

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50095116 ((R)-1-(3-Amino-propyl)-6-(1H-indol-3-ylmethyl)-10-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration required for somatostatin 4 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) | Bioorg Med Chem Lett 10: 2731-3 (2000) BindingDB Entry DOI: 10.7270/Q2QC02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50095116 ((R)-1-(3-Amino-propyl)-6-(1H-indol-3-ylmethyl)-10-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration required for somatostatin 5 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) | Bioorg Med Chem Lett 10: 2731-3 (2000) BindingDB Entry DOI: 10.7270/Q2QC02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50095115 ((1R,9R)-5-(4-Amino-butyl)-2-(1H-indol-3-ylmethyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration required for somatostatin 5 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) | Bioorg Med Chem Lett 10: 2731-3 (2000) BindingDB Entry DOI: 10.7270/Q2QC02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311424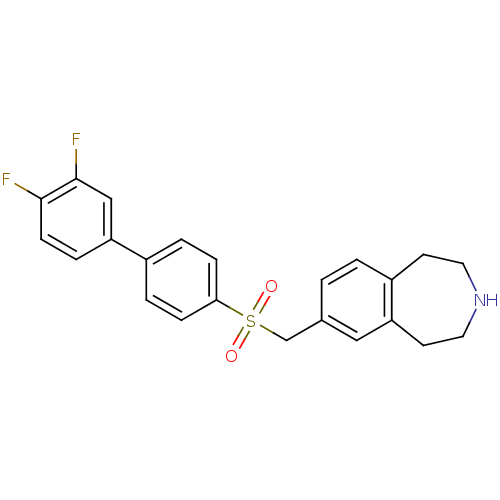 (7-((3',4'-difluorobiphenyl-4-ylsulfonyl)methyl)-2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50344942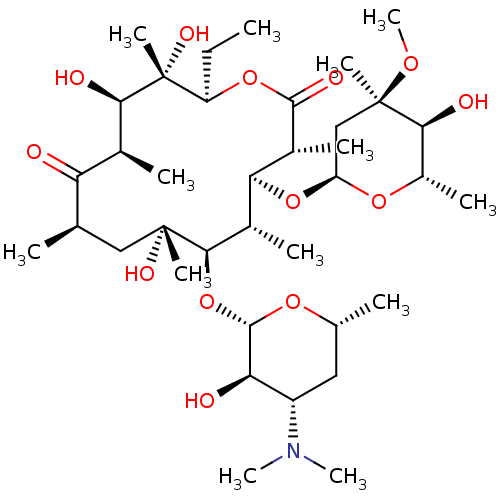 (CHEMBL532 | E-MYCIN E | ERYTHROMYCIN | ERYTHROMYCI...