 Found 413 hits with Last Name = 'jeay' and Initial = 's'
Found 413 hits with Last Name = 'jeay' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Canis lupus familiaris) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of dog MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Mus musculus) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Canis lupus familiaris) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of dog MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Mus musculus) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50467282
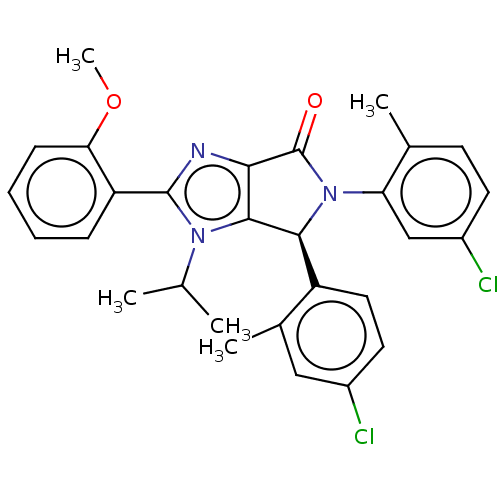
(CHEMBL4290086)Show SMILES COc1ccccc1-c1nc2C(=O)N([C@H](c2n1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C |r| Show InChI InChI=1S/C29H27Cl2N3O2/c1-16(2)33-27-25(32-28(33)22-8-6-7-9-24(22)36-5)29(35)34(23-15-20(31)11-10-17(23)3)26(27)21-13-12-19(30)14-18(21)4/h6-16,26H,1-5H3/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay |
Bioorg Med Chem Lett 28: 3404-3408 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.027
BindingDB Entry DOI: 10.7270/Q2C82D0H |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM129796

(US8815926, 75)Show SMILES COc1ncc(-c2nc3C(=O)N([C@H](c3n2C(C)C)c2ccc(Cl)cc2C)c2cc(Cl)ccc2C)c(OC)n1 |r| Show InChI InChI=1S/C28H27Cl2N5O3/c1-14(2)34-24-22(32-25(34)20-13-31-28(38-6)33-26(20)37-5)27(36)35(21-12-18(30)8-7-15(21)3)23(24)19-10-9-17(29)11-16(19)4/h7-14,23H,1-6H3/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay |
Bioorg Med Chem Lett 28: 3404-3408 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.027
BindingDB Entry DOI: 10.7270/Q2C82D0H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM143546
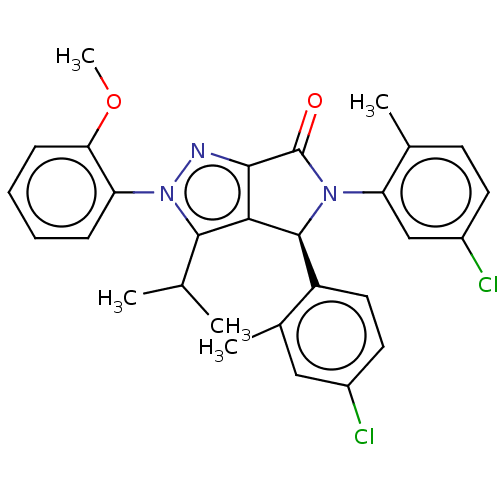
(US8969341, 141)Show SMILES COc1ccccc1-n1nc2C(=O)N([C@H](c2c1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C |r| Show InChI InChI=1S/C29H27Cl2N3O2/c1-16(2)27-25-26(32-34(27)22-8-6-7-9-24(22)36-5)29(35)33(23-15-20(31)11-10-17(23)3)28(25)21-13-12-19(30)14-18(21)4/h6-16,28H,1-5H3/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay |
Bioorg Med Chem Lett 28: 3404-3408 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.027
