 Found 390 hits with Last Name = 'jayawickreme' and Initial = 'c'
Found 390 hits with Last Name = 'jayawickreme' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Free fatty acid receptor 4
(Rattus norvegicus) | BDBM50044874
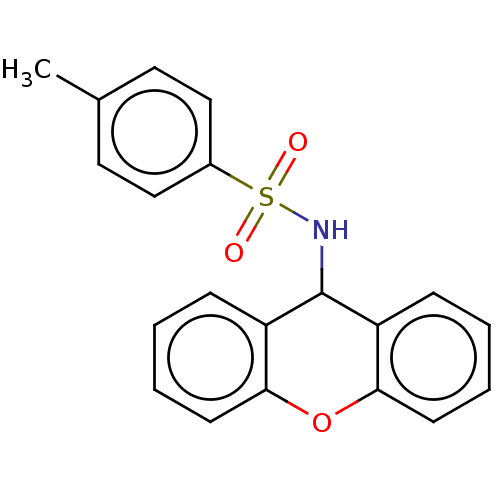
(CHEMBL3311302)Show InChI InChI=1S/C20H17NO3S/c1-14-10-12-15(13-11-14)25(22,23)21-20-16-6-2-4-8-18(16)24-19-9-5-3-7-17(19)20/h2-13,20-21H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat FFA4 receptor expressed in U2OS cells |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Mus musculus) | BDBM50044874

(CHEMBL3311302)Show InChI InChI=1S/C20H17NO3S/c1-14-10-12-15(13-11-14)25(22,23)21-20-16-6-2-4-8-18(16)24-19-9-5-3-7-17(19)20/h2-13,20-21H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of mouse FFA4 receptor expressed in U2OS cells |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159550

(CHEMBL3785336)Show SMILES COc1c(NC(=O)C(=O)c2ccc(OCCN3CCOCC3)c3ccccc23)cc(cc1NS(=O)(=O)CCCN(CCO)CCO)C(C)(C)C Show InChI InChI=1S/C36H50N4O9S/c1-36(2,3)26-24-30(34(47-4)31(25-26)38-50(45,46)23-7-12-39(13-18-41)14-19-42)37-35(44)33(43)29-10-11-32(28-9-6-5-8-27(28)29)49-22-17-40-15-20-48-21-16-40/h5-6,8-11,24-25,38,41-42H,7,12-23H2,1-4H3,(H,37,44) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159533

(CHEMBL3786653)Show SMILES COc1c(NC(=O)C(=O)c2ccc(OCCNC(=O)CN)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C28H34N4O7S/c1-28(2,3)17-14-21(26(38-4)22(15-17)32-40(5,36)37)31-27(35)25(34)20-10-11-23(19-9-7-6-8-18(19)20)39-13-12-30-24(33)16-29/h6-11,14-15,32H,12-13,16,29H2,1-5H3,(H,30,33)(H,31,35) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159545
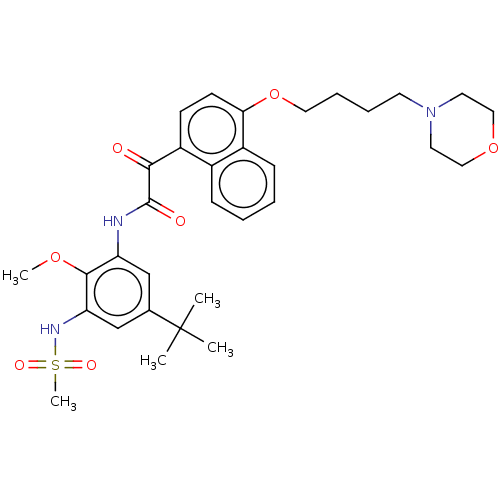
(CHEMBL3785828)Show SMILES COc1c(NC(=O)C(=O)c2ccc(OCCCCN3CCOCC3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C32H41N3O7S/c1-32(2,3)22-20-26(30(40-4)27(21-22)34-43(5,38)39)33-31(37)29(36)25-12-13-28(24-11-7-6-10-23(24)25)42-17-9-8-14-35-15-18-41-19-16-35/h6-7,10-13,20-21,34H,8-9,14-19H2,1-5H3,(H,33,37) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159548
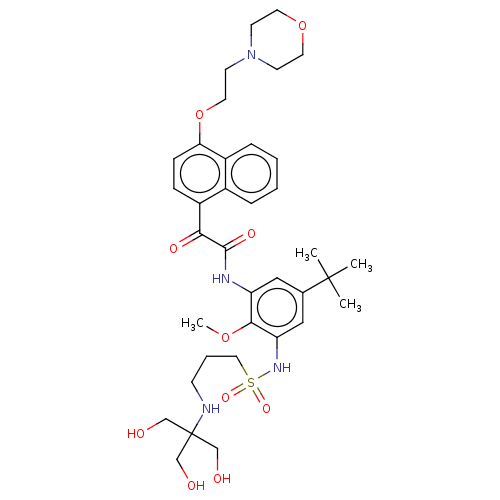
(CHEMBL3785750)Show SMILES COc1c(NC(=O)C(=O)c2ccc(OCCN3CCOCC3)c3ccccc23)cc(cc1NS(=O)(=O)CCCNC(CO)(CO)CO)C(C)(C)C Show InChI InChI=1S/C36H50N4O10S/c1-35(2,3)25-20-29(33(48-4)30(21-25)39-51(46,47)19-7-12-37-36(22-41,23-42)24-43)38-34(45)32(44)28-10-11-31(27-9-6-5-8-26(27)28)50-18-15-40-13-16-49-17-14-40/h5-6,8-11,20-21,37,39,41-43H,7,12-19,22-24H2,1-4H3,(H,38,45) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159554

