

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434188 (CHEMBL2385281 | US9346814, Cmpd No 2 Example 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... | ACS Med Chem Lett 4: 491-6 (2013) Article DOI: 10.1021/ml300410d BindingDB Entry DOI: 10.7270/Q2KP83J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434169 (CHEMBL2385300 | US9346814, Cmpd No 22 Example 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... | ACS Med Chem Lett 4: 491-6 (2013) Article DOI: 10.1021/ml300410d BindingDB Entry DOI: 10.7270/Q2KP83J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50430745 (CHEMBL2333951) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 56: 3121-47 (2013) Article DOI: 10.1021/jm3015926 BindingDB Entry DOI: 10.7270/Q23T9JKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50430742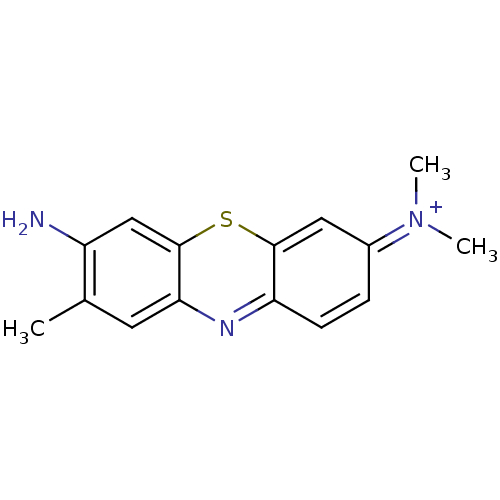 (Blutene | TOLONIUM CHLORIDE | Toluidine Blue O) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of recombinant tau (unknown origin) aggregation | J Med Chem 56: 3121-47 (2013) Article DOI: 10.1021/jm3015926 BindingDB Entry DOI: 10.7270/Q23T9JKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM21961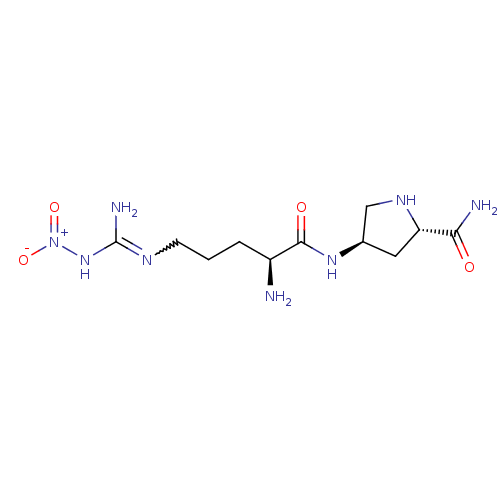 ((2S,4R)-4-[(2S)-2-amino-5-(1-nitrocarbamimidamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 56: 3121-47 (2013) Article DOI: 10.1021/jm3015926 BindingDB Entry DOI: 10.7270/Q23T9JKG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50430746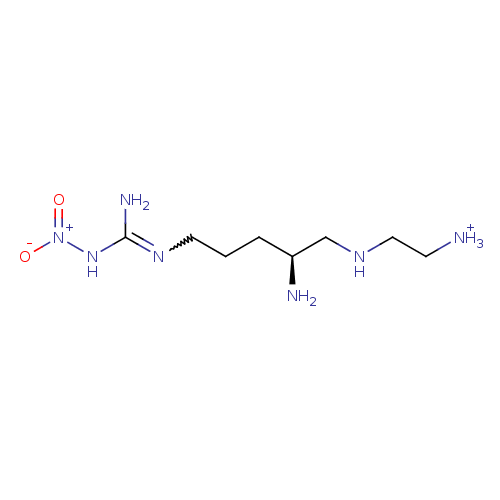 (CHEMBL2333950) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 56: 3121-47 (2013) Article DOI: 10.1021/jm3015926 BindingDB Entry DOI: 10.7270/Q23T9JKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50430747 (CHEMBL2333949) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 56: 3121-47 (2013) Article DOI: 10.1021/jm3015926 BindingDB Entry DOI: 10.7270/Q23T9JKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50230993 ((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 56: 3121-47 (2013) Article DOI: 10.1021/jm3015926 BindingDB Entry DOI: 10.7270/Q23T9JKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50240716 ((S)-2-Amino-5-(N'-propyl-guanidino)-pentanoic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 56: 3121-47 (2013) Article DOI: 10.1021/jm3015926 BindingDB Entry DOI: 10.7270/Q23T9JKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50002450 ((2S)-2-ammonio-5-{[(cyclopropylamino)(iminio)methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 56: 3121-47 (2013) Article DOI: 10.1021/jm3015926 BindingDB Entry DOI: 