BindingDB Entry DOI: 10.7270/Q2C82D0H |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM129722
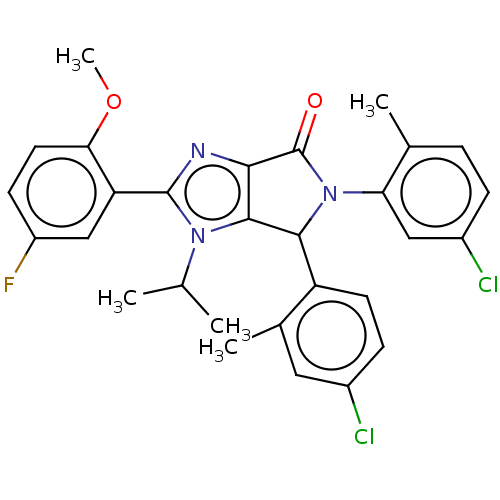
(US8815926, 1)Show SMILES COc1ccc(F)cc1-c1nc2C(=O)N(C(c2n1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C Show InChI InChI=1S/C29H26Cl2FN3O2/c1-15(2)34-27-25(33-28(34)22-14-20(32)9-11-24(22)37-5)29(36)35(23-13-19(31)7-6-16(23)3)26(27)21-10-8-18(30)12-17(21)4/h6-15,26H,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay |
Bioorg Med Chem Lett 28: 3404-3408 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.027
BindingDB Entry DOI: 10.7270/Q2C82D0H |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM129728
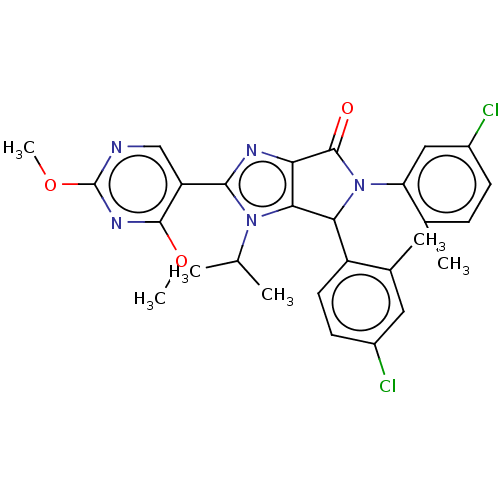
(US8815926, 7)Show SMILES COc1ncc(-c2nc3C(=O)N(C(c3n2C(C)C)c2ccc(Cl)cc2C)c2cc(Cl)ccc2C)c(OC)n1 Show InChI InChI=1S/C28H27Cl2N5O3/c1-14(2)34-24-22(32-25(34)20-13-31-28(38-6)33-26(20)37-5)27(36)35(21-12-18(30)8-7-15(21)3)23(24)19-10-9-17(29)11-16(19)4/h7-14,23H,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay |
Bioorg Med Chem Lett 28: 3404-3408 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.027
BindingDB Entry DOI: 10.7270/Q2C82D0H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM129723
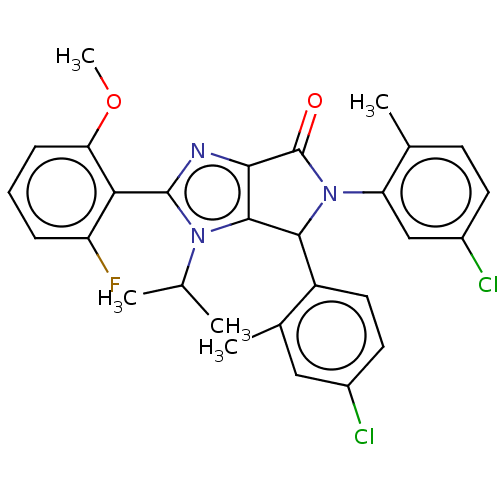
(US8815926, 2)Show SMILES COc1cccc(F)c1-c1nc2C(=O)N(C(c2n1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C |(4.68,5.6,;3.91,4.26,;4.68,2.93,;6.22,2.93,;6.99,1.6,;6.22,.26,;4.68,.26,;3.91,-1.07,;3.91,1.6,;2.37,1.6,;1.46,2.84,;,2.37,;-1.46,2.84,;-2.23,4.18,;-2.37,1.6,;-1.46,.35,;,.83,;1.46,.35,;1.86,-1.14,;.77,-2.22,;3.35,-1.53,;-1.86,-1.14,;-.77,-2.22,;-1.17,-3.71,;-2.66,-4.11,;-3.06,-5.6,;-3.75,-3.02,;-3.35,-1.53,;-4.44,-.45,;-3.91,1.6,;-4.68,.26,;-6.22,.26,;-6.99,-1.07,;-6.99,1.6,;-6.22,2.93,;-4.68,2.93,;-3.91,4.26,)| Show InChI InChI=1S/C29H26Cl2FN3O2/c1-15(2)34-27-25(33-28(34)24-21(32)7-6-8-23(24)37-5)29(36)35(22-14-19(31)10-9-16(22)3)26(27)20-12-11-18(30)13-17(20)4/h6-15,26H,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay |
Bioorg Med Chem Lett 28: 3404-3408 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.027