(CHEMBL3787085)Show SMILES CCCS(=O)(=O)Nc1cc(cc(NC(=O)C(=O)c2ccc(OCCN3CCOCC3)c3ccccc23)c1OC)C(C)(C)C Show InChI InChI=1S/C32H41N3O7S/c1-6-19-43(38,39)34-27-21-22(32(2,3)4)20-26(30(27)40-5)33-31(37)29(36)25-11-12-28(24-10-8-7-9-23(24)25)42-18-15-35-13-16-41-17-14-35/h7-12,20-21,34H,6,13-19H2,1-5H3,(H,33,37) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50044874

(CHEMBL3311302)Show InChI InChI=1S/C20H17NO3S/c1-14-10-12-15(13-11-14)25(22,23)21-20-16-6-2-4-8-18(16)24-19-9-5-3-7-17(19)20/h2-13,20-21H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human FFA4 receptor expressed in U2OS cells |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159556
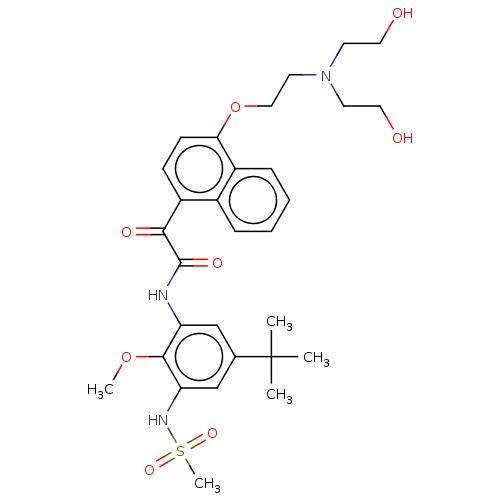
(CHEMBL3785219)Show SMILES COc1c(NC(=O)C(=O)c2ccc(OCCN(CCO)CCO)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C30H39N3O8S/c1-30(2,3)20-18-24(28(40-4)25(19-20)32-42(5,38)39)31-29(37)27(36)23-10-11-26(22-9-7-6-8-21(22)23)41-17-14-33(12-15-34)13-16-35/h6-11,18-19,32,34-35H,12-17H2,1-5H3,(H,31,37) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50323654
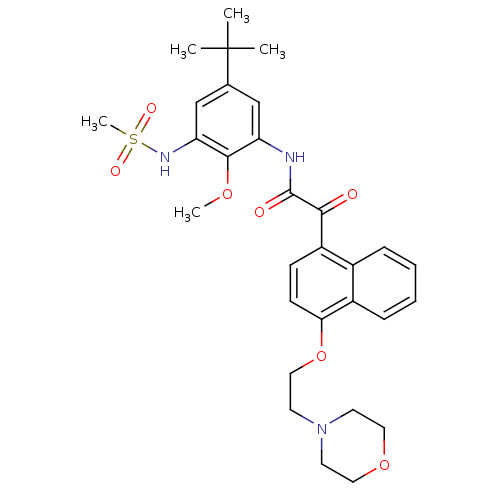
(CHEMBL1208829 | N-(5-tert-butyl-2-methoxy-3-(methy...)Show SMILES COc1c(NC(=O)C(=O)c2ccc(OCCN3CCOCC3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C30H37N3O7S/c1-30(2,3)20-18-24(28(38-4)25(19-20)32-41(5,36)37)31-29(35)27(34)23-10-11-26(22-9-7-6-8-21(22)23)40-17-14-33-12-15-39-16-13-33/h6-11,18-19,32H,12-17H2,1-5H3,(H,31,35) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159547
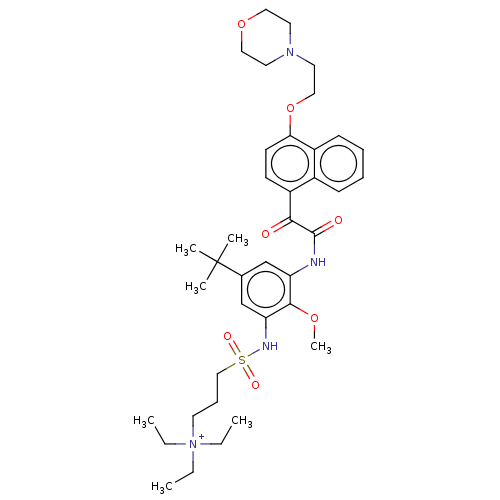
(CHEMBL3786659)Show SMILES [O-]C(=O)C(F)(F)F.CC[N+](CC)(CC)CCCS(=O)(=O)Nc1cc(cc(NC(=O)C(=O)c2ccc(OCCN3CCOCC3)c3ccccc23)c1OC)C(C)(C)C Show InChI InChI=1S/C38H54N4O7S.C2HF3O2/c1-8-42(9-2,10-3)21-13-25-50(45,46)40-33-27-28(38(4,5)6)26-32(36(33)47-7)39-37(44)35(43)31-16-17-34(30-15-12-11-14-29(30)31)49-24-20-41-18-22-48-23-19-41;3-2(4,5)1(6)7/h11-12,14-17,26-27,40H,8-10,13,18-25H2,1-7H3;(H,6,7) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159551

