 Found 284 hits with Last Name = 'jarvie' and Initial = 'e'
Found 284 hits with Last Name = 'jarvie' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(RAT) | BDBM81767
(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85671
(CAS_183658-72-2 | CYN 154806 | CYN-154806)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CSSC[C@H](NC1=O)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O |r| Show InChI InChI=1S/C56H68N12O14S2/c1-30(69)48-56(80)66-47(54(78)62-42(49(58)73)23-33-12-18-37(71)19-13-33)29-84-83-28-46(65-51(75)43(60-31(2)70)24-32-10-16-36(17-11-32)68(81)82)55(79)63-44(25-34-14-20-38(72)21-15-34)52(76)64-45(26-35-27-59-40-8-4-3-7-39(35)40)53(77)61-41(50(74)67-48)9-5-6-22-57/h3-4,7-8,10-21,27,30,41-48,59,69,71-72H,5-6,9,22-26,28-29,57H2,1-2H3,(H2,58,73)(H,60,70)(H,61,77)(H,62,78)(H,63,79)(H,64,76)(H,65,75)(H,66,80)(H,67,74)/t30-,41+,42-,43+,44+,45-,46-,47+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM85671

(CAS_183658-72-2 | CYN 154806 | CYN-154806)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CSSC[C@H](NC1=O)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O |r| Show InChI InChI=1S/C56H68N12O14S2/c1-30(69)48-56(80)66-47(54(78)62-42(49(58)73)23-33-12-18-37(71)19-13-33)29-84-83-28-46(65-51(75)43(60-31(2)70)24-32-10-16-36(17-11-32)68(81)82)55(79)63-44(25-34-14-20-38(72)21-15-34)52(76)64-45(26-35-27-59-40-8-4-3-7-39(35)40)53(77)61-41(50(74)67-48)9-5-6-22-57/h3-4,7-8,10-21,27,30,41-48,59,69,71-72H,5-6,9,22-26,28-29,57H2,1-2H3,(H2,58,73)(H,60,70)(H,61,77)(H,62,78)(H,63,79)(H,64,76)(H,65,75)(H,66,80)(H,67,74)/t30-,41+,42-,43+,44+,45-,46-,47+,48+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM85671

(CAS_183658-72-2 | CYN 154806 | CYN-154806)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CSSC[C@H](NC1=O)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O |r| Show InChI InChI=1S/C56H68N12O14S2/c1-30(69)48-56(80)66-47(54(78)62-42(49(58)73)23-33-12-18-37(71)19-13-33)29-84-83-28-46(65-51(75)43(60-31(2)70)24-32-10-16-36(17-11-32)68(81)82)55(79)63-44(25-34-14-20-38(72)21-15-34)52(76)64-45(26-35-27-59-40-8-4-3-7-39(35)40)53(77)61-41(50(74)67-48)9-5-6-22-57/h3-4,7-8,10-21,27,30,41-48,59,69,71-72H,5-6,9,22-26,28-29,57H2,1-2H3,(H2,58,73)(H,60,70)(H,61,77)(H,62,78)(H,63,79)(H,64,76)(H,65,75)(H,66,80)(H,67,74)/t30-,41+,42-,43+,44+,45-,46-,47+,48+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM85671

(CAS_183658-72-2 | CYN 154806 | CYN-154806)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CSSC[C@H](NC1=O)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O |r| Show InChI InChI=1S/C56H68N12O14S2/c1-30(69)48-56(80)66-47(54(78)62-42(49(58)73)23-33-12-18-37(71)19-13-33)29-84-83-28-46(65-51(75)43(60-31(2)70)24-32-10-16-36(17-11-32)68(81)82)55(79)63-44(25-34-14-20-38(72)21-15-34)52(76)64-45(26-35-27-59-40-8-4-3-7-39(35)40)53(77)61-41(50(74)67-48)9-5-6-22-57/h3-4,7-8,10-21,27,30,41-48,59,69,71-72H,5-6,9,22-26,28-29,57H2,1-2H3,(H2,58,73)(H,60,70)(H,61,77)(H,62,78)(H,63,79)(H,64,76)(H,65,75)(H,66,80)(H,67,74)/t30-,41+,42-,43+,44+,45-,46-,47+,48+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM85671

(CAS_183658-72-2 | CYN 154806 | CYN-154806)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CSSC[C@H](NC1=O)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O |r| Show InChI InChI=1S/C56H68N12O14S2/c1-30(69)48-56(80)66-47(54(78)62-42(49(58)73)23-33-12-18-37(71)19-13-33)29-84-83-28-46(65-51(75)43(60-31(2)70)24-32-10-16-36(17-11-32)68(81)82)55(79)63-44(25-34-14-20-38(72)21-15-34)52(76)64-45(26-35-27-59-40-8-4-3-7-39(35)40)53(77)61-41(50(74)67-48)9-5-6-22-57/h3-4,7-8,10-21,27,30,41-48,59,69,71-72H,5-6,9,22-26,28-29,57H2,1-2H3,(H2,58,73)(H,60,70)(H,61,77)(H,62,78)(H,63,79)(H,64,76)(H,65,75)(H,66,80)(H,67,74)/t30-,41+,42-,43+,44+,45-,46-,47+,48+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM85671