BindingDB Entry DOI: 10.7270/Q2C82D0H |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM129750

(US8815926, 29)Show SMILES COc1ccc(-c2nc3C(=O)N(C(c3n2C(C)C)c2ccc(Cl)cc2C)c2cc(Cl)ccc2C)c(OC)n1 Show InChI InChI=1S/C29H28Cl2N4O3/c1-15(2)34-26-24(33-27(34)21-11-12-23(37-5)32-28(21)38-6)29(36)35(22-14-19(31)8-7-16(22)3)25(26)20-10-9-18(30)13-17(20)4/h7-15,25H,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay |
Bioorg Med Chem Lett 28: 3404-3408 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.027
BindingDB Entry DOI: 10.7270/Q2C82D0H |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50467283

(CHEMBL4293896)Show SMILES COc1ccncc1-c1nc2C(=O)N([C@H](c2n1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C |r| Show InChI InChI=1S/C28H26Cl2N4O2/c1-15(2)33-26-24(32-27(33)21-14-31-11-10-23(21)36-5)28(35)34(22-13-19(30)7-6-16(22)3)25(26)20-9-8-18(29)12-17(20)4/h6-15,25H,1-5H3/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay |
Bioorg Med Chem Lett 28: 3404-3408 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.027
BindingDB Entry DOI: 10.7270/Q2C82D0H |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM129751
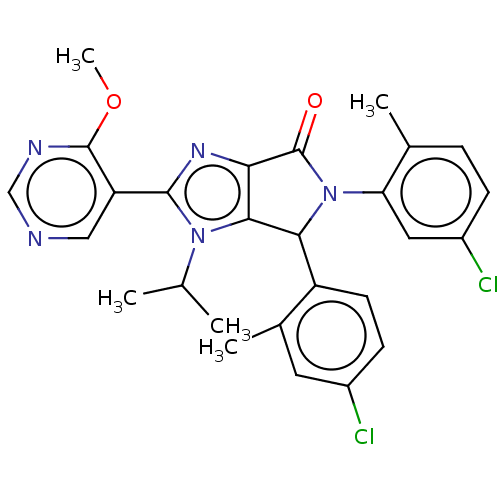
(US8815926, 30)Show SMILES COc1ncncc1-c1nc2C(=O)N(C(c2n1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C Show InChI InChI=1S/C27H25Cl2N5O2/c1-14(2)33-24-22(32-25(33)20-12-30-13-31-26(20)36-5)27(35)34(21-11-18(29)7-6-15(21)3)23(24)19-9-8-17(28)10-16(19)4/h6-14,23H,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay |
Bioorg Med Chem Lett 28: 3404-3408 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.027
BindingDB Entry DOI: 10.7270/Q2C82D0H |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887
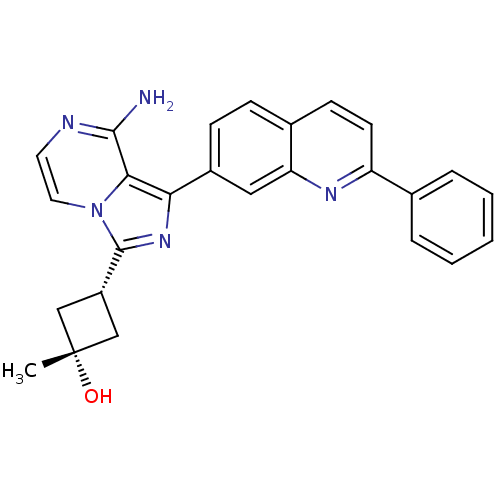
((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 st... |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158492
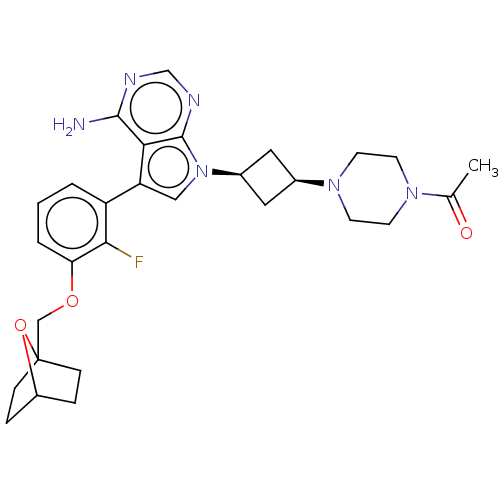
