399766
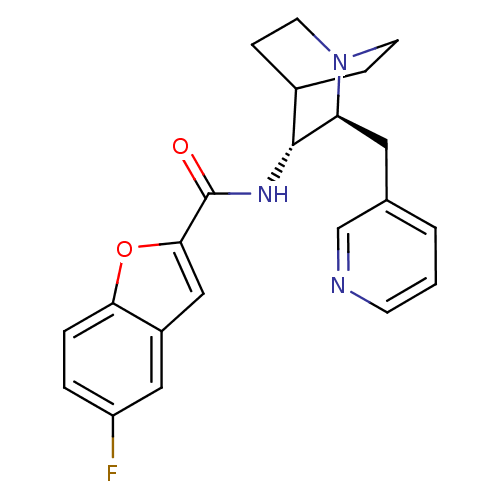
(CHEMBL2180254)Show SMILES Fc1ccc2oc(cc2c1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:13.14,wD:20.24,(11.82,-24.93,;11.19,-26.33,;12.09,-27.58,;11.45,-28.98,;9.93,-29.13,;9.02,-30.37,;7.56,-29.88,;7.57,-28.35,;9.04,-27.88,;9.67,-26.49,;6.41,-30.92,;6.41,-32.46,;5.08,-30.14,;3.74,-30.91,;2.41,-30.13,;1.08,-30.91,;1.08,-32.45,;2.41,-33.21,;1.62,-31.88,;3,-31.38,;3.74,-32.45,;5.07,-33.22,;5.07,-34.77,;3.73,-35.53,;3.73,-37.07,;5.07,-37.85,;6.41,-37.07,;6.4,-35.53,)| Show InChI InChI=1S/C22H22FN3O2/c23-17-3-4-19-16(11-17)12-20(28-19)22(27)25-21-15-5-8-26(9-6-15)18(21)10-14-2-1-7-24-13-14/h1-4,7,11-13,15,18,21H,5-6,8-10H2,(H,25,27)/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50164924
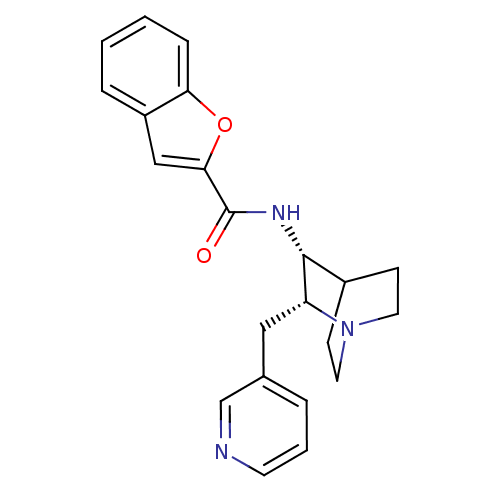
(Benzofuran-2-carboxylic acid ((2R,3R)-2-pyridin-3-...)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@@H]1Cc1cccnc1)c1cc2ccccc2o1 |wU:3.2,10.12,(5.23,-1.38,;5.23,.16,;3.9,.93,;2.55,.16,;1.22,.93,;.45,-.4,;1.78,-1.17,;1.22,-2.15,;-.09,-1.38,;-.09,.16,;2.55,-1.38,;3.9,-2.15,;3.88,-3.69,;2.55,-4.46,;2.55,-6,;3.88,-6.77,;5.23,-6,;5.21,-4.46,;6.56,.93,;8.03,.44,;8.94,1.7,;10.46,1.86,;11.08,3.27,;10.17,4.52,;8.64,4.36,;8.03,2.94,;6.56,2.47,)| Show InChI InChI=1S/C22H23N3O2/c26-22(20-13-17-5-1-2-6-19(17)27-20)24-21-16-7-10-25(11-8-16)18(21)12-15-4-3-9-23-14-15/h1-6,9,13-14,16,18,21H,7-8,10-12H2,(H,24,26)/t18-,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399769

(CHEMBL2180251)Show SMILES Cc1ccc2oc(cc2c1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:13.14,wD:20.24,(11.59,-12.88,;10.96,-14.29,;11.86,-15.54,;11.22,-16.93,;9.7,-17.08,;8.8,-18.32,;7.34,-17.84,;7.34,-16.3,;8.81,-15.83,;9.44,-14.44,;6.18,-18.87,;6.18,-20.41,;4.85,-18.09,;3.51,-18.86,;2.18,-18.08,;.85,-18.86,;.85,-20.4,;2.18,-21.17,;1.4,-19.83,;2.77,-19.33,;3.51,-20.4,;4.85,-21.18,;4.84,-22.72,;3.51,-23.48,;3.5,-25.03,;4.84,-25.8,;6.18,-25.02,;6.18,-23.48,)| Show InChI InChI=1S/C23H25N3O2/c1-15-4-5-20-18(11-15)13-21(28-20)23(27)25-22-17-6-9-26(10-7-17)19(22)12-16-3-2-8-24-14-16/h2-5,8,11,13-14,17,19,22H,6-7,9-10,12H2,1H3,(H,25,27)/t19-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399768