(CAS_183658-72-2 | CYN 154806 | CYN-154806)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CSSC[C@H](NC1=O)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O |r| Show InChI InChI=1S/C56H68N12O14S2/c1-30(69)48-56(80)66-47(54(78)62-42(49(58)73)23-33-12-18-37(71)19-13-33)29-84-83-28-46(65-51(75)43(60-31(2)70)24-32-10-16-36(17-11-32)68(81)82)55(79)63-44(25-34-14-20-38(72)21-15-34)52(76)64-45(26-35-27-59-40-8-4-3-7-39(35)40)53(77)61-41(50(74)67-48)9-5-6-22-57/h3-4,7-8,10-21,27,30,41-48,59,69,71-72H,5-6,9,22-26,28-29,57H2,1-2H3,(H2,58,73)(H,60,70)(H,61,77)(H,62,78)(H,63,79)(H,64,76)(H,65,75)(H,66,80)(H,67,74)/t30-,41+,42-,43+,44+,45-,46-,47+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50095116

((R)-1-(3-Amino-propyl)-6-(1H-indol-3-ylmethyl)-10-...)Show SMILES NCCCC1CCCN2C1C(=O)N(Cc1cccc3ccccc13)CCS[C@H](Cc1c[nH]c3ccccc13)C2=O Show InChI InChI=1S/C33H38N4O2S/c34-16-6-11-24-12-7-17-37-31(24)33(39)36(22-25-10-5-9-23-8-1-2-13-27(23)25)18-19-40-30(32(37)38)20-26-21-35-29-15-4-3-14-28(26)29/h1-5,8-10,13-15,21,24,30-31,35H,6-7,11-12,16-20,22,34H2/t24?,30-,31?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for somatostatin 4 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) |
Bioorg Med Chem Lett 10: 2731-3 (2000)
BindingDB Entry DOI: 10.7270/Q2QC02RM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50095116

((R)-1-(3-Amino-propyl)-6-(1H-indol-3-ylmethyl)-10-...)Show SMILES NCCCC1CCCN2C1C(=O)N(Cc1cccc3ccccc13)CCS[C@H](Cc1c[nH]c3ccccc13)C2=O Show InChI InChI=1S/C33H38N4O2S/c34-16-6-11-24-12-7-17-37-31(24)33(39)36(22-25-10-5-9-23-8-1-2-13-27(23)25)18-19-40-30(32(37)38)20-26-21-35-29-15-4-3-14-28(26)29/h1-5,8-10,13-15,21,24,30-31,35H,6-7,11-12,16-20,22,34H2/t24?,30-,31?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for somatostatin 5 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) |
Bioorg Med Chem Lett 10: 2731-3 (2000)
BindingDB Entry DOI: 10.7270/Q2QC02RM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50095115