(CHEMBL3786730)Show SMILES [Cl-].COc1c(NC(=O)C(=O)c2ccc(OCCN3CCOCC3)c3ccccc23)cc(cc1NS(=O)(=O)C[N+]12CCN(CC1)CC2)C(C)(C)C Show InChI InChI=1S/C36H47N5O7S.ClH/c1-36(2,3)26-23-30(34(46-4)31(24-26)38-49(44,45)25-41-17-11-39(12-18-41)13-19-41)37-35(43)33(42)29-9-10-32(28-8-6-5-7-27(28)29)48-22-16-40-14-20-47-21-15-40;/h5-10,23-24,38H,11-22,25H2,1-4H3;1H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159553
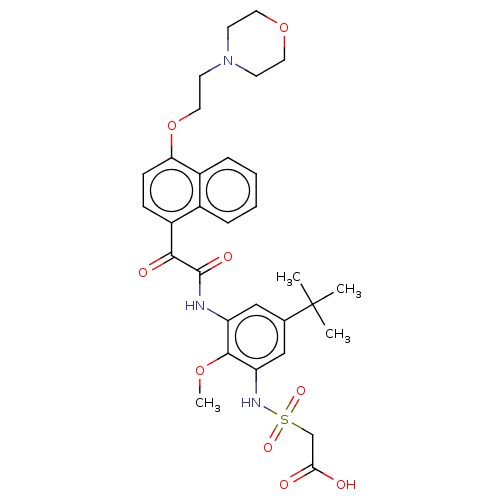
(CHEMBL3785530)Show SMILES COc1c(NC(=O)C(=O)c2ccc(OCCN3CCOCC3)c3ccccc23)cc(cc1NS(=O)(=O)CC(O)=O)C(C)(C)C Show InChI InChI=1S/C31H37N3O9S/c1-31(2,3)20-17-24(29(41-4)25(18-20)33-44(39,40)19-27(35)36)32-30(38)28(37)23-9-10-26(22-8-6-5-7-21(22)23)43-16-13-34-11-14-42-15-12-34/h5-10,17-18,33H,11-16,19H2,1-4H3,(H,32,38)(H,35,36) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50414955

(CHEMBL576138)Show SMILES CN(C(=O)c1c(C)onc1-c1ccccc1Cl)c1ccc(C)c(Cl)c1 Show InChI InChI=1S/C19H16Cl2N2O2/c1-11-8-9-13(10-16(11)21)23(3)19(24)17-12(2)25-22-18(17)14-6-4-5-7-15(14)20/h4-10H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 52: 7962-5 (2009)
Article DOI: 10.1021/jm901434t
BindingDB Entry DOI: 10.7270/Q2Z89DPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50414954