(CHEMBL2180252)Show SMILES Cc1ccc2cc(oc2c1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:13.14,wD:20.24,(27.77,-15.84,;26.23,-15.99,;25.33,-14.75,;23.81,-14.9,;23.19,-16.29,;21.72,-16.76,;21.71,-18.3,;23.17,-18.78,;24.08,-17.54,;25.6,-17.39,;20.56,-19.33,;20.56,-20.87,;19.23,-18.55,;17.89,-19.32,;16.56,-18.54,;15.23,-19.32,;15.23,-20.86,;16.56,-21.63,;15.77,-20.29,;17.15,-19.79,;17.89,-20.86,;19.22,-21.64,;19.22,-23.18,;17.88,-23.94,;17.88,-25.48,;19.22,-26.26,;20.56,-25.48,;20.55,-23.94,)| Show InChI InChI=1S/C23H25N3O2/c1-15-4-5-18-13-21(28-20(18)11-15)23(27)25-22-17-6-9-26(10-7-17)19(22)12-16-3-2-8-24-14-16/h2-5,8,11,13-14,17,19,22H,6-7,9-10,12H2,1H3,(H,25,27)/t19-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399786
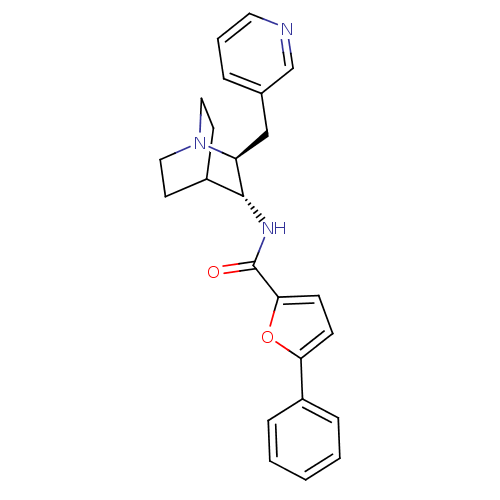
(CHEMBL2179868)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1ccc(o1)-c1ccccc1 |r,wU:3.2,wD:10.12,(22.82,-56.93,;22.83,-55.39,;21.5,-54.61,;20.16,-55.38,;18.83,-54.6,;17.5,-55.38,;17.5,-56.92,;18.83,-57.68,;18.05,-56.34,;19.42,-55.85,;20.16,-56.92,;21.49,-57.69,;21.49,-59.23,;20.15,-60,;20.15,-61.54,;21.49,-62.31,;22.82,-61.53,;22.82,-60,;24.16,-54.62,;24.17,-53.09,;25.62,-52.62,;26.52,-53.86,;25.62,-55.1,;28.05,-53.86,;28.82,-55.2,;30.36,-55.2,;31.13,-53.86,;30.35,-52.53,;28.82,-52.53,)| Show InChI InChI=1S/C24H25N3O2/c28-24(22-9-8-21(29-22)18-6-2-1-3-7-18)26-23-19-10-13-27(14-11-19)20(23)15-17-5-4-12-25-16-17/h1-9,12,16,19-20,23H,10-11,13-15H2,(H,26,28)/t20-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399772
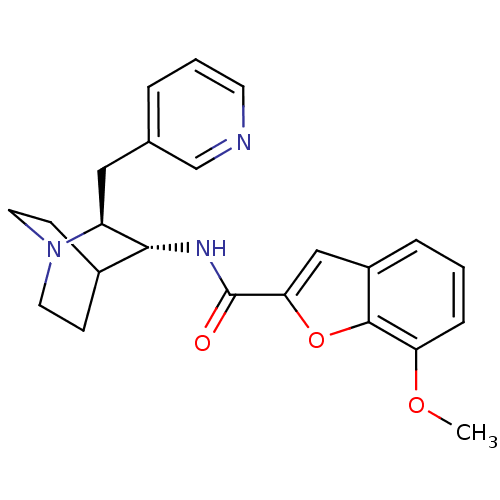
(CHEMBL2179882)Show SMILES COc1cccc2cc(oc12)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:14.15,wD:21.25,(11.42,-6.5,;12.06,-5.09,;11.16,-3.84,;11.8,-2.44,;10.9,-1.19,;9.38,-1.35,;8.75,-2.74,;7.28,-3.21,;7.28,-4.75,;8.74,-5.23,;9.64,-3.99,;6.12,-5.78,;6.12,-7.32,;4.79,-5,;3.45,-5.77,;2.12,-4.99,;.79,-5.77,;.79,-7.31,;2.12,-8.08,;1.33,-6.74,;2.71,-6.24,;3.45,-7.31,;4.79,-8.09,;4.78,-9.63,;3.44,-10.4,;3.44,-11.94,;4.78,-12.71,;6.12,-11.93,;6.12,-10.4,)| Show