

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine--tRNA ligase (Staphylococcus aureus) | BDBM18131 (SB-243545 | butyl (2S)-2-[(2S)-2-amino-3-(4-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.9 | 37 |
GSK | Assay Description The concentration of inhibitor which results in 50% inhibition (IC50) of enzyme activity was determined by preincubating recombinant S. aureus TyrRS ... | Protein Sci 10: 2008-16 (2001) Article DOI: 10.1110/ps.18001 BindingDB Entry DOI: 10.7270/Q2NG4NXT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM18128 ((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 11: 2499-502 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50097749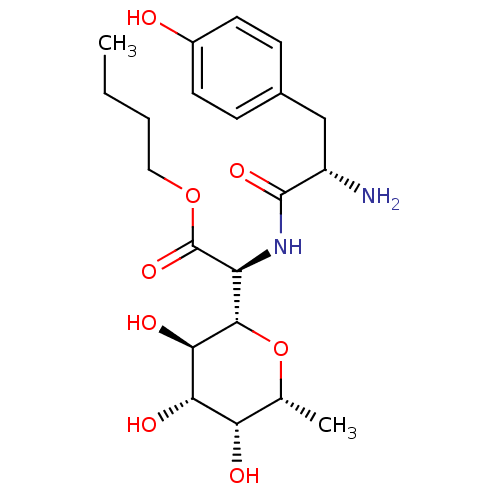 (CHEMBL163375 | [2-Amino-3-((S)-4-hydroxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 715-8 (2001) BindingDB Entry DOI: 10.7270/Q20C4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase (Staphylococcus aureus) | BDBM18128 ((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.9 | 37 |
GSK | Assay Description The concentration of inhibitor which results in 50% inhibition (IC50) of enzyme activity was determined by preincubating recombinant S. aureus TyrRS ... | Protein Sci 10: 2008-16 (2001) Article DOI: 10.1110/ps.18001 BindingDB Entry DOI: 10.7270/Q2NG4NXT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50112575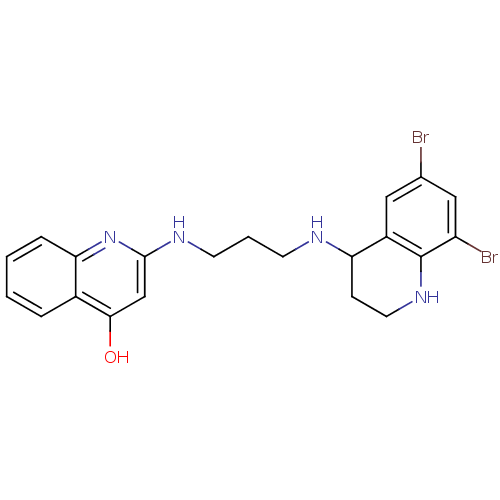 (2-[3-(6,8-Dibromo-1,2,3,4-tetrahydro-quinolin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Methionyl-tRNA synthetase in a pyrophosphate exchange assay | J Med Chem 45: 1959-62 (2002) BindingDB Entry DOI: 10.7270/Q2HQ3Z6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50091497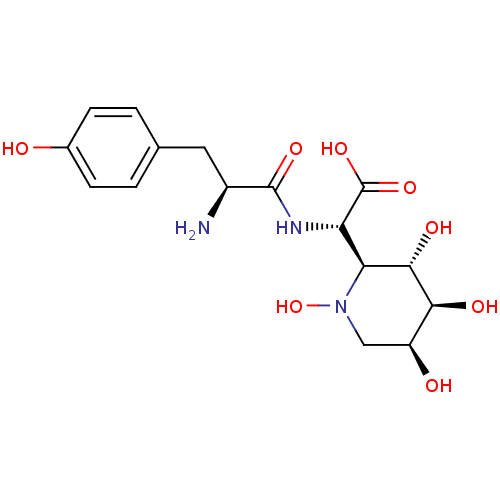 ((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity of compound against tyrosyl tRNA synthetase from Staphylococcus aureus was determined | Bioorg Med Chem Lett 10: 1811-4 (2000) BindingDB Entry DOI: 10.7270/Q23F4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50091497 ((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 711-4 (2001) BindingDB Entry DOI: 10.7270/Q2445KR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM18128 ((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity of compound against tyrosyl tRNA synthetase from Staphylococcus aureus was determined | Bioorg Med Chem Lett 10: 1811-4 (2000) BindingDB Entry DOI: 10.7270/Q23F4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50097746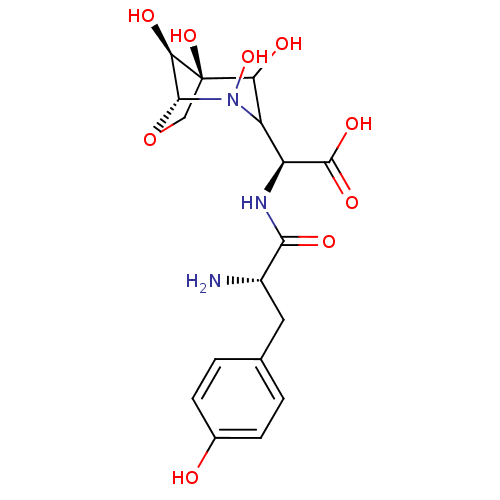 ((S)-[2-Amino-3-((S)-4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 711-4 (2001) BindingDB Entry DOI: 10.7270/Q2445KR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50222703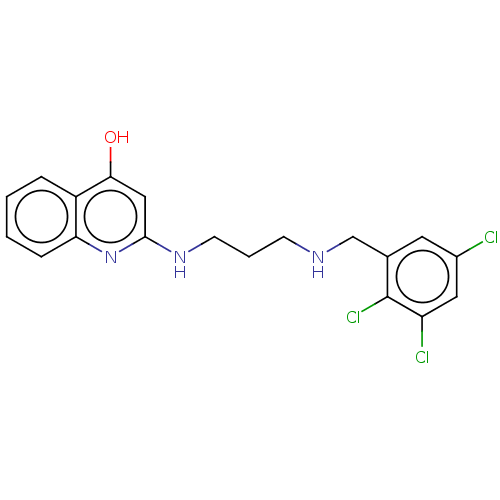 (CHEMBL160841) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50222702 (CHEMBL161347) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50222705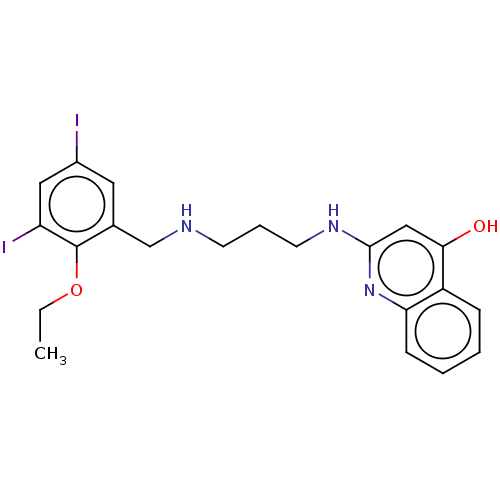 (CHEMBL161312) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50222700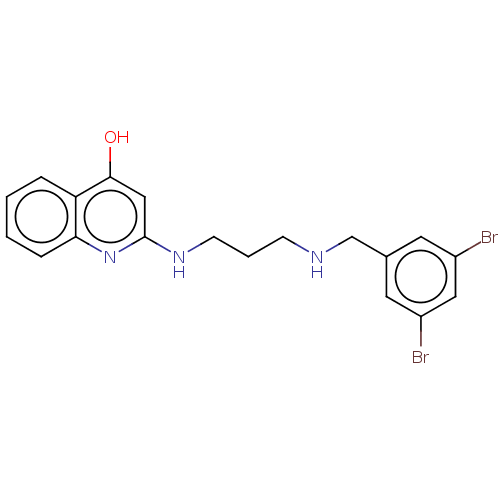 (CHEMBL161864) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50222701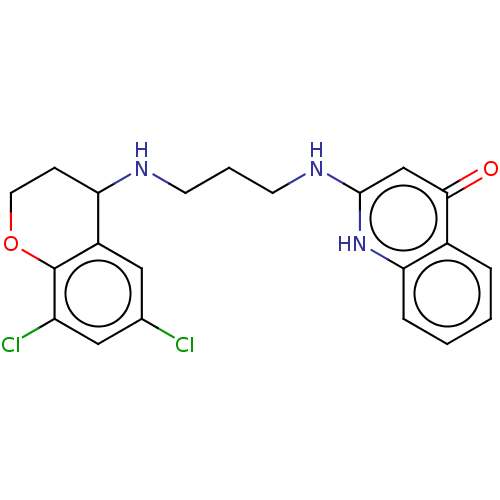 (CHEMBL158051) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50222699 (CHEMBL161300) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50222704 (CHEMBL345835) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase (Staphylococcus aureus) | BDBM18130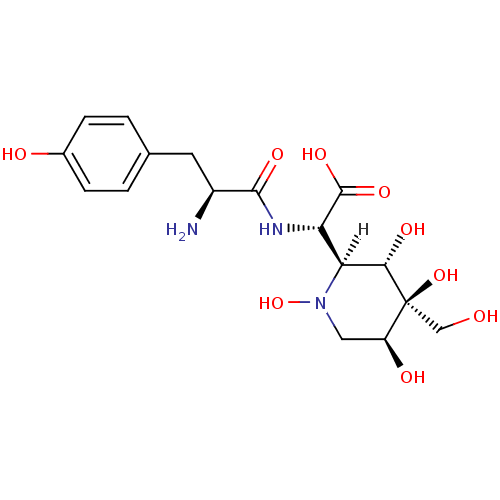 ((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 37 |