((1R,9R)-5-(4-Amino-butyl)-2-(1H-indol-3-ylmethyl)-...)Show SMILES NCCCC[C@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)SCCN(Cc2cccc3ccccc23)C1=O |r| Show InChI InChI=1S/C30H34N4O2S/c31-15-6-5-14-27-30(36)34(20-22-10-7-9-21-8-1-2-11-24(21)22)16-17-37-28(29(35)33-27)18-23-19-32-26-13-4-3-12-25(23)26/h1-4,7-13,19,27-28,32H,5-6,14-18,20,31H2,(H,33,35)/t27-,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for somatostatin 5 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) |
Bioorg Med Chem Lett 10: 2731-3 (2000)
BindingDB Entry DOI: 10.7270/Q2QC02RM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311424
(7-((3',4'-difluorobiphenyl-4-ylsulfonyl)methyl)-2,...)Show SMILES Fc1ccc(cc1F)-c1ccc(cc1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C23H21F2NO2S/c24-22-8-5-19(14-23(22)25)17-3-6-21(7-4-17)29(27,28)15-16-1-2-18-9-11-26-12-10-20(18)13-16/h1-8,13-14,26H,9-12,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50344942
(CHEMBL532 | E-MYCIN E | ERYTHROMYCIN | ERYTHROMYCI...)Show SMILES CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O |r| Show InChI InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 25 to 30 mins by time dependent inhibition a... |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254851
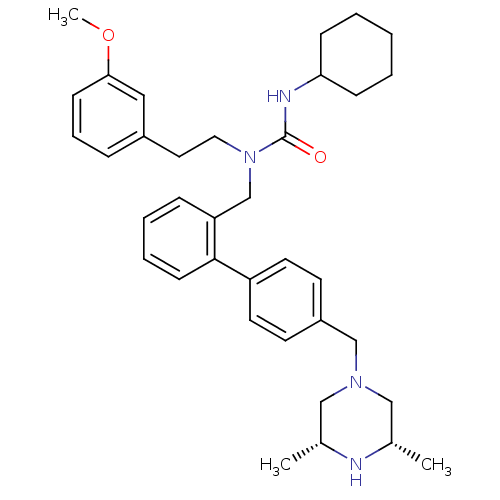
(3-Cyclohexyl-1-[4'-((3R,5S)-3,5-dimethyl-piperazin...)Show SMILES COc1cccc(CCN(Cc2ccccc2-c2ccc(CN3C[C@H](C)N[C@H](C)C3)cc2)C(=O)NC2CCCCC2)c1 |r| Show InChI InChI=1S/C36H48N4O2/c1-27-23-39(24-28(2)37-27)25-30-16-18-31(19-17-30)35-15-8-7-11-32(35)26-40(36(41)38-33-12-5-4-6-13-33)21-20-29-10-9-14-34(22-29)42-3/h7-11,14-19,22,27-28,33,37H,4-6,12-13,20-21,23-26H2,1-3H3,(H,38,41)/t27-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311425
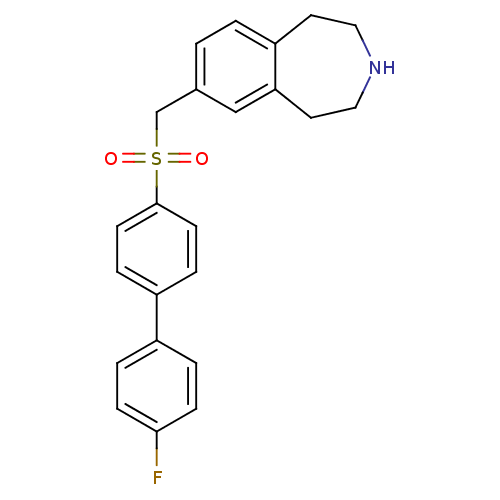
(7-((4'-fluorobiphenyl-4-ylsulfonyl)methyl)-2,3,4,5...)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C23H22FNO2S/c24-22-7-3-18(4-8-22)19-5-9-23(10-6-19)28(26,27)16-17-1-2-20-11-13-25-14-12-21(20)15-17/h1-10,15,25H,11-14,16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311414
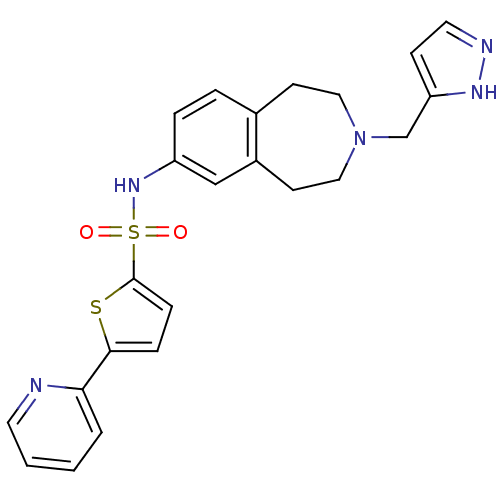
(CHEMBL1078492 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(s1)-c1ccccn1 Show InChI InChI=1S/C23H23N5O2S2/c29-32(30,23-7-6-22(31-23)21-3-1-2-11-24-21)27-19-5-4-17-9-13-28(14-10-18(17)15-19)16-20-8-12-25-26-20/h1-8,11-12,15,27H,9-10,13-14,16H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311413
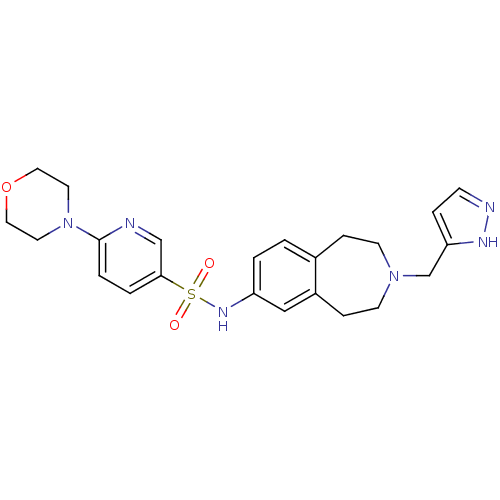
(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50095116