10.7270/Q23T9JKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50430748 (CHEMBL2333948) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 56: 3121-47 (2013) Article DOI: 10.1021/jm3015926 BindingDB Entry DOI: 10.7270/Q23T9JKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585273 (CHEMBL5076031) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 in HFL1 cells assessed as inhibition of EC80 S1P-induced IL8 release incubated for 16 to 24 hrs by ELISA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of DPP4 purified from human seminal plasma using Gly-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585274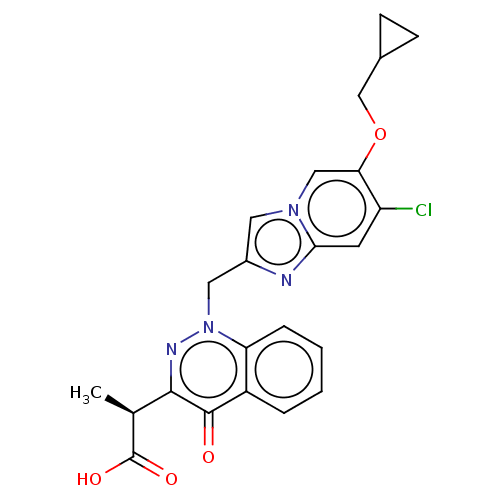 (CHEMBL5082556) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 in HFL1 cells assessed as inhibition of EC80 S1P-induced IL8 release incubated for 16 to 24 hrs by ELISA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585274 (CHEMBL5082556) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 receptor expressed in CHO cells assessed as inhibition of calcium flux | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585281 (CHEMBL5079623) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 receptor expressed in CHO cells assessed as inhibition of calcium flux | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50009364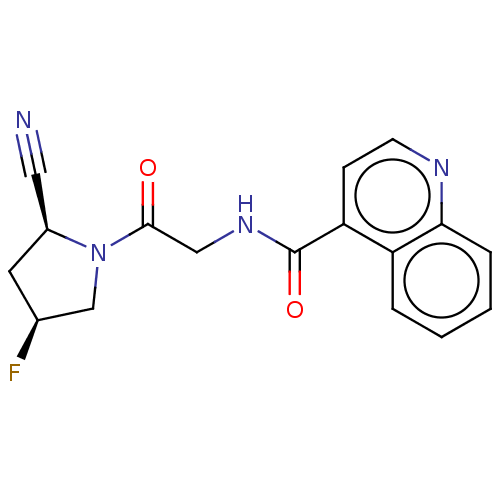 (CHEMBL3233840) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585280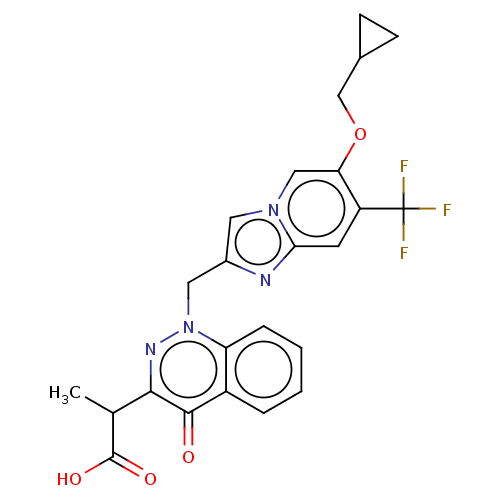 (CHEMBL5090966) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 receptor expressed in CHO cells assessed as inhibition of calcium flux | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585281 (CHEMBL5079623) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 in HFL1 cells assessed as inhibition of EC80 S1P-induced IL8 release incubated for 16 to 24 hrs by ELISA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50009370 (CHEMBL3233847) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50009360 (CHEMBL3233835 | US9346814, Cmpd No 23 Example 27) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50009360 (CHEMBL3233835 | US9346814, Cmpd No 23 Example 27) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585272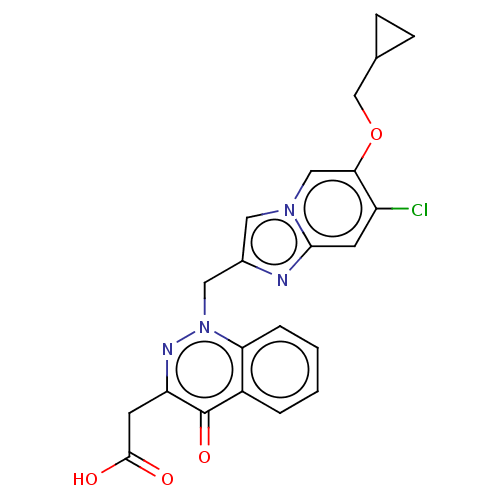 (CHEMBL5078844) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 in HFL1 cells assessed as inhibition of EC80 S1P-induced IL8 release incubated for 16 to 24 hrs by ELISA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585280 (CHEMBL5090966) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 in HFL1 cells assessed as inhibition of EC80 S1P-induced IL8 