

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0190 | -63.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0500 | -61.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.170 | -58.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.200 | -57.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,V583F] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.200 | -57.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.240 | -57.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.400 | -55.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A,V564F] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A,V564F] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.480 | -55.3 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,L579M] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,V583F] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.520 | -55.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,V583F] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.560 | -54.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A,V564F] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.700 | -54.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,V572F,L579M] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,L579M] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,V583F] (Human immunodeficiency virus type 1) | BDBM518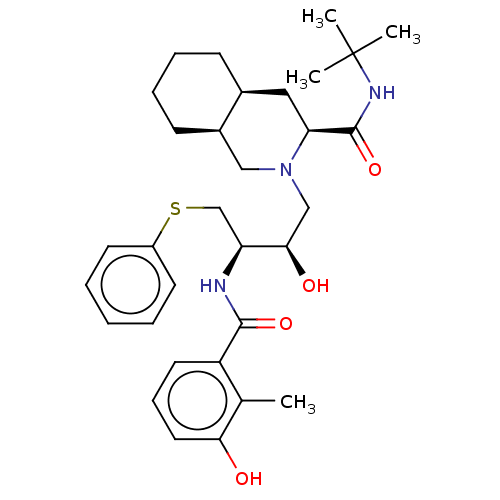 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.20 | -53.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A,V564F] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,L579M] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM518 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.20 | -53.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.30 | -52.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,L579M] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.80 | -51.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A] (Human immunodeficiency virus type 1) | BDBM518 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2 | -51.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,V583F] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.60 | -51.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A,V564F] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.10 | -50.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A,V564F] (Human immunodeficiency virus type 1) | BDBM518 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,V583F] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 4 | -49.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50173743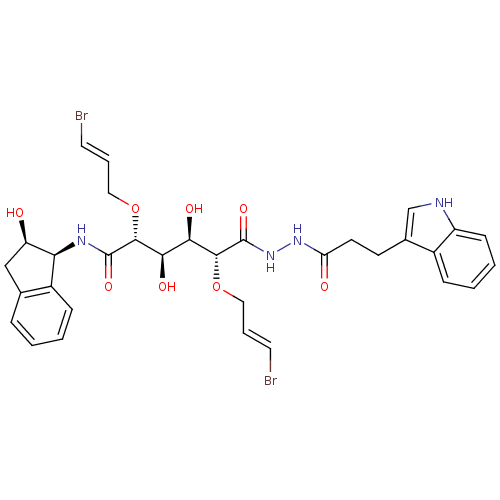 ((2R,3R,4R,5R)-2,5-Bis-((E)-3-bromo-allyloxy)-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 2 of Plasmodium falciparum 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50173737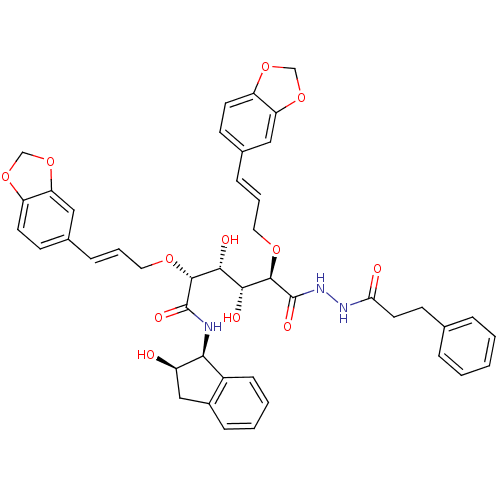 ((2R,3R,4R,5R)-2,5-Bis-((E)-3-benzo[1,3]dioxol-5-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 1 of Plasmodium falciparum, 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [482-580,I502K,I528M,T553A] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.90 | -48.