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 25 to 30 mins by time dependent inhibition a... | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254851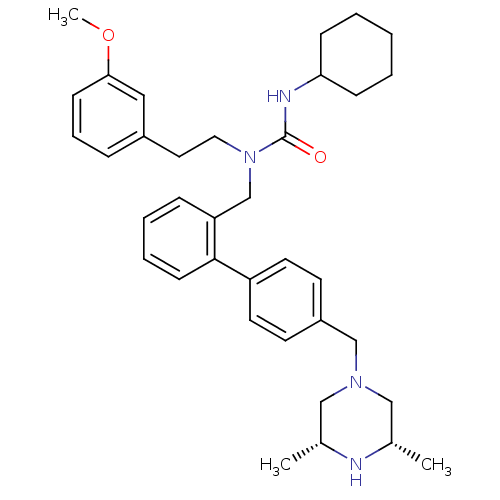 (3-Cyclohexyl-1-[4'-((3R,5S)-3,5-dimethyl-piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311425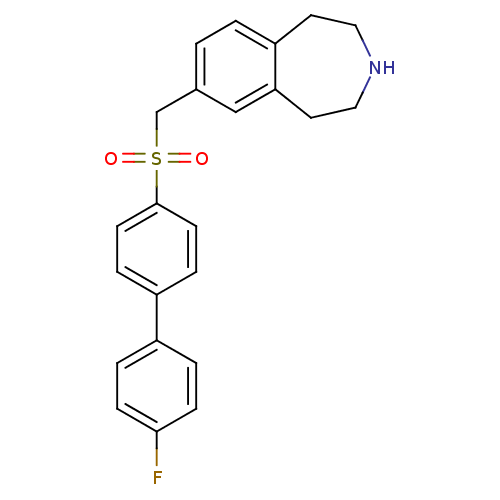 (7-((4'-fluorobiphenyl-4-ylsulfonyl)methyl)-2,3,4,5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311414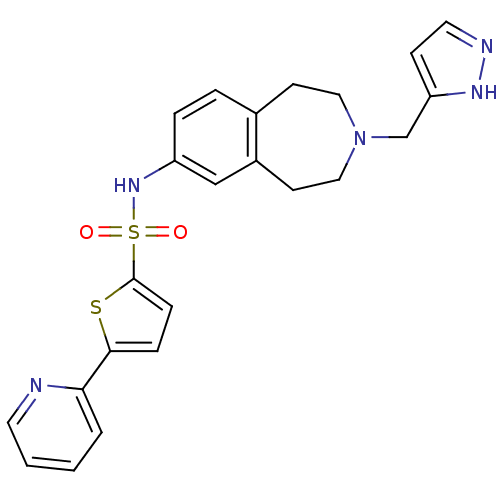 (CHEMBL1078492 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50311413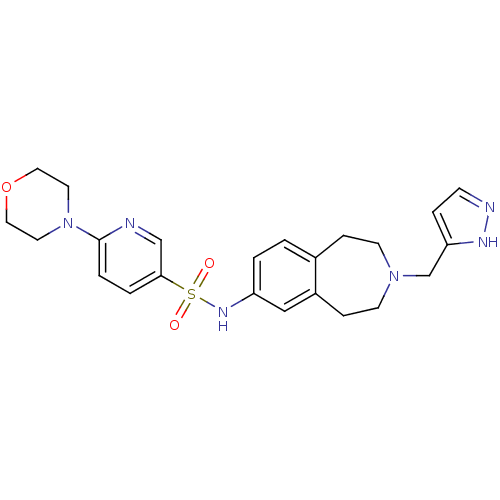 (CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C9 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50095116 ((R)-1-(3-Amino-propyl)-6-(1H-indol-3-ylmethyl)-10-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration required for somatostatin 2 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) | Bioorg Med Chem Lett 10: 2731-3 (2000) BindingDB Entry DOI: 10.7270/Q2QC02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311419 (CHEMBL1078464 | N-(4-fluorophenyl)-5-((2,3,4,5-tet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50450804 (CHEMBL123190) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Cambridge Chemistry Laboratory Curated by ChEMBL | Assay Description Compound was tested for inhibition of specific [125I]Tyr11-SRIF binding to human recombinant SST5 (somatostatin) receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 8: 3609-14 (1999) BindingDB Entry DOI: 10.7270/Q2V12690 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50450803 (CHEMBL332512) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Cambridge Chemistry Laboratory Curated