

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matripase (unknown origin) using Boc-QAR-AMC as substrate incubated for 30 mins prior to substrate addition by fluorescence assay | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50518599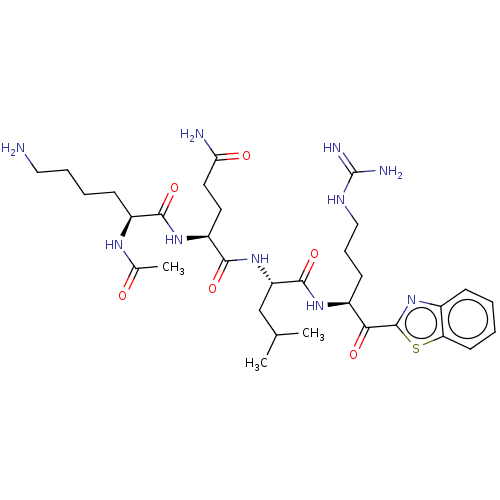 (CHEMBL4454016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged hepsin catalytic domain preincubated for 30 mins followed by Boc-QAR-AMC substrate addition a... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518590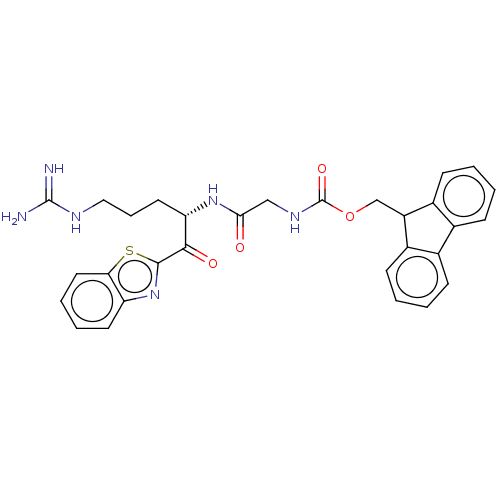 (CHEMBL4548036) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50518598 (CHEMBL4540950) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged hepsin catalytic domain preincubated for 30 mins followed by Boc-QAR-AMC substrate addition a... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032697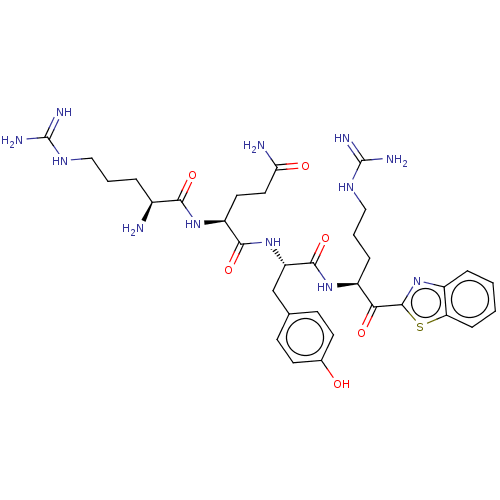 (CHEMBL3354675) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032934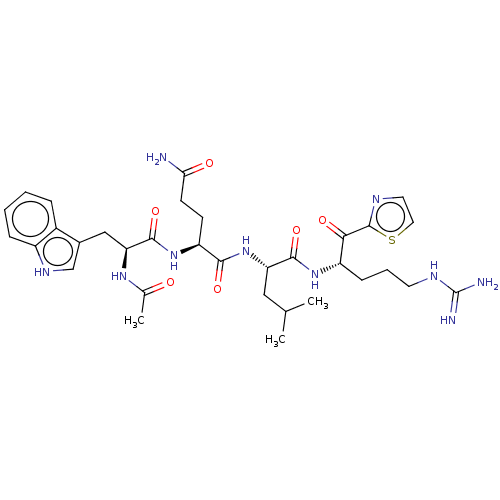 (CHEMBL3356591) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032999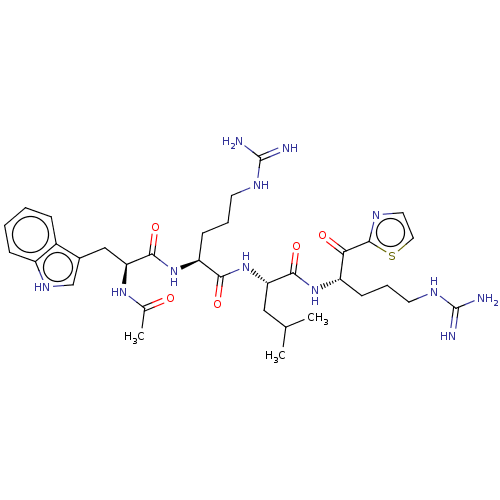 (CHEMBL3356597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518597 (CHEMBL4469801) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032936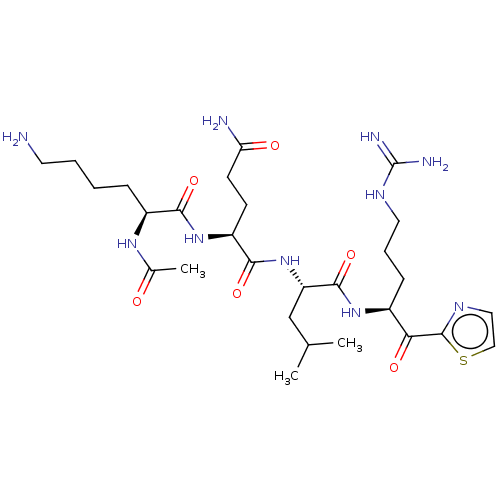 (CHEMBL3356589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032931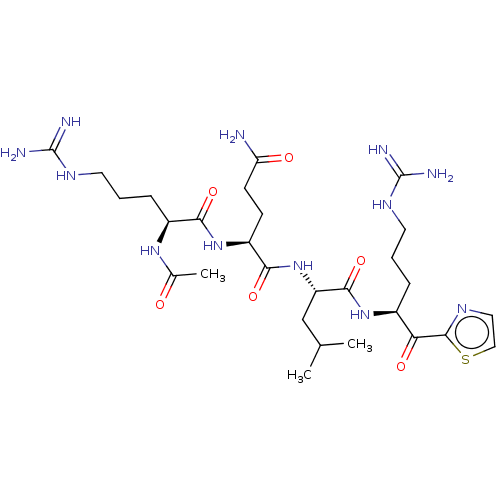 (CHEMBL3356594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032931 (CHEMBL3356594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032930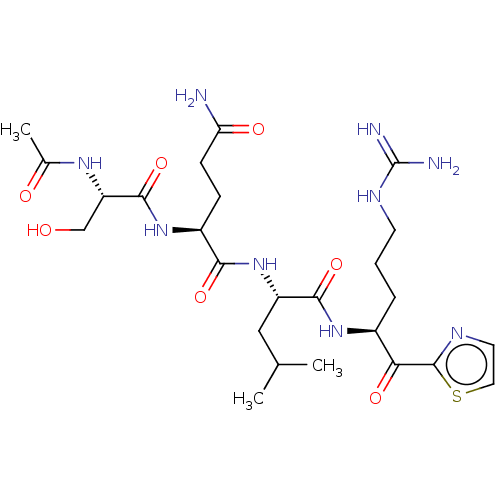 (CHEMBL3356595) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518606 (CHEMBL4562668) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032935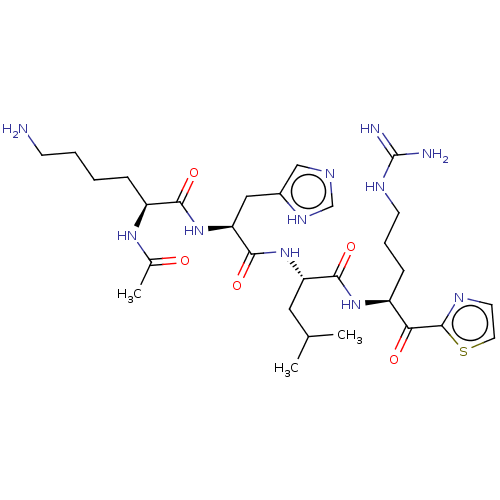 (CHEMBL3356590) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032947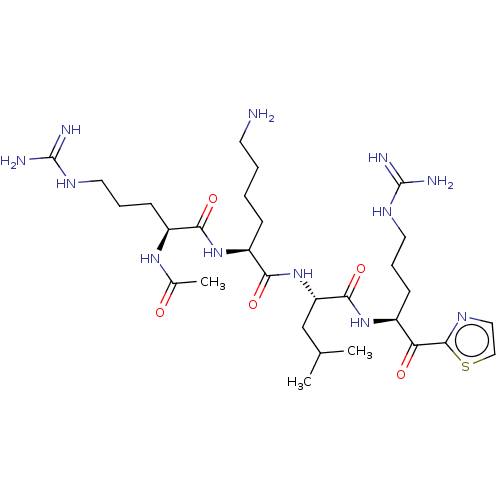 (CHEMBL3356606) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518609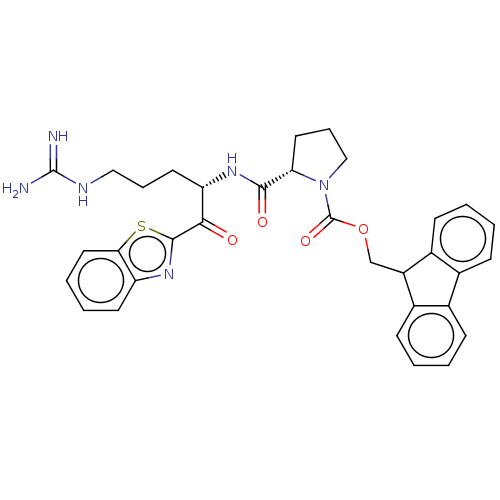 (CHEMBL4571215) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hepsin (unknown origin) using Boc-QAR-AMC as substrate after 30 mins prior to substrate addition by fluorescence assay | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518599 (CHEMBL4454016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032941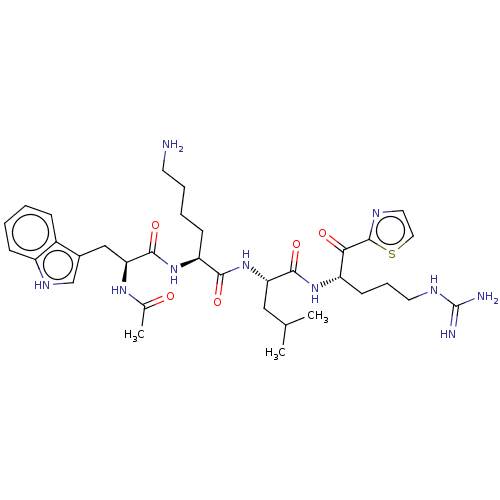 (CHEMBL3356600) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032991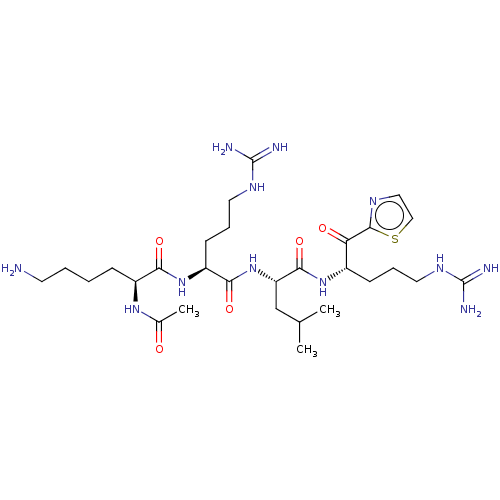 (CHEMBL3356596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032933 (CHEMBL3356592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032940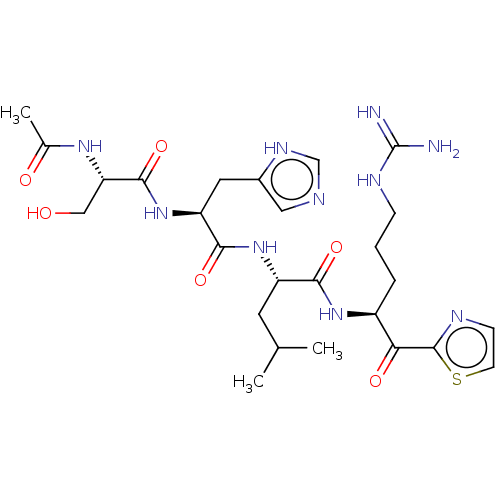 (CHEMBL3356599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032945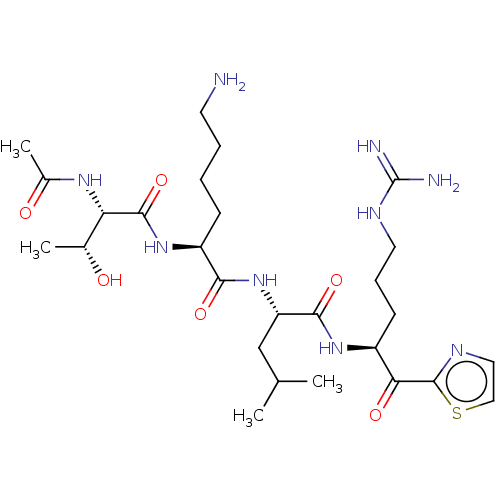 (CHEMBL3356604) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50518605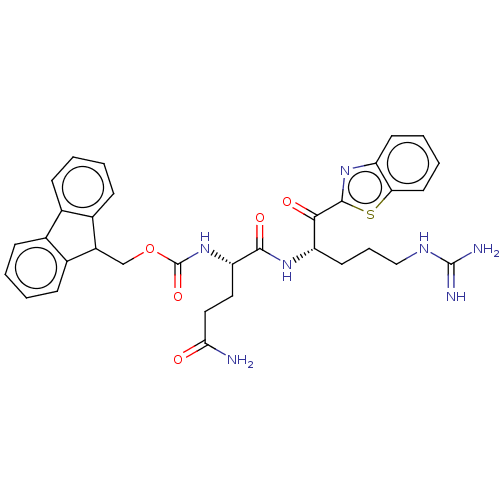 (CHEMBL4567317) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged matriptase catalytic domain expressed in Escherichia coli preincubated for 30 mins followed by... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032944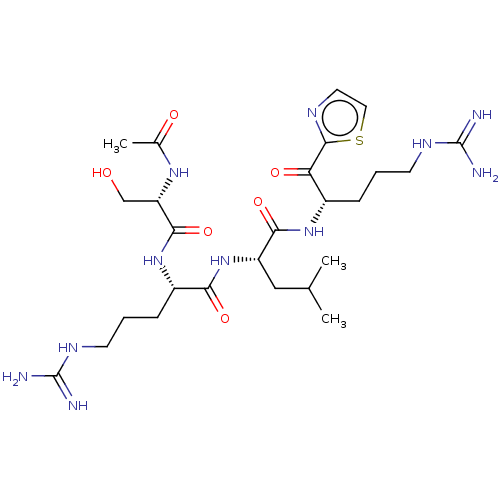 (CHEMBL3356603) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032933 (CHEMBL3356592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032933 (CHEMBL3356592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032947 (CHEMBL3356606) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032936 (CHEMBL3356589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032991 (CHEMBL3356596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032946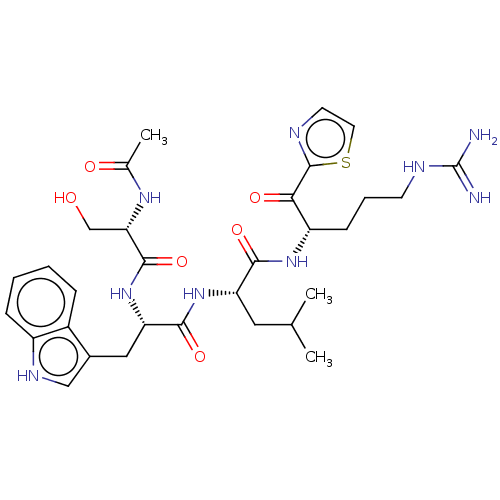 (CHEMBL3356605) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032939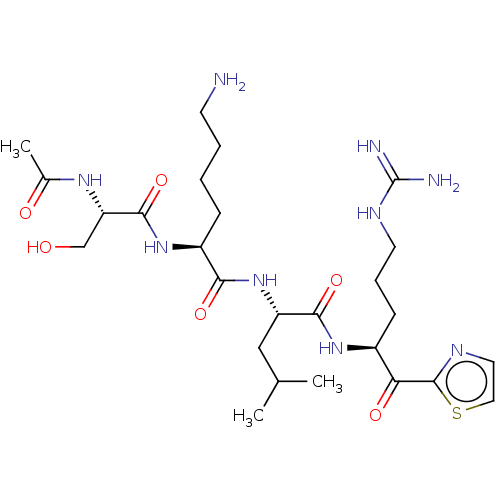 (CHEMBL3356598) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032943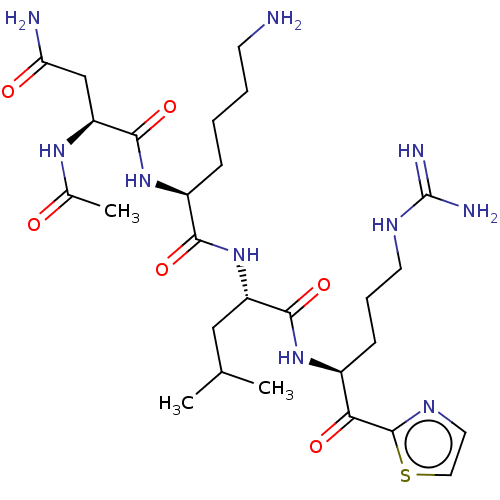 (CHEMBL3356602) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50518596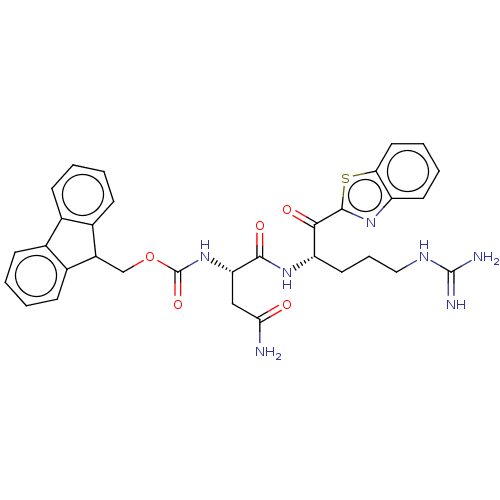 (CHEMBL4463174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged hepsin catalytic domain preincubated for 30 mins followed by Boc-QAR-AMC substrate addition a... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35654 (erk000526 | pyrimidylpyrrole, 11b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35657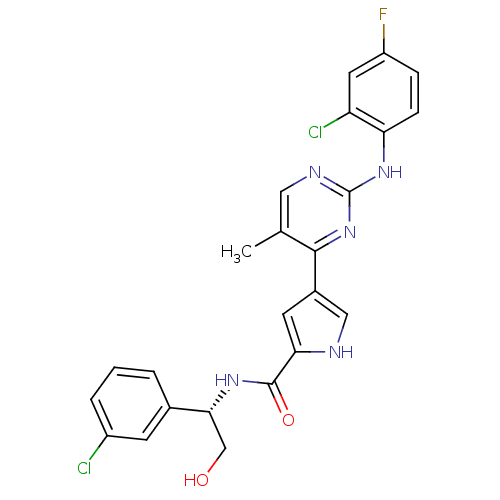 (pyrimidylpyrrole, 11e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35659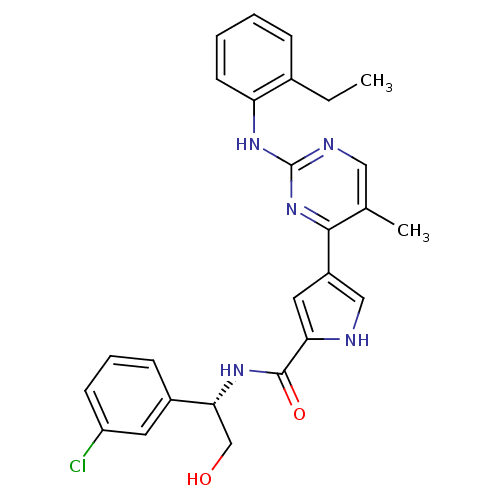 (pyrimidylpyrrole, 11g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35660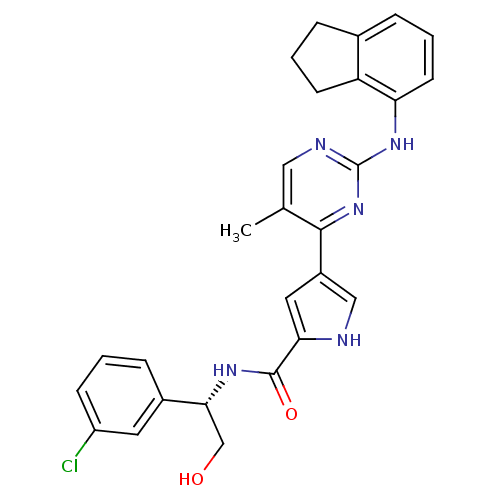 (pyrimidylpyrrole, 11h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35662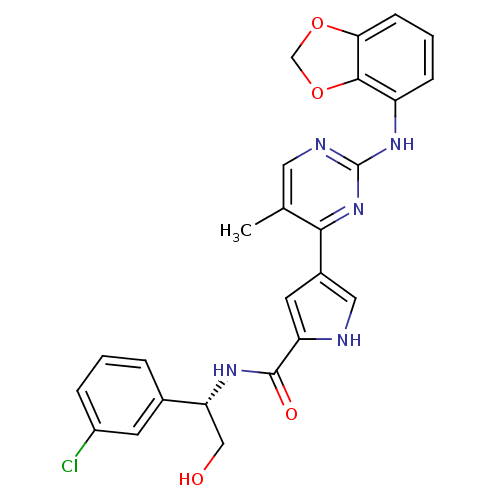 (pyrimidylpyrrole, 11j) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35663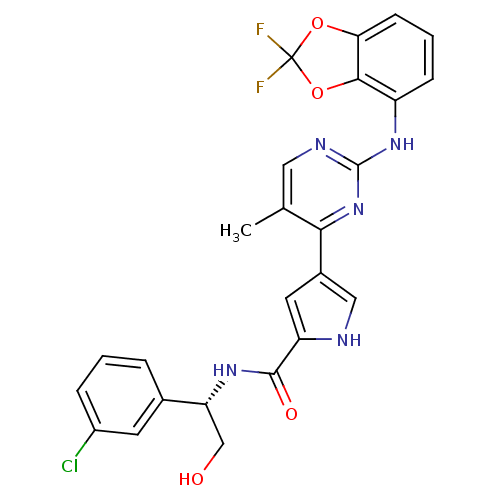 (pyrimidylpyrrole, 11k) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35647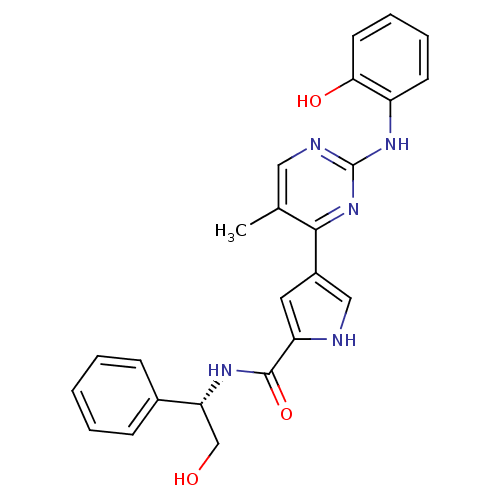 (erk000636 | pyrimidylpyrrole, 9f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35653 (pyrimidylpyrrole, 11a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM15645 (N-((S)-1-(3-Chloro-4-fluorophenyl)-2-hydroxyethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by