((R)-1-(3-Amino-propyl)-6-(1H-indol-3-ylmethyl)-10-...)Show SMILES NCCCC1CCCN2C1C(=O)N(Cc1cccc3ccccc13)CCS[C@H](Cc1c[nH]c3ccccc13)C2=O Show InChI InChI=1S/C33H38N4O2S/c34-16-6-11-24-12-7-17-37-31(24)33(39)36(22-25-10-5-9-23-8-1-2-13-27(23)25)18-19-40-30(32(37)38)20-26-21-35-29-15-4-3-14-28(26)29/h1-5,8-10,13-15,21,24,30-31,35H,6-7,11-12,16-20,22,34H2/t24?,30-,31?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for somatostatin 2 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) |
Bioorg Med Chem Lett 10: 2731-3 (2000)
BindingDB Entry DOI: 10.7270/Q2QC02RM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311419

(CHEMBL1078464 | N-(4-fluorophenyl)-5-((2,3,4,5-tet...)Show SMILES Fc1ccc(Nc2ccc(cn2)S(=O)(=O)Cc2ccc3CCNCCc3c2)cc1 Show InChI InChI=1S/C22H22FN3O2S/c23-19-3-5-20(6-4-19)26-22-8-7-21(14-25-22)29(27,28)15-16-1-2-17-9-11-24-12-10-18(17)13-16/h1-8,13-14,24H,9-12,15H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50450804

(CHEMBL123190)Show SMILES O=C1[C@H](Cc2ccc(OCc3ccccc3)cc2)N(CCCCc2ccccc2)C(=O)N1CCN1CCCC1 Show InChI InChI=1S/C33H39N3O3/c37-32-31(25-28-16-18-30(19-17-28)39-26-29-14-5-2-6-15-29)35(22-8-7-13-27-11-3-1-4-12-27)33(38)36(32)24-23-34-20-9-10-21-34/h1-6,11-12,14-19,31H,7-10,13,20-26H2/t31-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Cambridge Chemistry Laboratory
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of specific [125I]Tyr11-SRIF binding to human recombinant SST5 (somatostatin) receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 8: 3609-14 (1999)
BindingDB Entry DOI: 10.7270/Q2V12690 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50450803

(CHEMBL332512)Show SMILES O=C1[C@H](Cc2ccc(OCc3ccccc3)cc2)N(CCC#Cc2ccccc2)C(=O)N1CCN1CCCC1 Show InChI InChI=1S/C33H35N3O3/c37-32-31(25-28-16-18-30(19-17-28)39-26-29-14-5-2-6-15-29)35(22-8-7-13-27-11-3-1-4-12-27)33(38)36(32)24-23-34-20-9-10-21-34/h1-6,11-12,14-19,31H,8-10,20-26H2/t31-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Cambridge Chemistry Laboratory
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of specific [125I]Tyr11-SRIF binding to human recombinant SST5 (somatostatin) receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 8: 3609-14 (1999)
BindingDB Entry DOI: 10.7270/Q2V12690 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50450806
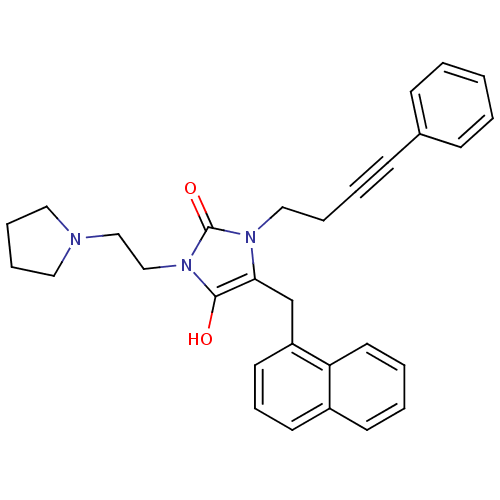
(CHEMBL324511)Show SMILES O=C1[C@H](Cc2cccc3ccccc23)N(CCC#Cc2ccccc2)C(=O)N1CCN1CCCC1 Show InChI InChI=1S/C30H31N3O2/c34-29-28(23-26-16-10-15-25-14-4-5-17-27(25)26)32(20-7-6-13-24-11-2-1-3-12-24)30(35)33(29)22-21-31-18-8-9-19-31/h1-5,10-12,14-17,28H,7-9,18-23H2/t28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Cambridge Chemistry Laboratory
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of specific [125I]Tyr11-SRIF binding to human recombinant SST5 (somatostatin) receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 8: 3609-14 (1999)
BindingDB Entry DOI: 10.7270/Q2V12690 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254851

(3-Cyclohexyl-1-[4'-((3R,5S)-3,5-dimethyl-piperazin...)Show SMILES COc1cccc(CCN(Cc2ccccc2-c2ccc(CN3C[C@H](C)N[C@H](C)C3)cc2)C(=O)NC2CCCCC2)c1 |r| Show InChI InChI=1S/C36H48N4O2/c1-27-23-39(24-28(2)37-27)25-30-16-18-31(19-17-30)35-15-8-7-11-32(35)26-40(36(41)38-33-12-5-4-6-13-33)21-20-29-10-9-14-34(22-29)42-3/h7-11,14-19,22,27-28,33,37H,4-6,12-13,20-21,23-26H2,1-3H3,(H,38,41)/t27-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311414