GSK | Assay Description The concentration of inhibitor which results in 50% inhibition (IC50) of enzyme activity was determined by preincubating recombinant S. aureus TyrRS ... | Protein Sci 10: 2008-16 (2001) Article DOI: 10.1110/ps.18001 BindingDB Entry DOI: 10.7270/Q2NG4NXT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124821 (2-[3-(3,5-Dichloro-benzylamino)-propylamino]-1H-qu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124843 (2-[3-(3-Bromo-2-ethoxy-5-methoxy-benzylamino)-prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124826 (2-[3-(2-Ethoxy-5-iodo-3-methyl-benzylamino)-propyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124811 (2-[3-(3-Bromo-5-iodo-benzylamino)-propylamino]-1H-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Staphylococcus aureus (strain MW2)) | BDBM50149651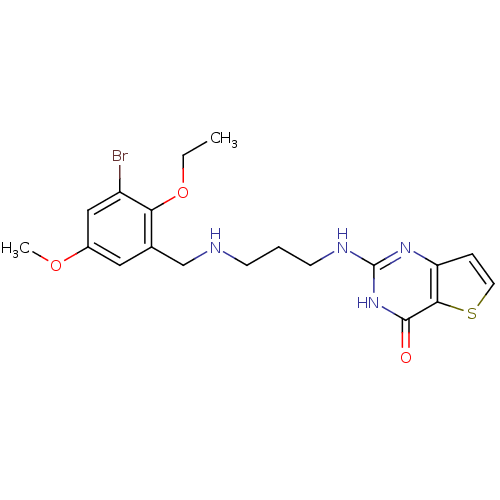 (2-[3-(3-Bromo-2-ethoxy-5-methoxy-benzylamino)-prop...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase | Bioorg Med Chem Lett 14: 3937-41 (2004) Article DOI: 10.1016/j.bmcl.2004.05.070 BindingDB Entry DOI: 10.7270/Q2QC02Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124808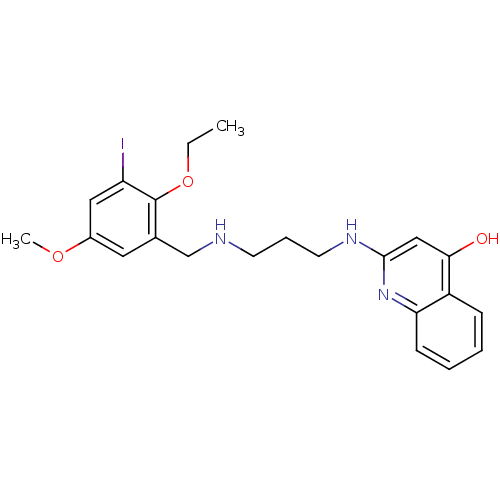 (2-[3-(2-Ethoxy-3-iodo-5-methoxy-benzylamino)-propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50104311 ((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 11: 2499-502 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50097748 (CHEMBL351127 | [2-Amino-3-((S)-4-hydroxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 715-8 (2001) BindingDB Entry DOI: 10.7270/Q20C4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Staphylococcus aureus) | BDBM18116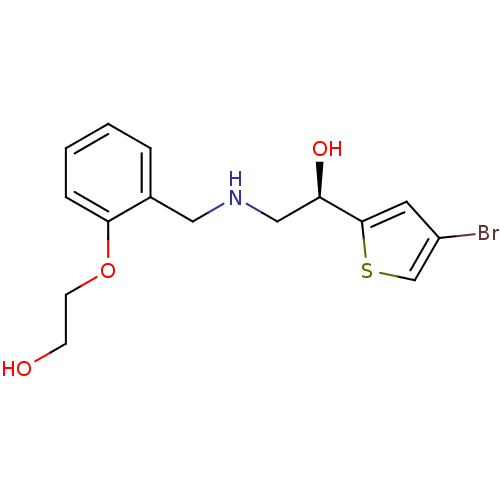 (2-[2-({[(2R)-2-(4-bromothiophen-2-yl)-2-hydroxyeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 2.0 | n/a |