InChI InChI=1S/C23H25N3O3/c1-28-19-6-2-5-17-13-20(29-22(17)19)23(27)25-21-16-7-10-26(11-8-16)18(21)12-15-4-3-9-24-14-15/h2-6,9,13-14,16,18,21H,7-8,10-12H2,1H3,(H,25,27)/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399789
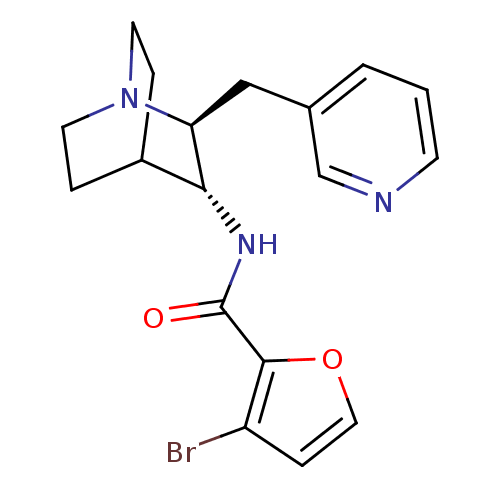
(CHEMBL2179865)Show SMILES Brc1ccoc1C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:9.9,wD:16.19,(30.28,-39.8,;31.52,-40.71,;32.98,-40.24,;33.88,-41.48,;32.98,-42.71,;31.52,-42.24,;30.19,-43.01,;30.18,-44.55,;28.86,-42.23,;27.52,-43,;26.19,-42.22,;24.86,-43,;24.86,-44.54,;26.19,-45.3,;25.41,-43.96,;26.78,-43.46,;27.52,-44.54,;28.85,-45.31,;28.85,-46.85,;27.51,-47.61,;27.51,-49.15,;28.84,-49.93,;30.18,-49.15,;30.18,-47.61,)| Show InChI InChI=1S/C18H20BrN3O2/c19-14-5-9-24-17(14)18(23)21-16-13-3-7-22(8-4-13)15(16)10-12-2-1-6-20-11-12/h1-2,5-6,9,11,13,15-16H,3-4,7-8,10H2,(H,21,23)/t15-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393248
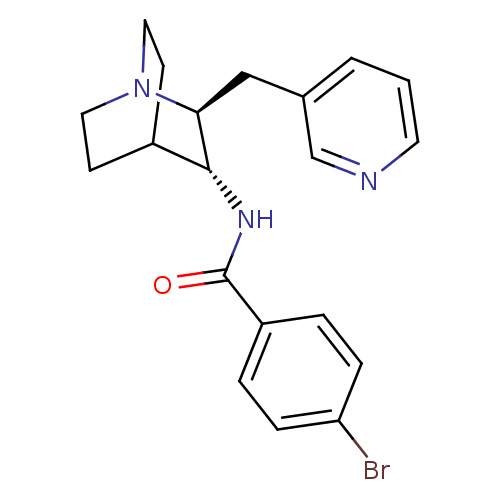
(CHEMBL1258005)Show SMILES Brc1ccc(cc1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:10.10,wD:17.20,TLB:18:17:13.12:15.16,THB:9:10:13.12:15.16,(-.69,-12.68,;-2.04,-13.43,;-2.06,-14.97,;-3.4,-15.72,;-4.72,-14.94,;-4.71,-13.4,;-3.37,-12.64,;-6.06,-15.69,;-6.08,-17.23,;-7.38,-14.9,;-8.73,-15.65,;-10.2,-15.01,;-11.56,-15.62,;-11.84,-17.01,;-10.46,-16.38,;-10.71,-14.48,;-10.27,-13.37,;-8.92,-17.03,;-8.16,-18.37,;-8.94,-19.7,;-10.48,-19.68,;-11.28,-21.01,;-10.51,-22.35,;-8.96,-22.36,;-8.18,-21.03,)| Show InChI InChI=1S/C20H22BrN3O/c21-17-5-3-16(4-6-17)20(25)23-19-15-7-10-24(11-8-15)18(19)12-14-2-1-9-22-13-14/h1-6,9,13,15,18-19H,7-8,10-12H2,(H,23,25)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399810
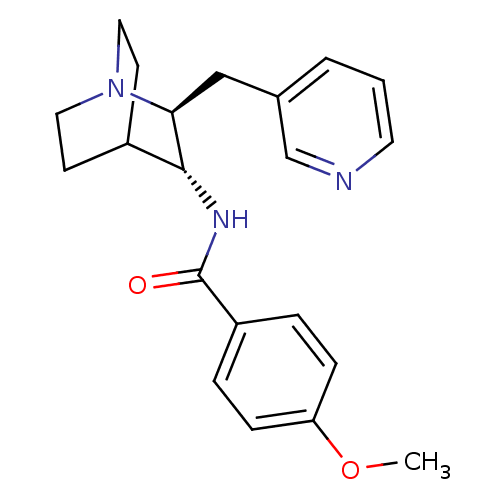