(CHEMBL1078492 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(s1)-c1ccccn1 Show InChI InChI=1S/C23H23N5O2S2/c29-32(30,23-7-6-22(31-23)21-3-1-2-11-24-21)27-19-5-4-17-9-13-28(14-10-18(17)15-19)16-20-8-12-25-26-20/h1-8,11-12,15,27H,9-10,13-14,16H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50095116

((R)-1-(3-Amino-propyl)-6-(1H-indol-3-ylmethyl)-10-...)Show SMILES NCCCC1CCCN2C1C(=O)N(Cc1cccc3ccccc13)CCS[C@H](Cc1c[nH]c3ccccc13)C2=O Show InChI InChI=1S/C33H38N4O2S/c34-16-6-11-24-12-7-17-37-31(24)33(39)36(22-25-10-5-9-23-8-1-2-13-27(23)25)18-19-40-30(32(37)38)20-26-21-35-29-15-4-3-14-28(26)29/h1-5,8-10,13-15,21,24,30-31,35H,6-7,11-12,16-20,22,34H2/t24?,30-,31?/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 407 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for somatostatin 1 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) |
Bioorg Med Chem Lett 10: 2731-3 (2000)
BindingDB Entry DOI: 10.7270/Q2QC02RM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311415
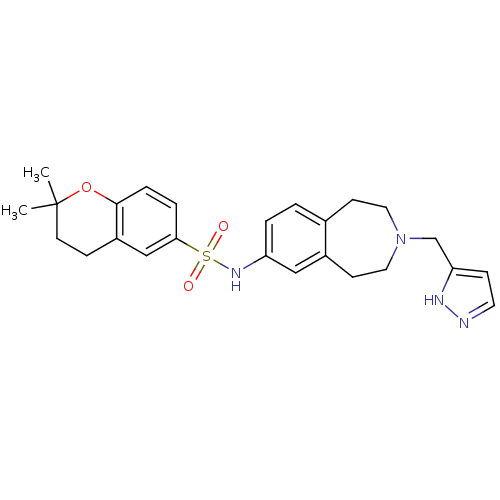
(CHEMBL1078490 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CC1(C)CCc2cc(ccc2O1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C25H30N4O3S/c1-25(2)11-7-20-16-23(5-6-24(20)32-25)33(30,31)28-21-4-3-18-9-13-29(14-10-19(18)15-21)17-22-8-12-26-27-22/h3-6,8,12,15-16,28H,7,9-11,13-14,17H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254968
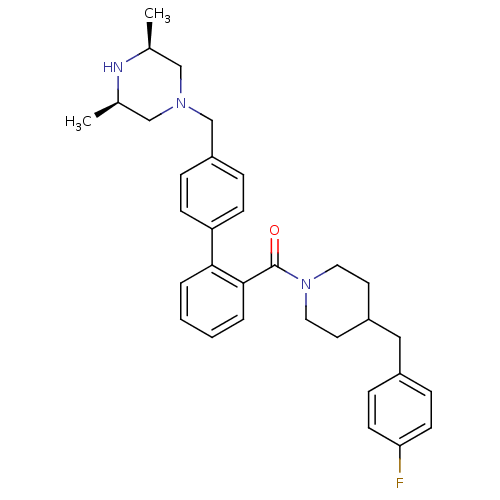
(CHEMBL479418 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(Cc3ccc(F)cc3)CC2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C32H38FN3O/c1-23-20-35(21-24(2)34-23)22-27-7-11-28(12-8-27)30-5-3-4-6-31(30)32(37)36-17-15-26(16-18-36)19-25-9-13-29(33)14-10-25/h3-14,23-24,26,34H,15-22H2,1-2H3/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50450805

(CHEMBL332786)Show SMILES COc1ccc(CCCCN2[C@@H](Cc3ccc(OCc4ccccc4)cc3)C(=O)N(CCN3CCCC3)C2=O)cc1 Show InChI InChI=1S/C34H41N3O4/c1-40-30-16-12-27(13-17-30)9-5-6-22-36-32(33(38)37(34(36)39)24-23-35-20-7-8-21-35)25-28-14-18-31(19-15-28)41-26-29-10-3-2-4-11-29/h2-4,10-19,32H,5-9,20-26H2,1H3/t32-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Cambridge Chemistry Laboratory
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of specific [125I]Tyr11-SRIF binding to human recombinant SST5 (somatostatin) receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 8: 3609-14 (1999)
BindingDB Entry DOI: 10.7270/Q2V12690 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50095115