GSK | Assay Description The reactions were performed in 96-well Packard 384-well Packard Optiplates. After 10 min of incubation, the reaction was stopped by addition of a co... | Bioorg Med Chem Lett 15: 2305-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.003 BindingDB Entry DOI: 10.7270/Q25D8Q3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Staphylococcus aureus (strain MW2)) | BDBM50149623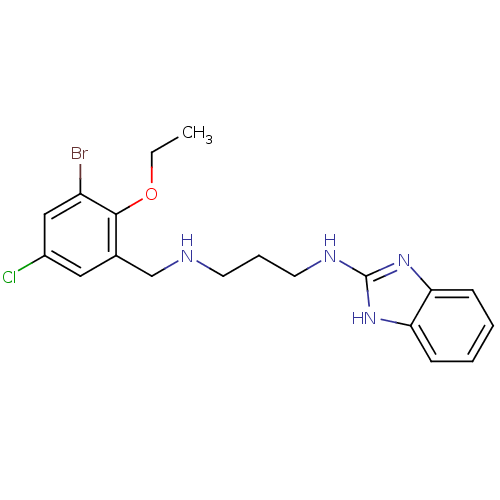 (CHEMBL424799 | N-(1H-Benzoimidazol-2-yl)-N''-(3-br...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase | Bioorg Med Chem Lett 14: 3937-41 (2004) Article DOI: 10.1016/j.bmcl.2004.05.070 BindingDB Entry DOI: 10.7270/Q2QC02Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124846 (2-[3-(5,7-Dichloro-1,2,3,4-tetrahydro-naphthalen-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124851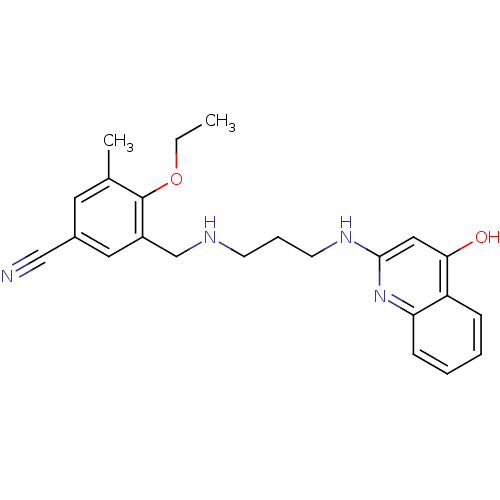 (4-Ethoxy-3-methyl-5-{[3-(4-oxo-1,4-dihydro-quinoli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Staphylococcus aureus (strain MW2)) | BDBM50149650 (2-[3-(3,5-Dibromo-benzylamino)-propylamino]-1H-thi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase | Bioorg Med Chem Lett 14: 3937-41 (2004) Article DOI: 10.1016/j.bmcl.2004.05.070 BindingDB Entry DOI: 10.7270/Q2QC02Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124803 (5-Bromo-2-ethoxy-3-{[3-(4-oxo-1,4-dihydro-quinolin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Staphylococcus aureus (strain MW2)) | BDBM50149646 (5-[3-(3,5-Dibromo-benzylamino)-propylamino]-4H-thi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase | Bioorg Med Chem Lett 14: 3937-41 (2004) Article DOI: 10.1016/j.bmcl.2004.05.070 BindingDB Entry DOI: 10.7270/Q2QC02Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM18132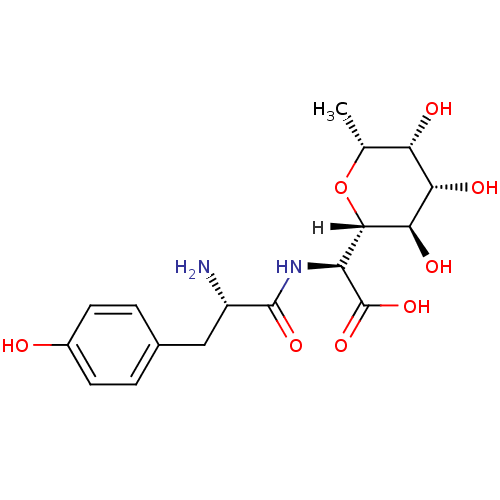 ((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 715-8 (2001) BindingDB Entry DOI: 10.7270/Q20C4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase (Staphylococcus aureus) | BDBM18132 ((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.9 | 37 |