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50173736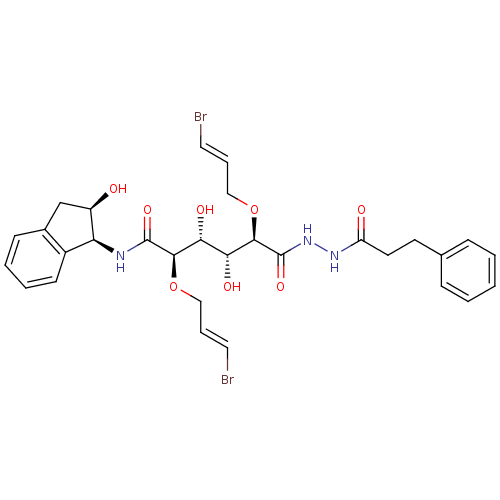 ((2R,3R,4R,5R)-2,5-Bis-((E)-3-bromo-allyloxy)-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 2 of Plasmodium falciparum 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,V572F,L579M] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50173736 ((2R,3R,4R,5R)-2,5-Bis-((E)-3-bromo-allyloxy)-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 1 of Plasmodium falciparum, 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50173749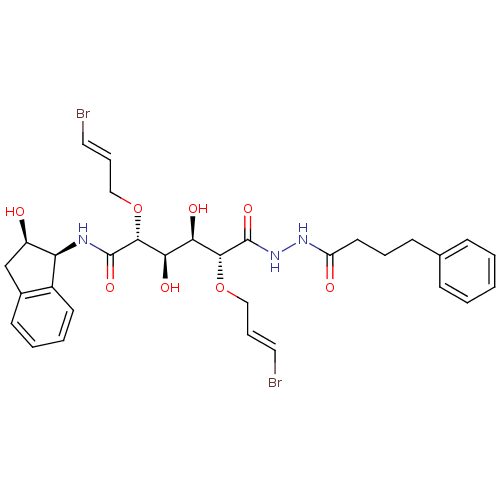 ((2R,3R,4R,5R)-2,5-Bis-((E)-3-bromo-allyloxy)-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 2 of Plasmodium falciparum 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50173749 ((2R,3R,4R,5R)-2,5-Bis-((E)-3-bromo-allyloxy)-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 1 of Plasmodium falciparum, 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50173737 ((2R,3R,4R,5R)-2,5-Bis-((E)-3-benzo[1,3]dioxol-5-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 2 of Plasmodium falciparum 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,V583F] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 12 | -47.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50173741 ((2R,3R,4R,5R)-2,5-Bis-((E)-3-bromo-allyloxy)-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 2 of Plasmodium falciparum 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,L579M] (Human immunodeficiency virus type 1) | BDBM518 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50173743 ((2R,3R,4R,5R)-2,5-Bis-((E)-3-bromo-allyloxy)-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 1 of Plasmodium falciparum, 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,V572F,L579M] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,V572F,L579M] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8001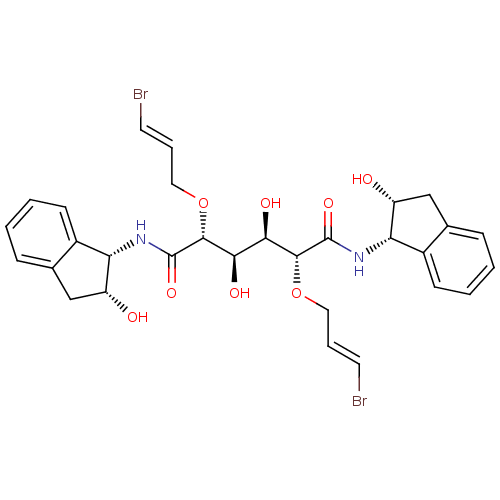 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-bromoprop-2-en-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 1 of Plasmodium falciparum, 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50173741 ((2R,3R,4R,5R)-2,5-Bis-((E)-3-bromo-allyloxy)-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 1 of Plasmodium falciparum, 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [490-588,L523I,E525D,M526I,I544V,L553H,H559K,V572F,L579M] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 45: 5468-77 (2006) Article DOI: 10.1021/bi051886s BindingDB Entry DOI: 10.7270/Q2QF8R33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50173747 ((1R,2R,3R,4R)-1,4-Bis-((E)-3-bromo-allyloxy)-1,4-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 4 of Plasmodium falciparum | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50173738 ((2R,3R,4R,5R)-2,5-Bis-[(E)-3-(4-acetyl-phenyl)-all...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition constant against plasmepsin 1 of Plasmodium falciparum, 10 min using 3 uM of DABCYL-Glu-Arg-Nle-Phe-Le u-Ser-Phe-Pro-EDANS as substrate | J Med Chem 48: 6090-106 (2005) Article DOI: 10.1021/jm050463l BindingDB Entry DOI: 10.7270/Q2Z037QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 120 total ) | Next | Last >> |