(CHEMBL575966)Show InChI InChI=1S/C18H14Cl2N2O2/c1-11-16(17(21-24-11)14-5-3-4-6-15(14)20)18(23)22(2)13-9-7-12(19)8-10-13/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 52: 7962-5 (2009)
Article DOI: 10.1021/jm901434t
BindingDB Entry DOI: 10.7270/Q2Z89DPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50414954

(CHEMBL575966)Show InChI InChI=1S/C18H14Cl2N2O2/c1-11-16(17(21-24-11)14-5-3-4-6-15(14)20)18(23)22(2)13-9-7-12(19)8-10-13/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 20: 1363-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.003
BindingDB Entry DOI: 10.7270/Q2028SS5 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159529
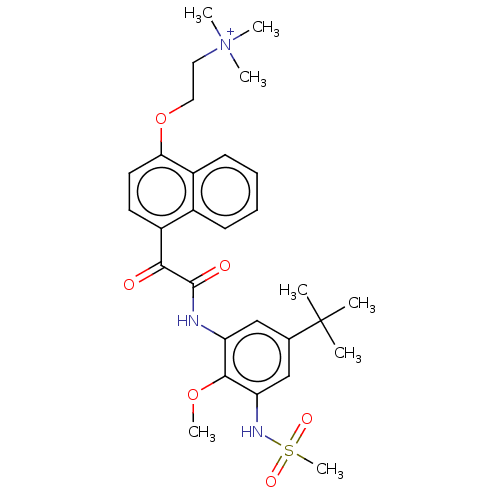
(CHEMBL3787449)Show SMILES [O-]C(=O)C(F)(F)F.COc1c(NC(=O)C(=O)c2ccc(OCC[N+](C)(C)C)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C29H37N3O6S.C2HF3O2/c1-29(2,3)19-17-23(27(37-7)24(18-19)31-39(8,35)36)30-28(34)26(33)22-13-14-25(38-16-15-32(4,5)6)21-12-10-9-11-20(21)22;3-2(4,5)1(6)7/h9-14,17-18,31H,15-16H2,1-8H3;(H,6,7) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159555

(CHEMBL3787277)Show SMILES COc1c(NC(=O)C(=O)c2ccc(OCCN3CCC(CC3)C(O)=O)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C32H39N3O8S/c1-32(2,3)21-18-25(29(42-4)26(19-21)34-44(5,40)41)33-30(37)28(36)24-10-11-27(23-9-7-6-8-22(23)24)43-17-16-35-14-12-20(13-15-35)31(38)39/h6-11,18-20,34H,12-17H2,1-5H3,(H,33,37)(H,38,39) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159546
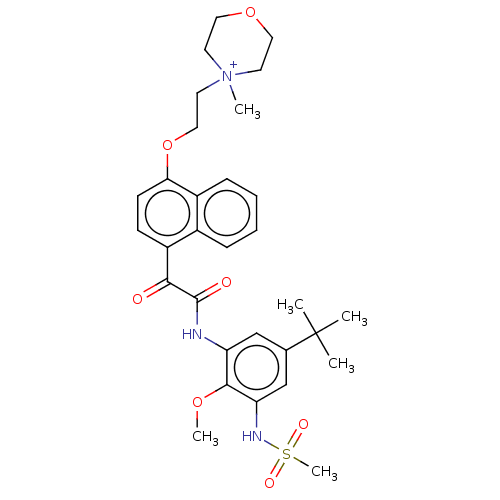
(CHEMBL3786591)Show SMILES [O-]C(=O)C(F)(F)F.COc1c(NC(=O)C(=O)c2ccc(OCC[N+]3(C)CCOCC3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C31H39N3O7S.C2HF3O2/c1-31(2,3)21-19-25(29(39-5)26(20-21)33-42(6,37)38)32-30(36)28(35)24-11-12-27(23-10-8-7-9-22(23)24)41-18-15-34(4)13-16-40-17-14-34;3-2(4,5)1(6)7/h7-12,19-20,33H,13-18H2,1-6H3;(H,6,7) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159532
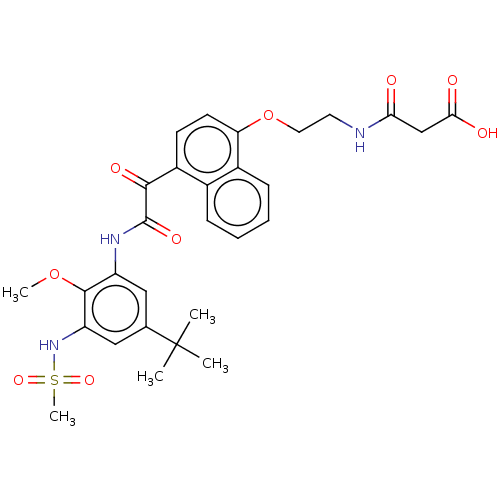
(CHEMBL3786485)Show SMILES COc1c(NC(=O)C(=O)c2ccc(OCCNC(=O)CC(O)=O)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C29H33N3O9S/c1-29(2,3)17-14-21(27(40-4)22(15-17)32-42(5,38)39)31-28(37)26(36)20-10-11-23(19-9-7-6-8-18(19)20)41-13-12-30-24(33)16-25(34)35/h6-11,14-15,32H,12-13,16H2,1-5H3,(H,30,33)(H,31,37)(H,34,35) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50414954