((1R,9R)-5-(4-Amino-butyl)-2-(1H-indol-3-ylmethyl)-...)Show SMILES NCCCC[C@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)SCCN(Cc2cccc3ccccc23)C1=O |r| Show InChI InChI=1S/C30H34N4O2S/c31-15-6-5-14-27-30(36)34(20-22-10-7-9-21-8-1-2-11-24(21)22)16-17-37-28(29(35)33-27)18-23-19-32-26-13-4-3-12-25(23)26/h1-4,7-13,19,27-28,32H,5-6,14-18,20,31H2,(H,33,35)/t27-,28-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for somatostatin 1 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) |
Bioorg Med Chem Lett 10: 2731-3 (2000)
BindingDB Entry DOI: 10.7270/Q2QC02RM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254968

(CHEMBL479418 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(Cc3ccc(F)cc3)CC2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C32H38FN3O/c1-23-20-35(21-24(2)34-23)22-27-7-11-28(12-8-27)30-5-3-4-6-31(30)32(37)36-17-15-26(16-18-36)19-25-9-13-29(33)14-10-25/h3-14,23-24,26,34H,15-22H2,1-2H3/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311423
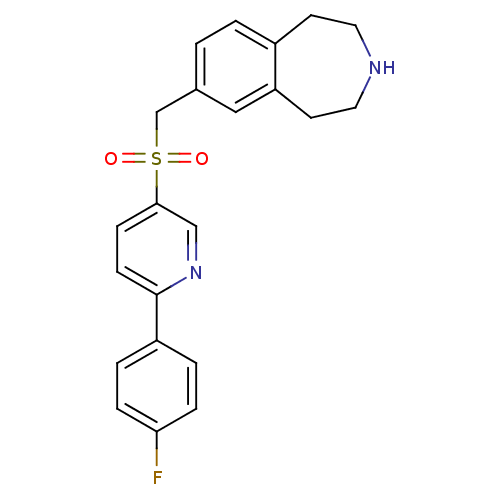
(7-((6-(4-fluorophenyl)pyridin-3-ylsulfonyl)methyl)...)Show SMILES Fc1ccc(cc1)-c1ccc(cn1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C22H21FN2O2S/c23-20-5-3-18(4-6-20)22-8-7-21(14-25-22)28(26,27)15-16-1-2-17-9-11-24-12-10-19(17)13-16/h1-8,13-14,24H,9-12,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311412
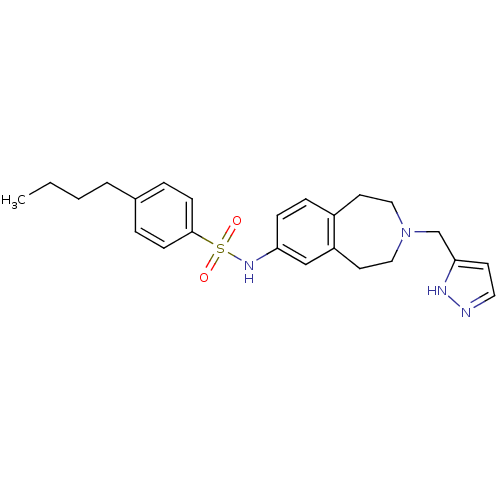
(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311412

(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 using diethoxyfluorescein as substrate |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254931
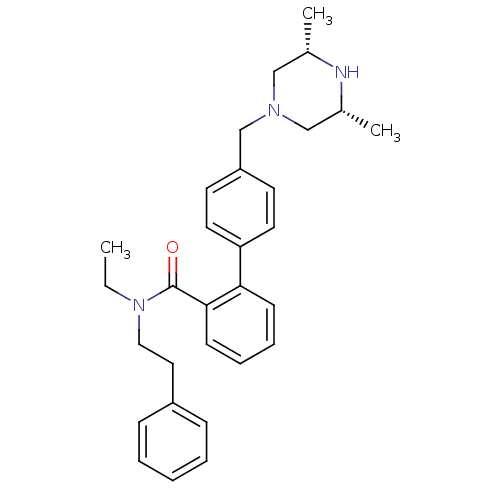
(4'-((3R,5S)-3,5-Dimethyl-piperazin-1-ylmethyl)-bip...)Show SMILES CCN(CCc1ccccc1)C(=O)c1ccccc1-c1ccc(CN2C[C@H](C)N[C@H](C)C2)cc1 |r| Show InChI InChI=1S/C30H37N3O/c1-4-33(19-18-25-10-6-5-7-11-25)30(34)29-13-9-8-12-28(29)27-16-14-26(15-17-27)22-32-20-23(2)31-24(3)21-32/h5-17,23-24,31H,4,18-22H2,1-3H3/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50095115