GSK | Assay Description The concentration of inhibitor which results in 50% inhibition (IC50) of enzyme activity was determined by preincubating recombinant S. aureus TyrRS ... | Protein Sci 10: 2008-16 (2001) Article DOI: 10.1110/ps.18001 BindingDB Entry DOI: 10.7270/Q2NG4NXT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124825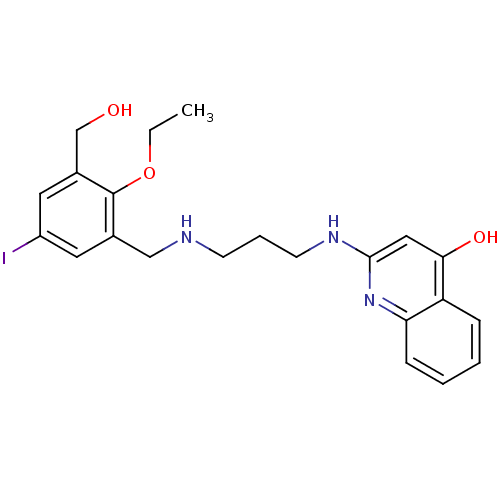 (2-[3-(2-Ethoxy-3-hydroxymethyl-5-iodo-benzylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124854 (2-[3-(3-Chloro-2-ethoxy-5-methoxy-benzylamino)-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124835 (2-[3-(6-Bromo-8-ethyl-1,2,3,4-tetrahydro-quinolin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124817 (2-[3-(3,5-Dibromo-2-ethoxy-benzylamino)-propylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50091515 (Aminoalkyl adenylate and aminoacyl sulfamate analo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against cognate Staphylococcus aureus Arginyl-tRNA synthetase | Bioorg Med Chem Lett 10: 1871-4 (2000) BindingDB Entry DOI: 10.7270/Q2TX3FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124824 (3-Bromo-4-ethoxy-5-{[3-(4-oxo-1,4-dihydro-quinolin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124813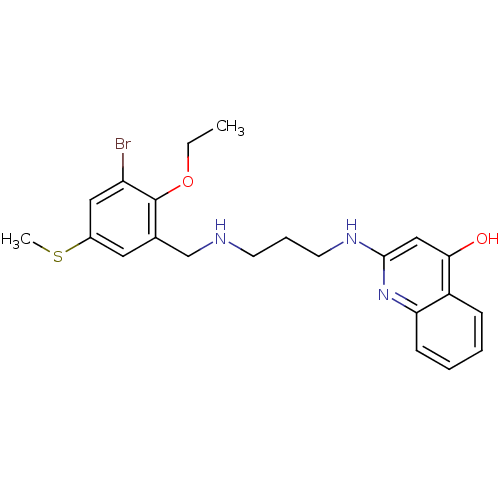 (2-[3-(3-Bromo-2-ethoxy-5-methylsulfanyl-benzylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Staphylococcus aureus (strain MW2)) | BDBM50149649 (2-[3-(3,5-Dibromo-2-ethoxy-benzylamino)-propylamin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase | Bioorg Med Chem Lett 14: 3937-41 (2004) Article DOI: 10.1016/j.bmcl.2004.05.070 BindingDB Entry DOI: 10.7270/Q2QC02Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124800 (2-[3-(3-Bromo-2-ethoxy-5-methyl-benzylamino)-propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Staphylococcus aureus (strain MW2)) | BDBM50149630 (CHEMBL182960 | N-(3,5-Dibromo-benzyl)-N''-(1H-imid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase | Bioorg Med Chem Lett 14: 3937-41 (2004) Article DOI: 10.1016/j.bmcl.2004.05.070 BindingDB Entry DOI: 10.7270/Q2QC02Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124801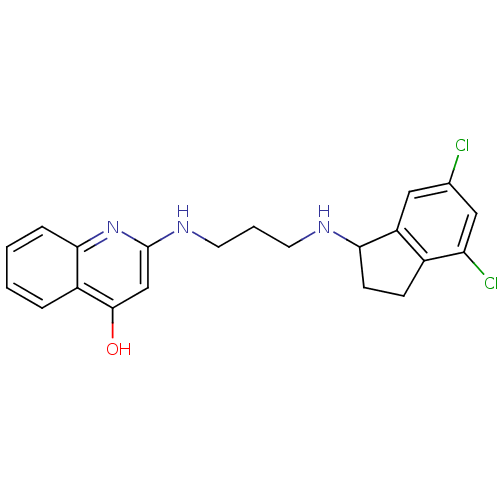 (2-[3-(4,6-Dichloro-indan-1-ylamino)-propylamino]-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124857 (2-[3-(2-Ethoxy-3-methyl-5-methylsulfanyl-benzylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124850 (2-[3-(5-Bromo-2-ethoxy-3-methyl-benzylamino)-propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124819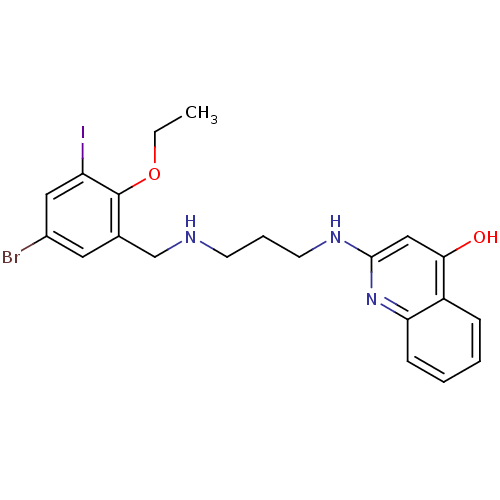 (2-[3-(5-Bromo-2-ethoxy-3-iodo-benzylamino)-propyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50124852 (2-[3-(5,7-Dichloro-2,3-dihydro-benzofuran-3-ylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay | Bioorg Med Chem Lett 13: 665-8 (2003) BindingDB Entry DOI: 10.7270/Q2KP81JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine--tRNA ligase (Staphylococcus aureus (strain MW2)) | BDBM50149643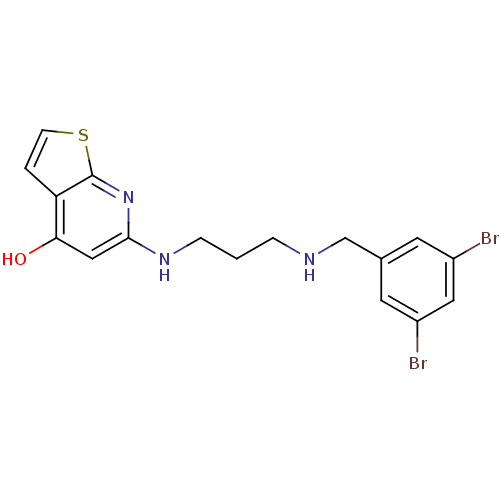 (6-[3-(3,5-Dibromo-benzylamino)-propylamino]-7H-thi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase | Bioorg Med Chem Lett 14: 3937-41 (2004) Article DOI: 10.1016/j.bmcl.2004.05.070 BindingDB Entry DOI: 10.7270/Q2QC02Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 370 total ) | Next | Last >> |