(CHEMBL2179844)Show SMILES COc1ccc(cc1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:11.11,wD:18.21,(23.91,-38.77,;22.58,-38,;21.25,-38.76,;21.24,-40.31,;19.9,-41.07,;18.58,-40.29,;18.57,-38.76,;19.9,-37.99,;17.24,-41.06,;17.24,-42.6,;15.91,-40.28,;14.58,-41.05,;13.25,-40.27,;11.92,-41.05,;11.92,-42.59,;13.25,-43.35,;12.46,-42.01,;13.84,-41.52,;14.58,-42.59,;15.91,-43.36,;15.91,-44.9,;14.57,-45.67,;14.57,-47.2,;15.9,-47.98,;17.24,-47.2,;17.24,-45.67,)| Show InChI InChI=1S/C21H25N3O2/c1-26-18-6-4-17(5-7-18)21(25)23-20-16-8-11-24(12-9-16)19(20)13-15-3-2-10-22-14-15/h2-7,10,14,16,19-20H,8-9,11-13H2,1H3,(H,23,25)/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393255
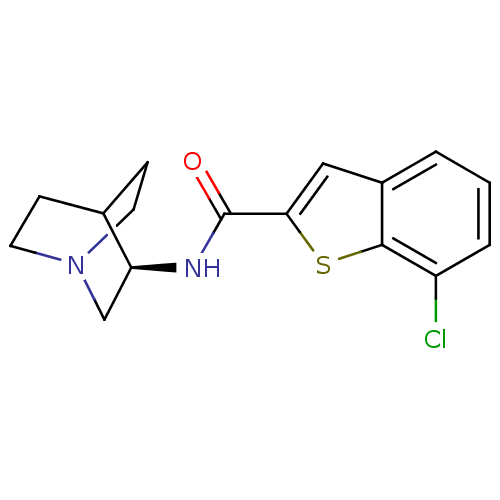
(CHEMBL2151572)Show SMILES Clc1cccc2cc(sc12)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:13.14,(9.9,-24.54,;9.14,-23.2,;9.91,-21.88,;9.15,-20.54,;7.62,-20.54,;6.85,-21.86,;5.34,-22.17,;5.18,-23.71,;6.58,-24.33,;7.61,-23.19,;3.84,-24.47,;3.84,-26.01,;2.51,-23.7,;1.17,-24.46,;1.17,-26,;-.16,-26.76,;-1.49,-26,;-1.49,-24.46,;-.16,-23.68,;.6,-25.01,;-.89,-25.41,)| Show InChI InChI=1S/C16H17ClN2OS/c17-12-3-1-2-11-8-14(21-15(11)12)16(20)18-13-9-19-6-4-10(13)5-7-19/h1-3,8,10,13H,4-7,9H2,(H,18,20)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 9.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026089
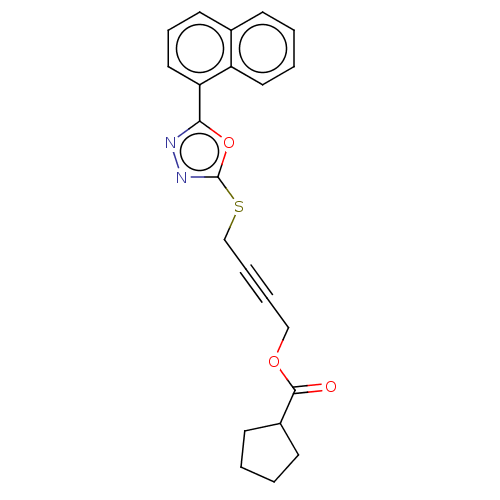
(CHEMBL1270505)Show SMILES O=C(OCC#CCSc1nnc(o1)-c1cccc2ccccc12)C1CCCC1 Show InChI InChI=1S/C22H20N2O3S/c25-21(17-9-1-2-10-17)26-14-5-6-15-28-22-24-23-20(27-22)19-13-7-11-16-8-3-4-12-18(16)19/h3-4,7-8,11-13,17H,1-2,9-10,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026085

(CHEMBL1269896)Show SMILES COc1ccc(cc1)C(=O)OCC#CCSc1nnc(o1)-c1cccc2ccccc12 Show InChI InChI=1S/C24H18N2O4S/c1-28-19-13-11-18(12-14-19)23(27)29-15-4-5-16-31-24-26-25-22(30-24)21-10-6-8-17-7-2-3-9-20(17)21/h2-3,6-14H,15-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026170

(CHEMBL1271429)Show SMILES Brc1ccc(cc1)C(=O)OCC#CCSc1nnc(o1)-c1cccc2ccccc12 Show InChI InChI=1S/C23H15BrN2O3S/c24-18-12-10-17(11-13-18)22(27)28-14-3-4-15-30-23-26-25-21(29-23)20-9-5-7-16-6-1-2-8-19(16)20/h1-2,5-13H,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026084