release incubated for 16 to 24 hrs by ELISA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585278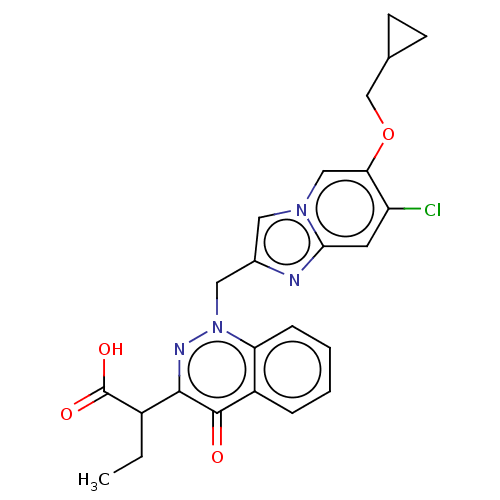 (CHEMBL5073747) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 receptor expressed in CHO cells assessed as inhibition of calcium flux | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585278 (CHEMBL5073747) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 in HFL1 cells assessed as inhibition of EC80 S1P-induced IL8 release incubated for 16 to 24 hrs by ELISA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50382287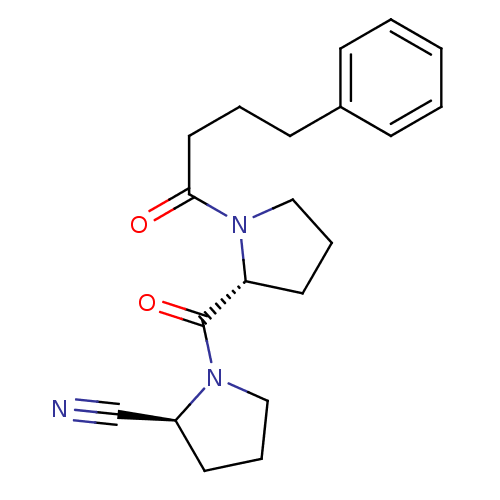 (CHEMBL2024671) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human recombinant PREP expressed in Escherichia coli assessed as pNA release from Z-Gly-Pro-p-nitroanilide pre-incubated with enzyme fo... | Bioorg Med Chem Lett 22: 3412-7 (2012) Article DOI: 10.1016/j.bmcl.2012.03.107 BindingDB Entry DOI: 10.7270/Q27D2W5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585273 (CHEMBL5076031) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 receptor expressed in CHO cells assessed as inhibition of calcium flux | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434163 (CHEMBL2385273 | US9346814, Cmpd No 12 Example 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434163 (CHEMBL2385273 | US9346814, Cmpd No 12 Example 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... | ACS Med Chem Lett 4: 491-6 (2013) Article DOI: 10.1021/ml300410d BindingDB Entry DOI: 10.7270/Q2KP83J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585262 (CHEMBL5081728) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 receptor expressed in CHO cells assessed as inhibition of calcium flux | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM233322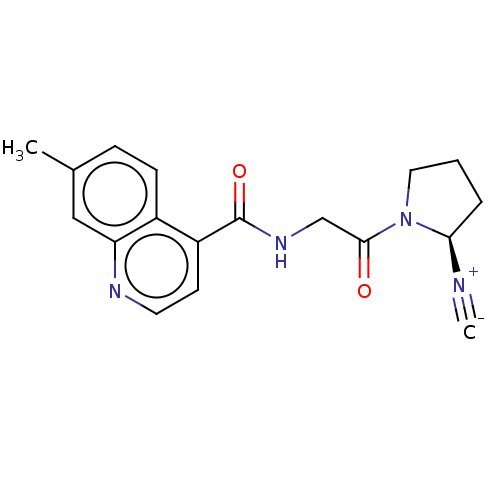 (US9346814, Cmpd No 27 Example 32) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50009359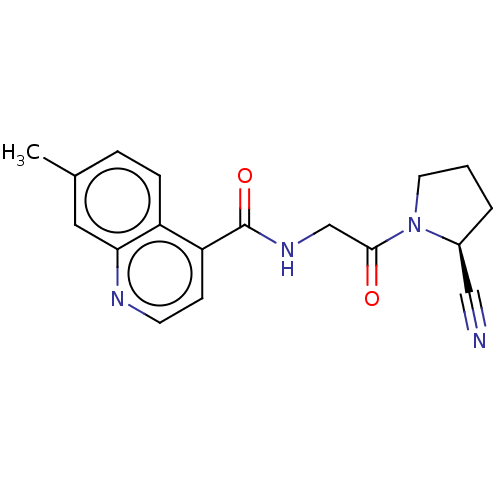 (CHEMBL3233834) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434164 (CHEMBL2385272 | US9346814, Cmpd No 13 Example 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434164 (CHEMBL2385272 | US9346814, Cmpd No 13 Example 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... | ACS Med Chem Lett 4: 491-6 (2013) Article DOI: 10.1021/ml300410d BindingDB Entry DOI: 10.7270/Q2KP83J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585271 (CHEMBL5070413) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 in HFL1 cells assessed as inhibition of EC80 S1P-induced IL8 release incubated for 16 to 24 hrs by