((1R,9R)-5-(4-Amino-butyl)-2-(1H-indol-3-ylmethyl)-...)Show SMILES NCCCC[C@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)SCCN(Cc2cccc3ccccc23)C1=O |r| Show InChI InChI=1S/C30H34N4O2S/c31-15-6-5-14-27-30(36)34(20-22-10-7-9-21-8-1-2-11-24(21)22)16-17-37-28(29(35)33-27)18-23-19-32-26-13-4-3-12-25(23)26/h1-4,7-13,19,27-28,32H,5-6,14-18,20,31H2,(H,33,35)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for somatostatin 4 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) |
Bioorg Med Chem Lett 10: 2731-3 (2000)
BindingDB Entry DOI: 10.7270/Q2QC02RM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311420
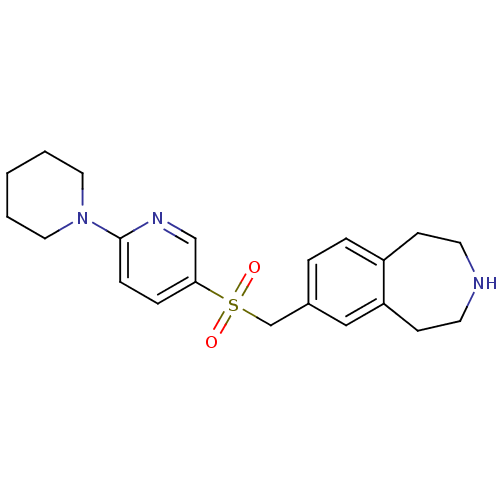
(7-((6-(piperidin-1-yl)pyridin-3-ylsulfonyl)methyl)...)Show SMILES O=S(=O)(Cc1ccc2CCNCCc2c1)c1ccc(nc1)N1CCCCC1 Show InChI InChI=1S/C21H27N3O2S/c25-27(26,16-17-4-5-18-8-10-22-11-9-19(18)14-17)20-6-7-21(23-15-20)24-12-2-1-3-13-24/h4-7,14-15,22H,1-3,8-13,16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50095116

((R)-1-(3-Amino-propyl)-6-(1H-indol-3-ylmethyl)-10-...)Show SMILES NCCCC1CCCN2C1C(=O)N(Cc1cccc3ccccc13)CCS[C@H](Cc1c[nH]c3ccccc13)C2=O Show InChI InChI=1S/C33H38N4O2S/c34-16-6-11-24-12-7-17-37-31(24)33(39)36(22-25-10-5-9-23-8-1-2-13-27(23)25)18-19-40-30(32(37)38)20-26-21-35-29-15-4-3-14-28(26)29/h1-5,8-10,13-15,21,24,30-31,35H,6-7,11-12,16-20,22,34H2/t24?,30-,31?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for somatostatin 3 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) |
Bioorg Med Chem Lett 10: 2731-3 (2000)
BindingDB Entry DOI: 10.7270/Q2QC02RM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311416
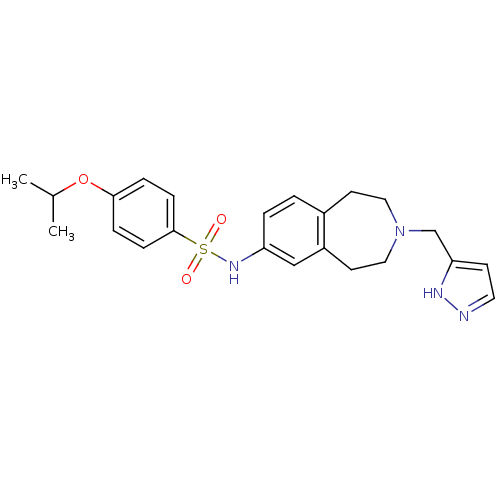
(CHEMBL1078208 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CC(C)Oc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C23H28N4O3S/c1-17(2)30-22-5-7-23(8-6-22)31(28,29)26-20-4-3-18-10-13-27(14-11-19(18)15-20)16-21-9-12-24-25-21/h3-9,12,15,17,26H,10-11,13-14,16H2,1-2H3,(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254892
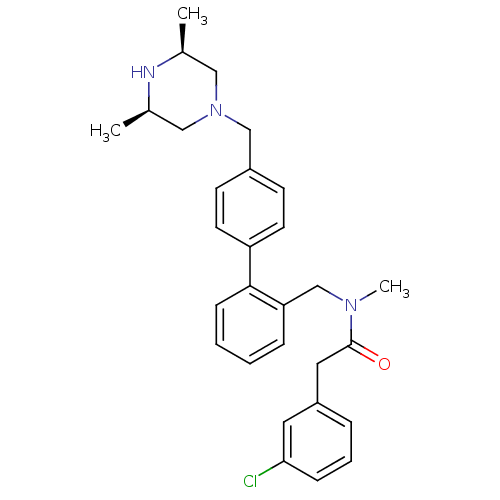
(2-(3-Chloro-phenyl)-N-[4'-((3R,5S)-3,5-dimethyl-pi...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)Cc2cccc(Cl)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-21-17-33(18-22(2)31-21)19-23-11-13-25(14-12-23)28-10-5-4-8-26(28)20-32(3)29(34)16-24-7-6-9-27(30)15-24/h4-15,21-22,31H,16-20H2,1-3H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50247157
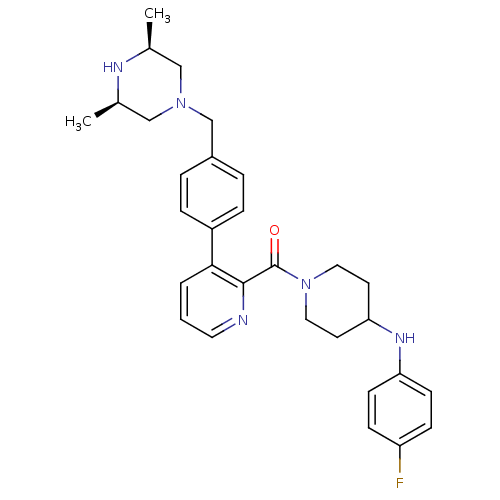
((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 25 to 30 mins by time dependent inhibition a... |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50450802