(CHEMBL1271012)Show SMILES Fc1ccccc1C(=O)OCC#CCSc1nnc(o1)-c1cccc2ccccc12 Show InChI InChI=1S/C23H15FN2O3S/c24-20-13-4-3-11-19(20)22(27)28-14-5-6-15-30-23-26-25-21(29-23)18-12-7-9-16-8-1-2-10-17(16)18/h1-4,7-13H,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026174

(CHEMBL1271117)Show SMILES Fc1ccc(cc1)C(=O)OCC#CCSc1nnc(o1)-c1cccc2ccccc12 Show InChI InChI=1S/C23H15FN2O3S/c24-18-12-10-17(11-13-18)22(27)28-14-3-4-15-30-23-26-25-21(29-23)20-9-5-7-16-6-1-2-8-19(16)20/h1-2,5-13H,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026078
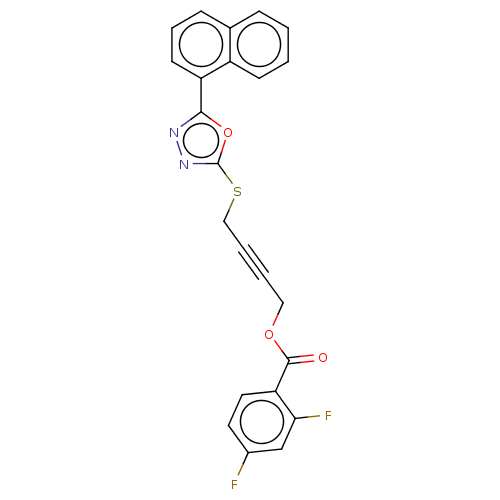
(CHEMBL1271219)Show SMILES Fc1ccc(C(=O)OCC#CCSc2nnc(o2)-c2cccc3ccccc23)c(F)c1 Show InChI InChI=1S/C23H14F2N2O3S/c24-16-10-11-19(20(25)14-16)22(28)29-12-3-4-13-31-23-27-26-21(30-23)18-9-5-7-15-6-1-2-8-17(15)18/h1-2,5-11,14H,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026083
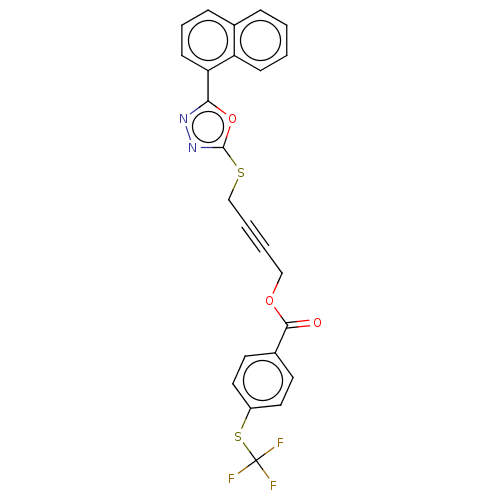
(CHEMBL1270004)Show SMILES FC(F)(F)Sc1ccc(cc1)C(=O)OCC#CCSc1nnc(o1)-c1cccc2ccccc12 Show InChI InChI=1S/C24H15F3N2O3S2/c25-24(26,27)34-18-12-10-17(11-13-18)22(30)31-14-3-4-15-33-23-29-28-21(32-23)20-9-5-7-16-6-1-2-8-19(16)20/h1-2,5-13H,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026079

(CHEMBL1270507)Show SMILES Fc1ccc(cc1)C(=O)OCC#CCSc1nnc(o1)-c1ccc(F)cc1 Show InChI InChI=1S/C19H12F2N2O3S/c20-15-7-3-13(4-8-15)17-22-23-19(26-17)27-12-2-1-11-25-18(24)14-5-9-16(21)10-6-14/h3-10H,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026080
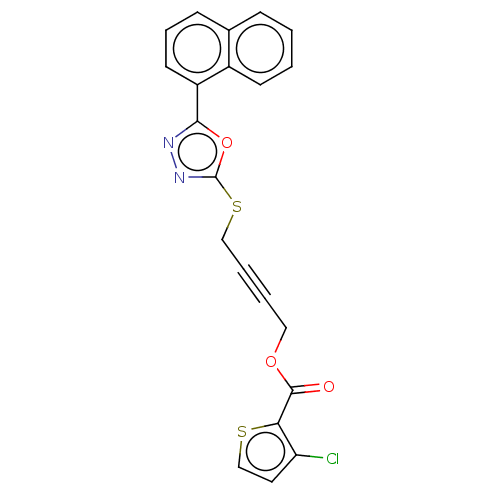
(CHEMBL1270311)Show SMILES Clc1ccsc1C(=O)OCC#CCSc1nnc(o1)-c1cccc2ccccc12 Show InChI InChI=1S/C21H13ClN2O3S2/c22-17-10-13-28-18(17)20(25)26-11-3-4-12-29-21-24-23-19(27-21)16-9-5-7-14-6-1-2-8-15(14)16/h1-2,5-10,13H,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026086
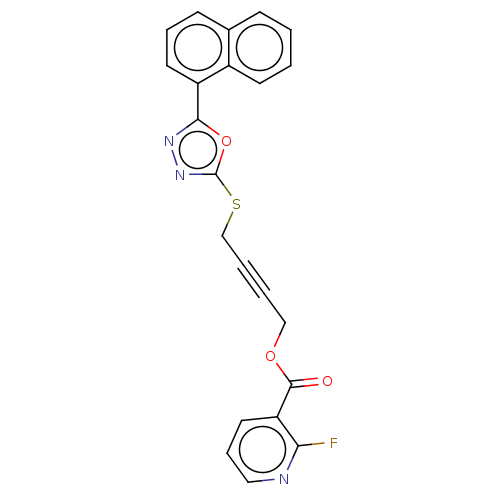
(CHEMBL1270606)Show SMILES Fc1ncccc1C(=O)OCC#CCSc1nnc(o1)-c1cccc2ccccc12 Show InChI InChI=1S/C22H14FN3O3S/c23-19-18(11-6-12-24-19)21(27)28-13-3-4-14-30-22-26-25-20(29-22)17-10-5-8-15-7-1-2-9-16(15)17/h1-2,5-12H,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026088