(CHEMBL575966)Show InChI InChI=1S/C18H14Cl2N2O2/c1-11-16(17(21-24-11)14-5-3-4-6-15(14)20)18(23)22(2)13-9-7-12(19)8-10-13/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 52: 7962-5 (2009)
Article DOI: 10.1021/jm901434t
BindingDB Entry DOI: 10.7270/Q2Z89DPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50414955

(CHEMBL576138)Show SMILES CN(C(=O)c1c(C)onc1-c1ccccc1Cl)c1ccc(C)c(Cl)c1 Show InChI InChI=1S/C19H16Cl2N2O2/c1-11-8-9-13(10-16(11)21)23(3)19(24)17-12(2)25-22-18(17)14-6-4-5-7-15(14)20/h4-10H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 52: 7962-5 (2009)
Article DOI: 10.1021/jm901434t
BindingDB Entry DOI: 10.7270/Q2Z89DPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50414954

(CHEMBL575966)Show InChI InChI=1S/C18H14Cl2N2O2/c1-11-16(17(21-24-11)14-5-3-4-6-15(14)20)18(23)22(2)13-9-7-12(19)8-10-13/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 1363-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.003
BindingDB Entry DOI: 10.7270/Q2028SS5 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159549

(CHEMBL3786247)Show SMILES COc1c(NC(=O)C(=O)c2ccc(OCCN3CCOCC3)c3ccccc23)cc(cc1NS(=O)(=O)CCCN(CC(O)=O)CC(O)=O)C(C)(C)C Show InChI InChI=1S/C36H46N4O11S/c1-36(2,3)24-20-28(34(49-4)29(21-24)38-52(47,48)19-7-12-40(22-31(41)42)23-32(43)44)37-35(46)33(45)27-10-11-30(26-9-6-5-8-25(26)27)51-18-15-39-13-16-50-17-14-39/h5-6,8-11,20-21,38H,7,12-19,22-23H2,1-4H3,(H,37,46)(H,41,42)(H,43,44) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50414955

(CHEMBL576138)Show SMILES CN(C(=O)c1c(C)onc1-c1ccccc1Cl)c1ccc(C)c(Cl)c1 Show InChI InChI=1S/C19H16Cl2N2O2/c1-11-8-9-13(10-16(11)21)23(3)19(24)17-12(2)25-22-18(17)14-6-4-5-7-15(14)20/h4-10H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 7962-5 (2009)
Article DOI: 10.1021/jm901434t
BindingDB Entry DOI: 10.7270/Q2Z89DPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50414955

(CHEMBL576138)Show SMILES CN(C(=O)c1c(C)onc1-c1ccccc1Cl)c1ccc(C)c(Cl)c1 Show InChI InChI=1S/C19H16Cl2N2O2/c1-11-8-9-13(10-16(11)21)23(3)19(24)17-12(2)25-22-18(17)14-6-4-5-7-15(14)20/h4-10H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 7962-5 (2009)
Article DOI: 10.1021/jm901434t
BindingDB Entry DOI: 10.7270/Q2Z89DPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50414955