by ChEMBL | Assay Description Compound was tested for inhibition of specific [125I]Tyr11-SRIF binding to human recombinant SST5 (somatostatin) receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 8: 3609-14 (1999) BindingDB Entry DOI: 10.7270/Q2V12690 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50450806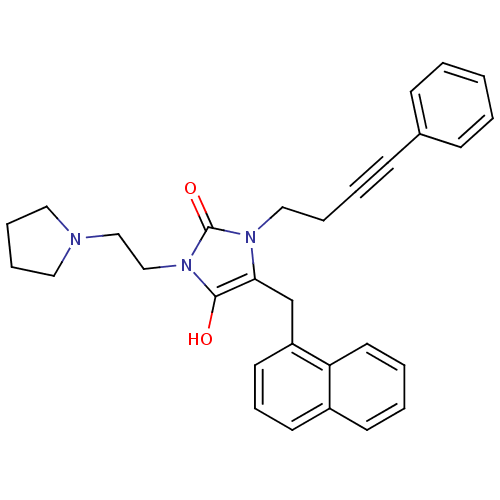 (CHEMBL324511) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Cambridge Chemistry Laboratory Curated by ChEMBL | Assay Description Compound was tested for inhibition of specific [125I]Tyr11-SRIF binding to human recombinant SST5 (somatostatin) receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 8: 3609-14 (1999) BindingDB Entry DOI: 10.7270/Q2V12690 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254851 (3-Cyclohexyl-1-[4'-((3R,5S)-3,5-dimethyl-piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50311414 (CHEMBL1078492 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C9 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50095116 ((R)-1-(3-Amino-propyl)-6-(1H-indol-3-ylmethyl)-10-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 407 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration required for somatostatin 1 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) | Bioorg Med Chem Lett 10: 2731-3 (2000) BindingDB Entry DOI: 10.7270/Q2QC02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311415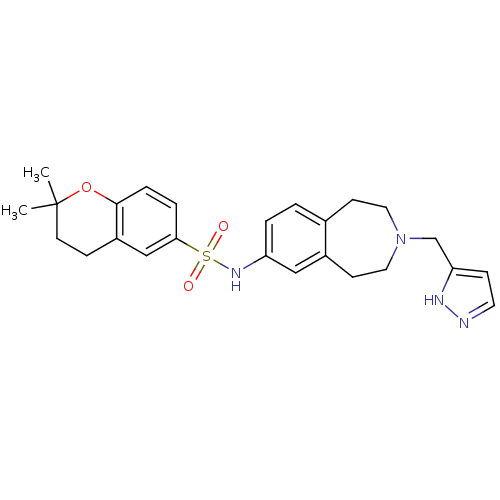 (CHEMBL1078490 