(CHEMBL1271322)Show SMILES Clc1ccc(cc1)C(=O)OCC#CCSc1nnc(o1)-c1cccc2ccccc12 Show InChI InChI=1S/C23H15ClN2O3S/c24-18-12-10-17(11-13-18)22(27)28-14-3-4-15-30-23-26-25-21(29-23)20-9-5-7-16-6-1-2-8-19(16)20/h1-2,5-13H,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50329322
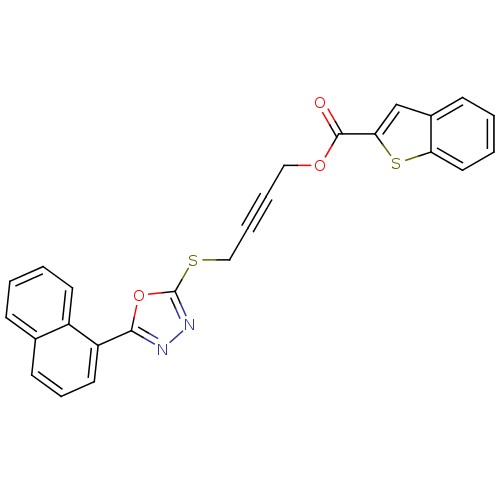
(4-(5-(Naphthalen-1-yl)-1,3,4-oxadiazol-2-ylthio)bu...)Show SMILES O=C(OCC#CCSc1nnc(o1)-c1cccc2ccccc12)c1cc2ccccc2s1 Show InChI InChI=1S/C25H16N2O3S2/c28-24(22-16-18-9-2-4-13-21(18)32-22)29-14-5-6-15-31-25-27-26-23(30-25)20-12-7-10-17-8-1-3-11-19(17)20/h1-4,7-13,16H,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50329323
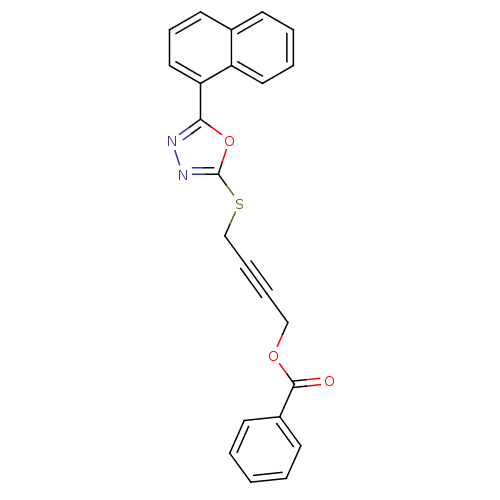
(4-(5-(Naphthalen-1-yl)-1,3,4-oxadiazol-2-ylthio)bu...)Show SMILES O=C(OCC#CCSc1nnc(o1)-c1cccc2ccccc12)c1ccccc1 Show InChI InChI=1S/C23H16N2O3S/c26-22(18-10-2-1-3-11-18)27-15-6-7-16-29-23-25-24-21(28-23)20-14-8-12-17-9-4-5-13-19(17)20/h1-5,8-14H,15-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026087
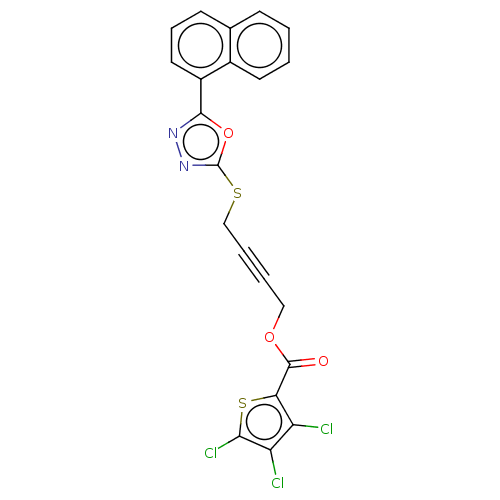
(CHEMBL1270218)Show SMILES Clc1sc(C(=O)OCC#CCSc2nnc(o2)-c2cccc3ccccc23)c(Cl)c1Cl Show InChI InChI=1S/C21H11Cl3N2O3S2/c22-15-16(23)18(24)31-17(15)20(27)28-10-3-4-11-30-21-26-25-19(29-21)14-9-5-7-12-6-1-2-8-13(12)14/h1-2,5-9H,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026082

(CHEMBL1270912)Show SMILES O=C(OCC#CCSc1nnc(o1)-c1cccc2ccccc12)c1csc2ccccc12 Show InChI InChI=1S/C25H16N2O3S2/c28-24(21-16-32-22-13-4-3-11-19(21)22)29-14-5-6-15-31-25-27-26-23(30-25)20-12-7-9-17-8-1-2-10-18(17)20/h1-4,7-13,16H,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026081

(CHEMBL1270112)Show SMILES FC(F)Oc1ccc(cc1)C(=O)OCC#CCSc1nnc(o1)-c1cccc2ccccc12 Show InChI InChI=1S/C24H16F2N2O4S/c25-23(26)31-18-12-10-17(11-13-18)22(29)30-14-3-4-15-33-24-28-27-21(32-24)20-9-5-7-16-6-1-2-8-19(16)20/h1-2,5-13,23H,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50026091
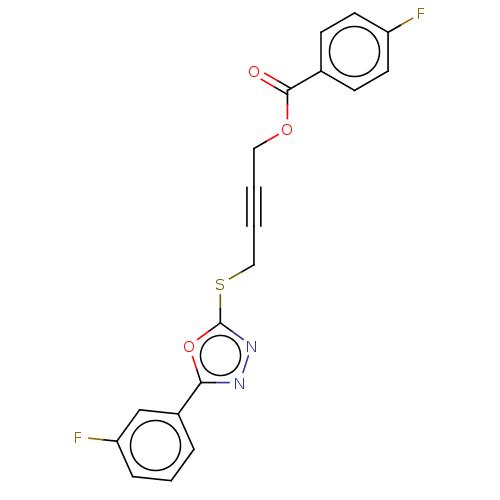
(CHEMBL1270506)Show SMILES Fc1ccc(cc1)C(=O)OCC#CCSc1nnc(o1)-c1cccc(F)c1 Show InChI InChI=1S/C19H12F2N2O3S/c20-15-8-6-13(7-9-15)18(24)25-10-1-2-11-27-19-23-22-17(26-19)14-4-3-5-16(21)12-14/h3-9,12H,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 after 15 mins by UV-vis spectrophotometer analysis |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399774