(CHEMBL576138)Show SMILES CN(C(=O)c1c(C)onc1-c1ccccc1Cl)c1ccc(C)c(Cl)c1 Show InChI InChI=1S/C19H16Cl2N2O2/c1-11-8-9-13(10-16(11)21)23(3)19(24)17-12(2)25-22-18(17)14-6-4-5-7-15(14)20/h4-10H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 52: 7962-5 (2009)
Article DOI: 10.1021/jm901434t
BindingDB Entry DOI: 10.7270/Q2Z89DPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50414954

(CHEMBL575966)Show InChI InChI=1S/C18H14Cl2N2O2/c1-11-16(17(21-24-11)14-5-3-4-6-15(14)20)18(23)22(2)13-9-7-12(19)8-10-13/h3-10H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 52: 7962-5 (2009)
Article DOI: 10.1021/jm901434t
BindingDB Entry DOI: 10.7270/Q2Z89DPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50414954

(CHEMBL575966)Show InChI InChI=1S/C18H14Cl2N2O2/c1-11-16(17(21-24-11)14-5-3-4-6-15(14)20)18(23)22(2)13-9-7-12(19)8-10-13/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 7962-5 (2009)
Article DOI: 10.1021/jm901434t
BindingDB Entry DOI: 10.7270/Q2Z89DPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50414954

(CHEMBL575966)Show InChI InChI=1S/C18H14Cl2N2O2/c1-11-16(17(21-24-11)14-5-3-4-6-15(14)20)18(23)22(2)13-9-7-12(19)8-10-13/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 52: 7962-5 (2009)
Article DOI: 10.1021/jm901434t
BindingDB Entry DOI: 10.7270/Q2Z89DPD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50044849
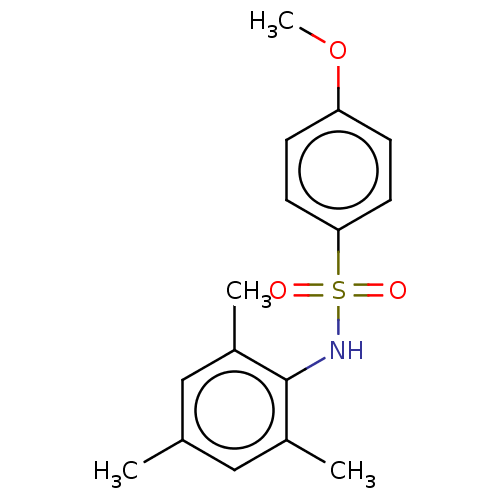
(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M2 receptor expressed in CHO cells assessed as intracellular calcium level by fluorescence/summary (Abse5) as... |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human CaV1.2 channel expressed in HEK293 cells by FLIPR/summary (Abse5) assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as intracellular calcium level by fluorescence/summary (Abse5) as... |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARdelta by SPA/summary (Abse5) assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARdelta by SPA/Abse5 assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARalpha by SPA/Abse5 assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K gamma by TR-FRET/summary (Abse5) assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B by luminescence assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4 receptor assessed as cAMP level by HTRF LANCE/summary (Abse5) assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3B by FP assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Activin receptor type-2B
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human ACVR2B by Ant A204 luciferase reporter/summary (Abse5) assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by SPA/Abse5 assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by SPA/summary (Abse5) assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50044874

(CHEMBL3311302)Show InChI InChI=1S/C20H17NO3S/c1-14-10-12-15(13-11-14)25(22,23)21-20-16-6-2-4-8-18(16)24-19-9-5-3-7-17(19)20/h2-13,20-21H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human FFA1 receptor expressed in U2OS cells |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARalpha by SPA/summary (Abse5) assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D2 receptor by GTPgS SPA assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50159558

(CHEMBL3786009)Show SMILES COc1c(NC(=O)C(=O)c2ccc(OCCN(CC(O)=O)CC(O)=O)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C30H35N3O10S/c1-30(2,3)18-14-22(28(42-4)23(15-18)32-44(5,40)41)31-29(39)27(38)21-10-11-24(20-9-7-6-8-19(20)21)43-13-12-33(16-25(34)35)17-26(36)37/h6-11,14-15,32H,12-13,16-17H2,1-5H3,(H,31,39)(H,34,35)(H,36,37) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... |
Bioorg Med Chem Lett 26: 1901-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.025
BindingDB Entry DOI: 10.7270/Q22R3TJ2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1B receptor by LEADseeker GTPgS/summary (Abse5) assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human beta 2 adrenergic receptor by TR FRET/summary (Abse5) assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50044849

(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D2 receptor by GTPgS SPA/summary (Abse5) assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data