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254968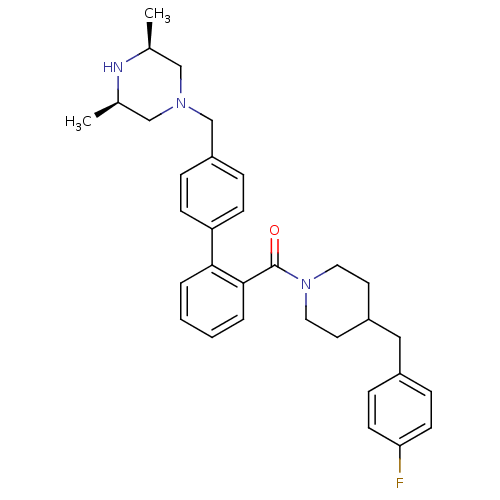 (CHEMBL479418 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50450805 (CHEMBL332786) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Cambridge Chemistry Laboratory Curated by ChEMBL | Assay Description Compound was tested for inhibition of specific [125I]Tyr11-SRIF binding to human recombinant SST5 (somatostatin) receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 8: 3609-14 (1999) BindingDB Entry DOI: 10.7270/Q2V12690 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50095115 ((1R,9R)-5-(4-Amino-butyl)-2-(1H-indol-3-ylmethyl)-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration required for somatostatin 1 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) | Bioorg Med Chem Lett 10: 2731-3 (2000) BindingDB Entry DOI: 10.7270/Q2QC02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254968 (CHEMBL479418 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311423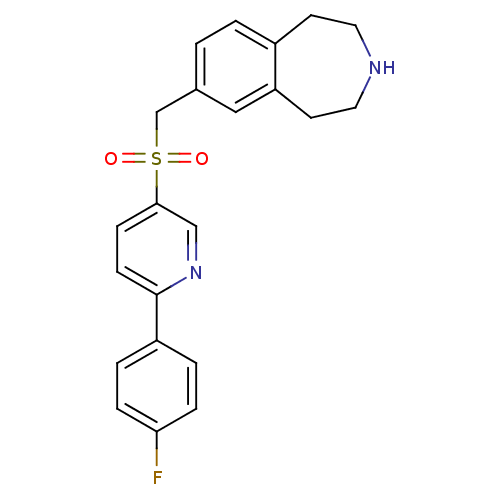 (7-((6-(4-fluorophenyl)pyridin-3-ylsulfonyl)methyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50311412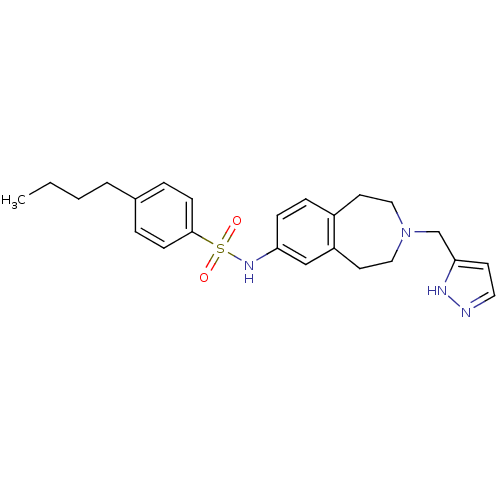 (CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C9 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50311412 (CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 using diethoxyfluorescein as substrate | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254931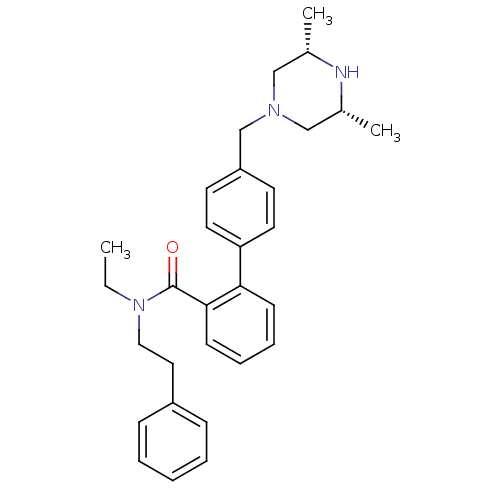 (4'-((3R,5S)-3,5-Dimethyl-piperazin-1-ylmethyl)-bip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50095115 ((1R,9R)-5-(4-Amino-butyl)-2-(1H-indol-3-ylmethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration required for somatostatin 4 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) | Bioorg Med Chem Lett 10: 2731-3 (2000) BindingDB Entry DOI: 10.7270/Q2QC02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311420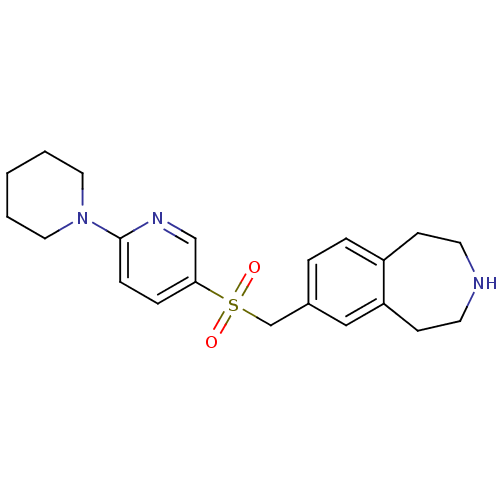 (7-((6-(piperidin-1-yl)pyridin-3-ylsulfonyl)methyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50095116 ((R)-1-(3-Amino-propyl)-6-(1H-indol-3-ylmethyl)-10-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration required for somatostatin 3 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) | Bioorg Med Chem Lett 10: 2731-3 (2000) BindingDB Entry DOI: 10.7270/Q2QC02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311416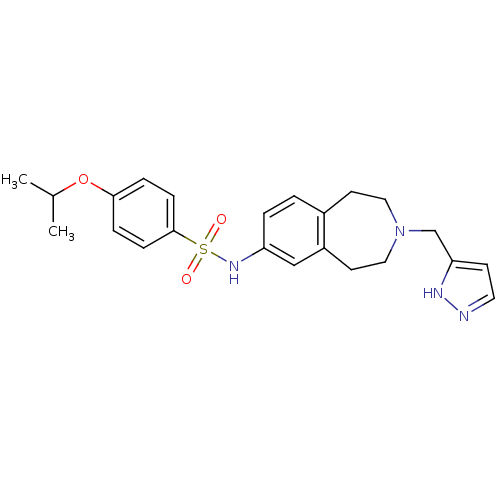 (CHEMBL1078208 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254892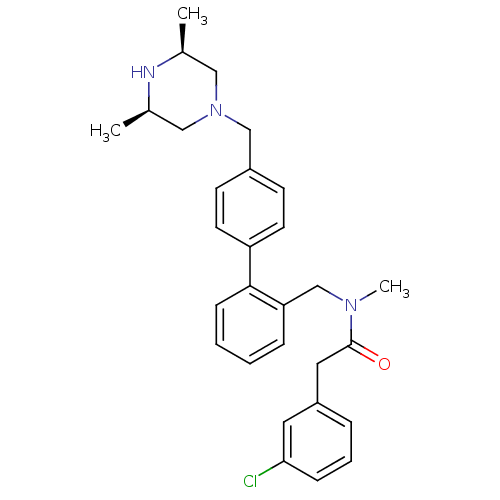 (2-(3-Chloro-phenyl)-N-[4'-((3R,5S)-3,5-dimethyl-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50247157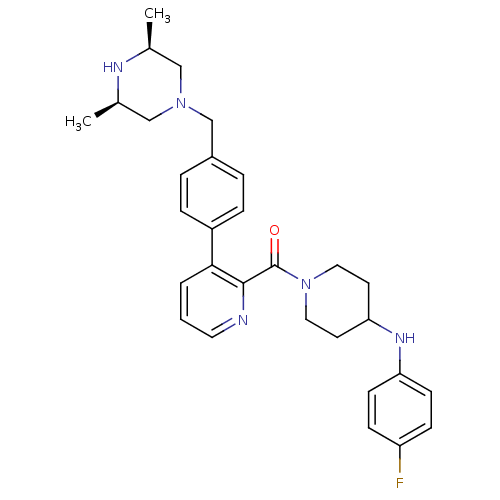 ((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 25 to 30 mins by time dependent inhibition a... | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50450802 (CHEMBL120607) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Cambridge Chemistry Laboratory Curated by ChEMBL | Assay Description Compound