(CHEMBL120607)Show SMILES NCCCC[C@@H](NC(=O)CC1SC(N([C@@H](Cc2ccc(cc2)-c2ccccc2)C(N)=O)C1=O)c1ccccc1)C(N)=O Show InChI InChI=1S/C32H37N5O4S/c33-18-8-7-13-25(29(34)39)36-28(38)20-27-31(41)37(32(42-27)24-11-5-2-6-12-24)26(30(35)40)19-21-14-16-23(17-15-21)22-9-3-1-4-10-22/h1-6,9-12,14-17,25-27,32H,7-8,13,18-20,33H2,(H2,34,39)(H2,35,40)(H,36,38)/t25-,26+,27?,32?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Cambridge Chemistry Laboratory
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of specific [125I]Tyr11-SRIF binding to human recombinant SST5 (somatostatin) receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 8: 3609-14 (1999)
BindingDB Entry DOI: 10.7270/Q2V12690 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254892

(2-(3-Chloro-phenyl)-N-[4'-((3R,5S)-3,5-dimethyl-pi...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)Cc2cccc(Cl)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-21-17-33(18-22(2)31-21)19-23-11-13-25(14-12-23)28-10-5-4-8-26(28)20-32(3)29(34)16-24-7-6-9-27(30)15-24/h4-15,21-22,31H,16-20H2,1-3H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254931

(4'-((3R,5S)-3,5-Dimethyl-piperazin-1-ylmethyl)-bip...)Show SMILES CCN(CCc1ccccc1)C(=O)c1ccccc1-c1ccc(CN2C[C@H](C)N[C@H](C)C2)cc1 |r| Show InChI InChI=1S/C30H37N3O/c1-4-33(19-18-25-10-6-5-7-11-25)30(34)29-13-9-8-12-28(29)27-16-14-26(15-17-27)22-32-20-23(2)31-24(3)21-32/h5-17,23-24,31H,4,18-22H2,1-3H3/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50095115

((1R,9R)-5-(4-Amino-butyl)-2-(1H-indol-3-ylmethyl)-...)Show SMILES NCCCC[C@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)SCCN(Cc2cccc3ccccc23)C1=O |r| Show InChI InChI=1S/C30H34N4O2S/c31-15-6-5-14-27-30(36)34(20-22-10-7-9-21-8-1-2-11-24(21)22)16-17-37-28(29(35)33-27)18-23-19-32-26-13-4-3-12-25(23)26/h1-4,7-13,19,27-28,32H,5-6,14-18,20,31H2,(H,33,35)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for somatostatin 2 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) |
Bioorg Med Chem Lett 10: 2731-3 (2000)
BindingDB Entry DOI: 10.7270/Q2QC02RM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311421
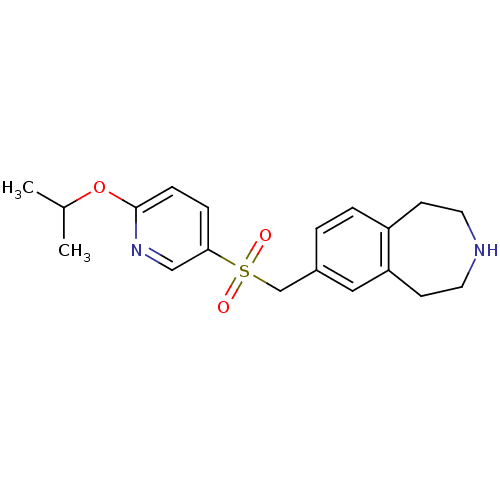
(7-((6-isopropoxypyridin-3-ylsulfonyl)methyl)-2,3,4...)Show InChI InChI=1S/C19H24N2O3S/c1-14(2)24-19-6-5-18(12-21-19)25(22,23)13-15-3-4-16-7-9-20-10-8-17(16)11-15/h3-6,11-12,14,20H,7-10,13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data