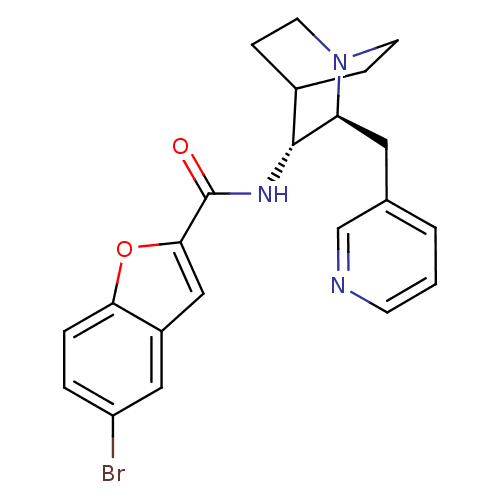
(CHEMBL2179880)Show SMILES Brc1ccc2oc(cc2c1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:13.14,wD:20.24,(24.79,-45.11,;24.16,-46.52,;25.06,-47.77,;24.43,-49.17,;22.9,-49.31,;22,-50.55,;20.54,-50.07,;20.55,-48.53,;22.01,-48.07,;22.64,-46.68,;19.39,-51.11,;19.38,-52.65,;18.05,-50.33,;16.71,-51.1,;15.38,-50.32,;14.05,-51.1,;14.05,-52.64,;15.38,-53.4,;14.6,-52.06,;15.98,-51.57,;16.71,-52.64,;18.05,-53.41,;18.05,-54.96,;16.71,-55.72,;16.7,-57.26,;18.04,-58.04,;19.38,-57.26,;19.38,-55.72,)| Show InChI InChI=1S/C22H22BrN3O2/c23-17-3-4-19-16(11-17)12-20(28-19)22(27)25-21-15-5-8-26(9-6-15)18(21)10-14-2-1-7-24-13-14/h1-4,7,11-13,15,18,21H,5-6,8-10H2,(H,25,27)/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399762

(CHEMBL2180258)Show SMILES Cc1c(oc2ccc(Cl)cc12)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:14.15,wD:21.25,(19.47,-38.89,;20.72,-39.8,;20.71,-41.34,;22.17,-41.82,;23.07,-40.58,;24.59,-40.43,;25.23,-39.04,;24.33,-37.79,;24.96,-36.39,;22.81,-37.95,;22.18,-39.34,;19.56,-42.37,;19.55,-43.91,;18.22,-41.6,;16.89,-42.36,;15.56,-41.59,;14.22,-42.36,;14.22,-43.91,;15.56,-44.67,;14.77,-43.33,;16.15,-42.83,;16.89,-43.91,;18.22,-44.68,;18.22,-46.22,;16.88,-46.99,;16.88,-48.53,;18.21,-49.3,;19.55,-48.52,;19.55,-46.99,)| Show InChI InChI=1S/C23H24ClN3O2/c1-14-18-12-17(24)4-5-20(18)29-22(14)23(28)26-21-16-6-9-27(10-7-16)19(21)11-15-3-2-8-25-13-15/h2-5,8,12-13,16,19,21H,6-7,9-11H2,1H3,(H,26,28)/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399765
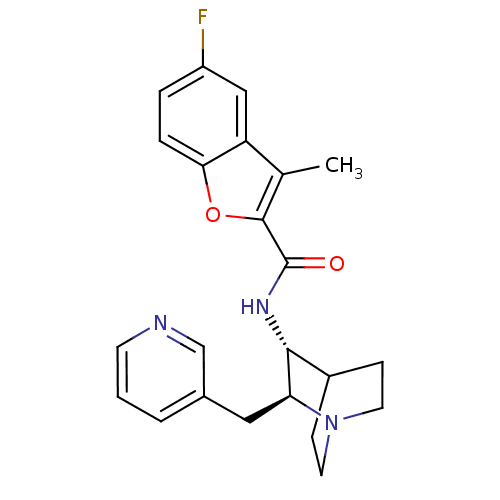
(CHEMBL2180255)Show SMILES Cc1c(oc2ccc(F)cc12)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:14.15,wD:21.25,(19.29,-27.28,;20.54,-28.19,;20.53,-29.72,;21.99,-30.21,;22.89,-28.97,;24.41,-28.82,;25.05,-27.42,;24.15,-26.18,;24.78,-24.77,;22.63,-26.33,;22,-27.72,;19.38,-30.76,;19.37,-32.3,;18.04,-29.98,;16.71,-30.75,;15.38,-29.97,;14.05,-30.75,;14.05,-32.29,;15.38,-33.05,;14.59,-31.72,;15.97,-31.22,;16.71,-32.29,;18.04,-33.06,;18.04,-34.61,;16.7,-35.37,;16.7,-36.91,;18.03,-37.69,;19.37,-36.91,;19.37,-35.37,)| Show