was tested for inhibition of specific [125I]Tyr11-SRIF binding to human recombinant SST5 (somatostatin) receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 8: 3609-14 (1999) BindingDB Entry DOI: 10.7270/Q2V12690 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254892 (2-(3-Chloro-phenyl)-N-[4'-((3R,5S)-3,5-dimethyl-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254931 (4'-((3R,5S)-3,5-Dimethyl-piperazin-1-ylmethyl)-bip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50095115 ((1R,9R)-5-(4-Amino-butyl)-2-(1H-indol-3-ylmethyl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration required for somatostatin 2 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) | Bioorg Med Chem Lett 10: 2731-3 (2000) BindingDB Entry DOI: 10.7270/Q2QC02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311421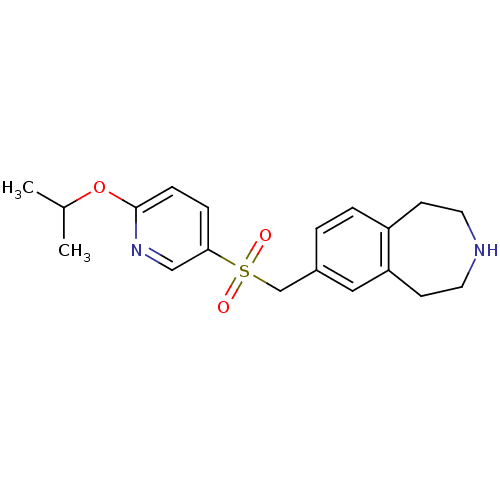 (7-((6-isopropoxypyridin-3-ylsulfonyl)methyl)-2,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311422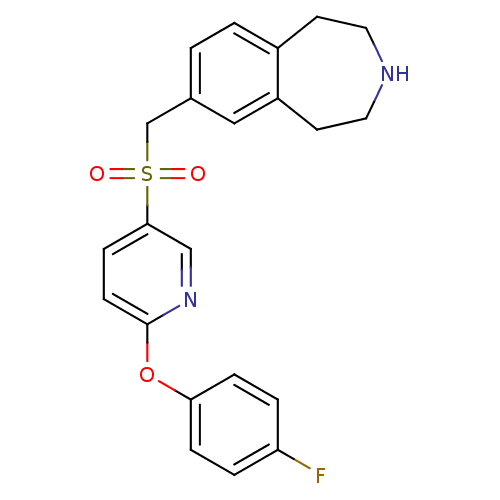 (7-((6-(4-fluorophenoxy)pyridin-3-ylsulfonyl)methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50311412 (CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 using 7-{3-(4-phenylpiperazin-1-ylmethyl)benzyl}resorufin as substrate | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50344942 (CHEMBL532 | E-MYCIN E | ERYTHROMYCIN | ERYTHROMYCI...