ERK2 was converted to ATP by pyruvate kinase with the production of pyruvate fr... | J Med Chem 50: 1280-7 (2007) Article DOI: 10.1021/jm061381f BindingDB Entry DOI: 10.7270/Q2BR8QFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35641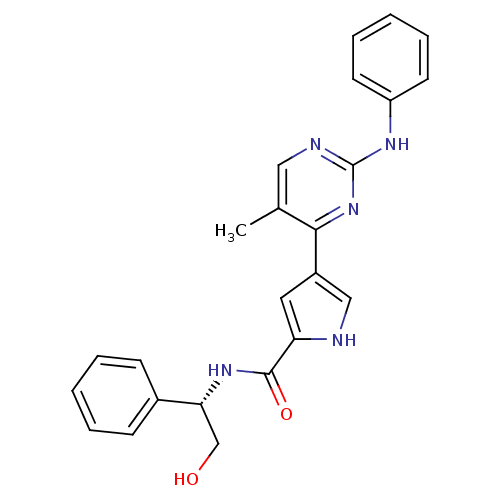 (erk000040 | pyrimidylpyrrole, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35642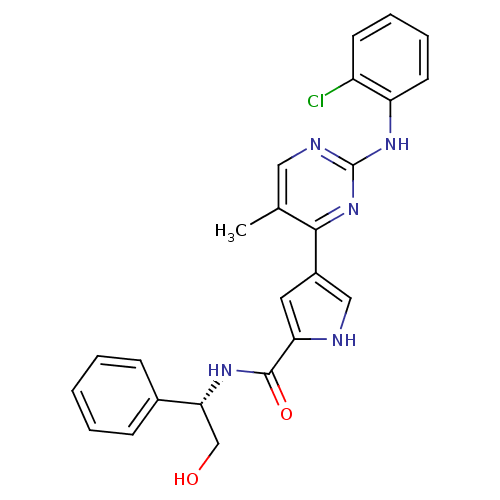 (pyrimidylpyrrole, 9a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35643 (pyrimidylpyrrole, 9b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35645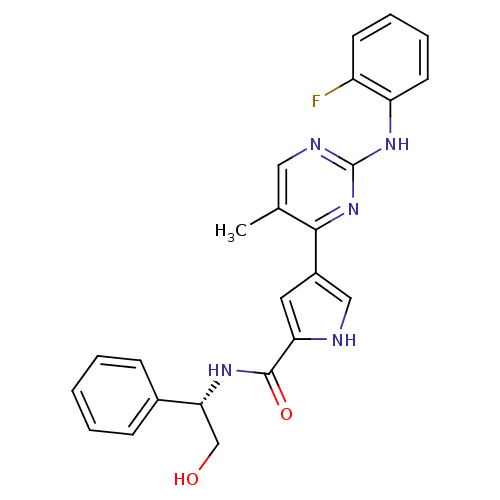 (pyrimidylpyrrole, 9d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35649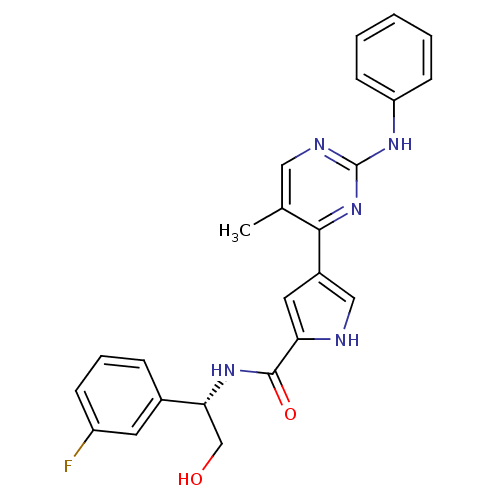 (erk000537 | pyrimidylpyrrole, 10b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1002 total ) | Next | Last >> |