InChI InChI=1S/C23H24FN3O2/c1-14-18-12-17(24)4-5-20(18)29-22(14)23(28)26-21-16-6-9-27(10-7-16)19(21)11-15-3-2-8-25-13-15/h2-5,8,12-13,16,19,21H,6-7,9-11H2,1H3,(H,26,28)/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50059171
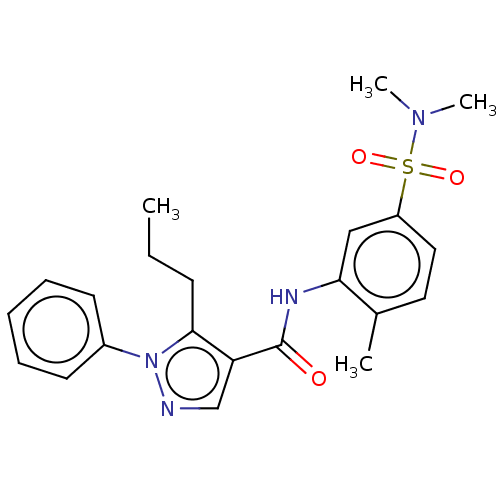
(CHEMBL3393296)Show SMILES CCCc1c(cnn1-c1ccccc1)C(=O)Nc1cc(ccc1C)S(=O)(=O)N(C)C Show InChI InChI=1S/C22H26N4O3S/c1-5-9-21-19(15-23-26(21)17-10-7-6-8-11-17)22(27)24-20-14-18(13-12-16(20)2)30(28,29)25(3)4/h6-8,10-15H,5,9H2,1-4H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46-400) as substrate |
J Med Chem 58: 278-93 (2015)
Article DOI: 10.1021/jm501038s
BindingDB Entry DOI: 10.7270/Q269758W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50173952
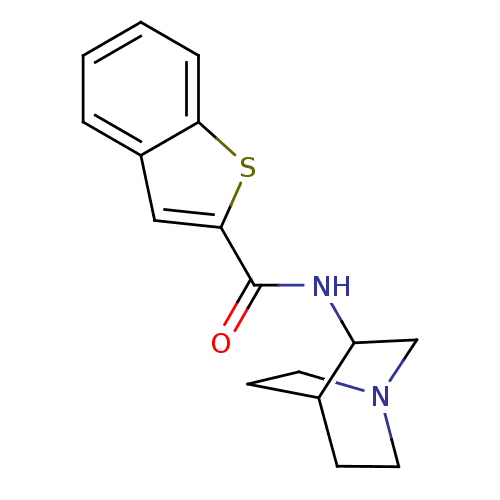
(Benzo[b]thiophene-2-carboxylic acid (1-aza-bicyclo...)Show SMILES O=C(NC1CN2CCC1CC2)c1cc2ccccc2s1 |THB:2:3:9.10:7.6,(2.61,.02,;2.05,-1.43,;.52,-1.65,;-.44,-.43,;-.73,.98,;-2.08,1.59,;-2.02,3.23,;-1.56,2.11,;-1.82,.21,;-3.37,-.45,;-3.58,.93,;3.01,-2.63,;2.59,-4.13,;3.87,-4.99,;4.09,-6.52,;5.52,-7.09,;6.74,-6.13,;6.52,-4.6,;5.09,-4.03,;4.55,-2.58,)| Show InChI InChI=1S/C16H18N2OS/c19-16(15-9-12-3-1-2-4-14(12)20-15)17-13-10-18-7-5-11(13)6-8-18/h1-4,9,11,13H,5-8,10H2,(H,17,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50417172

(CHEMBL1269897)Show SMILES CN(C)c1ccc(cc1)C(=O)OCC#CCSc1nnc(o1)-c1cccc2ccccc12 Show InChI InChI=1S/C25H21N3O3S/c1-28(2)20-14-12-19(13-15-20)24(29)30-16-5-6-17-32-25-27-26-23(31-25)22-11-7-9-18-8-3-4-10-21(18)22/h3-4,7-15H,16-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged reticulocyte 15-lipoxygenase-1 catalytic site assessed as 15-HPETE formation by Michaelis-Menten equation ... |
J Med Chem 53: 7392-404 (2010)
Article DOI: 10.1021/jm1008852
BindingDB Entry DOI: 10.7270/Q2SJ1KVW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data