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 0 to 5 mins by time dependent inhibition ass... | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50311417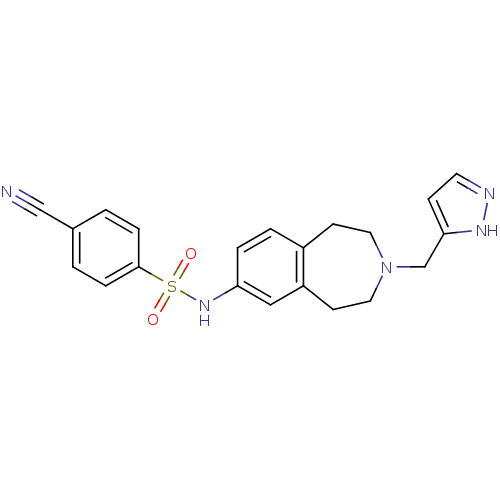 (CHEMBL1081860 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C9 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50311412 (CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254893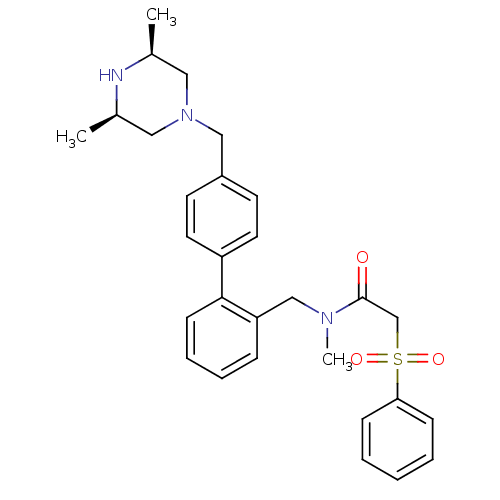 (2-Benzenesulfonyl-N-[4'-((3R,5S)-3,5-dimethyl-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50095115 ((1R,9R)-5-(4-Amino-butyl)-2-(1H-indol-3-ylmethyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration required for somatostatin 3 receptor in radioligand binding assay ([125I]-Tyr11-SRIF) | Bioorg Med Chem Lett 10: 2731-3 (2000) BindingDB Entry DOI: 10.7270/Q2QC02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254971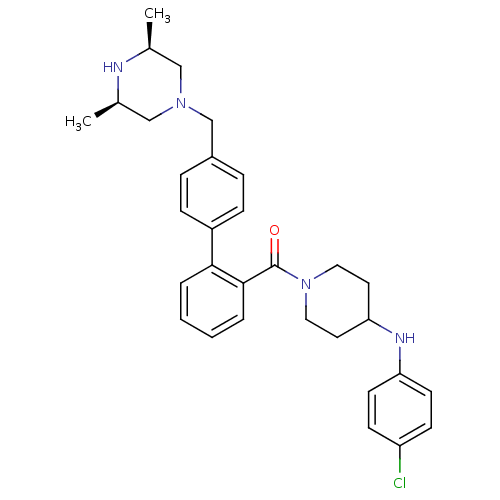 (CHEMBL481575 | [4-(4-Chloro-phenylamino)-piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254999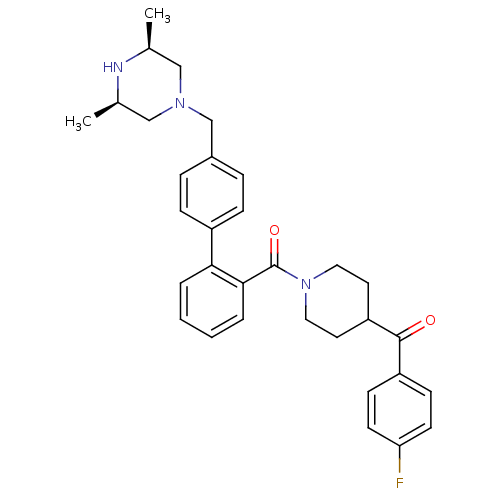 (CHEMBL480210 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292984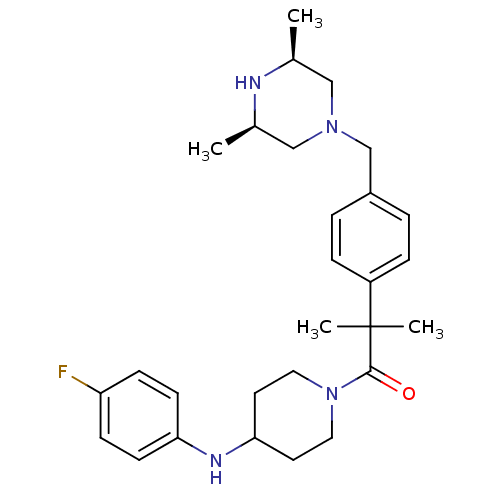 (1-[2-(4-([(3R,5S)-3,5-Dimethyl-1-piperazinyl]methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254999 (CHEMBL480210 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292981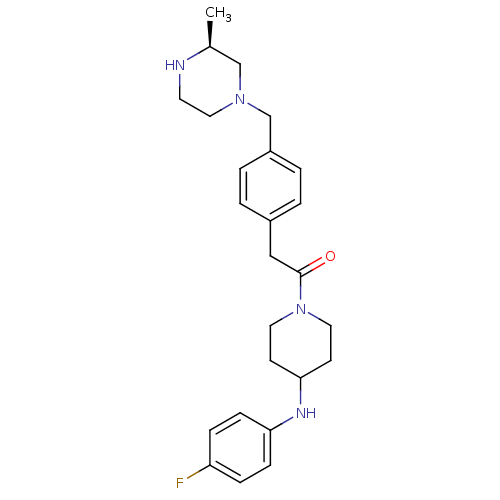 (CHEMBL489094 | N-(4-Fluorophenyl)-1-[(4-([(3S)-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254971 (CHEMBL481575 | [4-(4-Chloro-phenylamino)-piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 271 total ) | Next | Last >> |