ELISA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585281 (CHEMBL5079623) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against human S1P2 assessed as inhibition of beta-arrestin 2 recruitment | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585276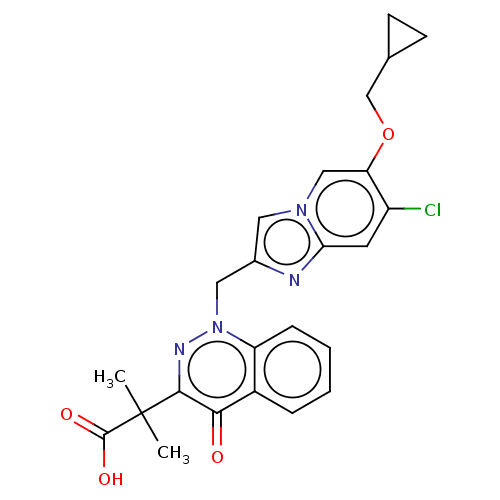 (CHEMBL5093130) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 receptor expressed in CHO cells assessed as inhibition of calcium flux | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585270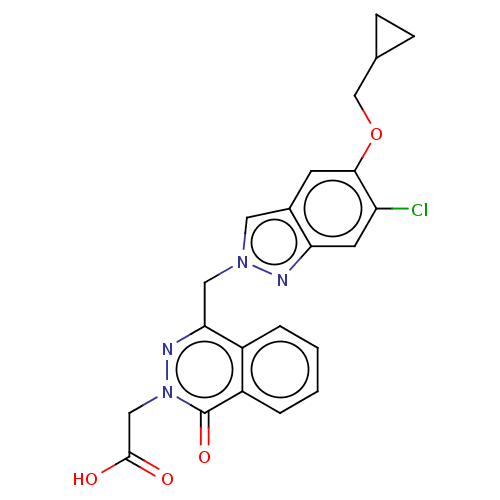 (CHEMBL5079608) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 in HFL1 cells assessed as inhibition of EC80 S1P-induced IL8 release incubated for 16 to 24 hrs by ELISA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50009331 (CHEMBL3233843 | US9346814, Cmpd No 24 Example 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50009331 (CHEMBL3233843 | US9346814, Cmpd No 24 Example 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585261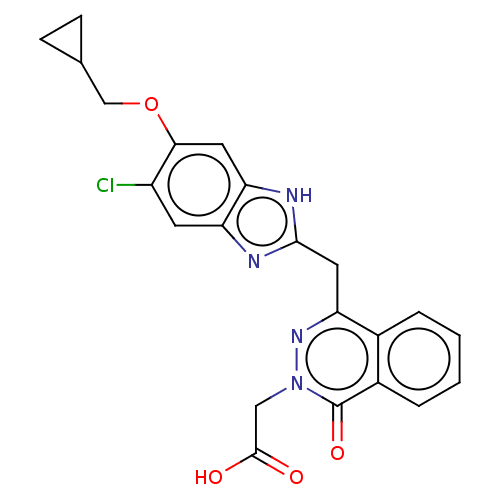 (CHEMBL5076737) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 receptor expressed in CHO cells assessed as inhibition of calcium flux | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50585271 (CHEMBL5070413) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human S1P2 receptor expressed in CHO cells assessed as inhibition of calcium flux | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01066 BindingDB Entry DOI: 10.7270/Q2VM4H5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434165 (CHEMBL2385271 | US9346814, Cmpd No 11 Example 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... | ACS Med Chem Lett 4: 491-6 (2013) Article DOI: 10.1021/ml300410d BindingDB Entry DOI: 10.7270/Q2KP83J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434165 (CHEMBL2385271 | US9346814, Cmpd No 11 Example 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434170 (CHEMBL2385299 | US9346814, Cmpd No 21 Example 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434170 (CHEMBL2385299 | US9346814, Cmpd No 21 Example 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... | ACS Med Chem Lett 4: 491-6 (2013) Article DOI: 10.1021/ml300410d BindingDB Entry DOI: 10.7270/Q2KP83J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434166 (CHEMBL2385270 | US9346814, Cmpd No 6 Example 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... | ACS Med Chem Lett 4: 491-6 (2013) Article DOI: 10.1021/ml300410d BindingDB Entry DOI: 10.7270/Q2KP83J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 857 total ) | Next | Last >> |