(CHEMBL3785376)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cccc(OCC34CCC(CC3)O4)c2F)c2c(N)ncnc12 |r,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.78,8.6,;-1.87,9.66,;-1.5,11.15,;-.02,11.55,;1.07,10.49,;-.08,10.33,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C29H35FN6O3/c1-18(37)34-9-11-35(12-10-34)19-13-20(14-19)36-15-23(25-27(31)32-17-33-28(25)36)22-3-2-4-24(26(22)30)38-16-29-7-5-21(39-29)6-8-29/h2-4,15,17,19-21H,5-14,16H2,1H3,(H2,31,32,33)/t19-,20+,21?,29? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 st... |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158493
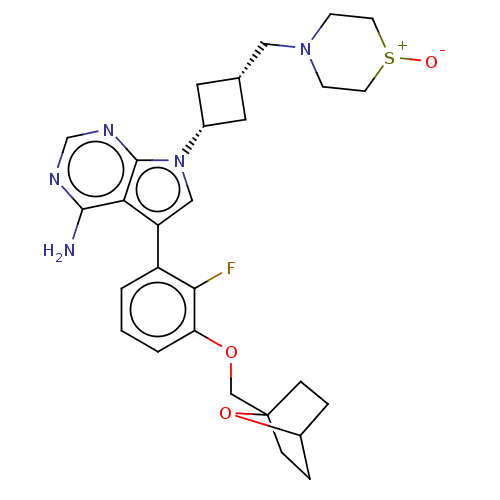
(CHEMBL3787360)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCC45CCC(CC4)O5)c3F)c12)[C@@H]1C[C@H](CN2CC[S+]([O-])CC2)C1 |r,wD:26.30,28.33,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.78,8.6,;-1.87,9.66,;-1.5,11.15,;-.02,11.55,;1.07,10.49,;-.08,10.33,;1.29,3.91,;.07,3.74,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;4.94,-8.18,;6.42,-8.61,;7.53,-7.55,;8.72,-7.9,;7.17,-6.05,;5.69,-5.62,;1.42,-3.96,)| Show InChI InChI=1S/C28H34FN5O3S/c29-25-21(2-1-3-23(25)36-16-28-6-4-20(37-28)5-7-28)22-15-34(27-24(22)26(30)31-17-32-27)19-12-18(13-19)14-33-8-10-38(35)11-9-33/h1-3,15,17-20H,4-14,16H2,(H2,30,31,32)/t18-,19+,20?,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158492

(CHEMBL3785376)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cccc(OCC34CCC(CC3)O4)c2F)c2c(N)ncnc12 |r,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.78,8.6,;-1.87,9.66,;-1.5,11.15,;-.02,11.55,;1.07,10.49,;-.08,10.33,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C29H35FN6O3/c1-18(37)34-9-11-35(12-10-34)19-13-20(14-19)36-15-23(25-27(31)32-17-33-28(25)36)22-3-2-4-24(26(22)30)38-16-29-7-5-21(39-29)6-8-29/h2-4,15,17,19-21H,5-14,16H2,1H3,(H2,31,32,33)/t19-,20+,21?,29? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50157880
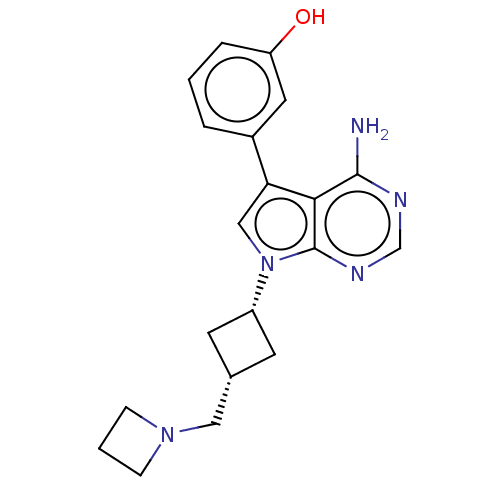
(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158505
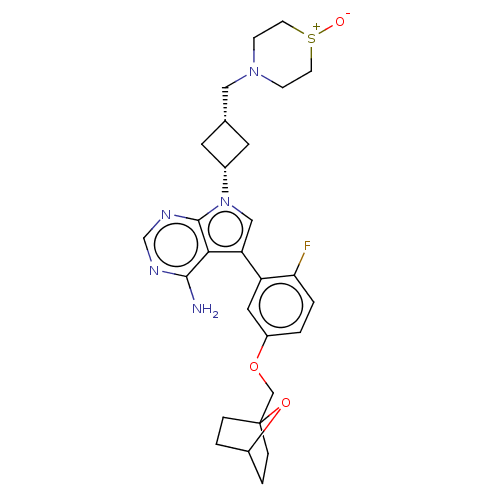
(CHEMBL3786100)Show SMILES Nc1ncnc2n(cc(-c3cc(OCC45CCC(CC4)O5)ccc3F)c12)[C@@H]1C[C@H](CN2CC[S+]([O-])CC2)C1 |r,wD:26.30,28.33,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;8.5,4.87,;10.02,4.88,;10.78,6.2,;9.99,7.52,;8.47,7.51,;9.19,6.61,;3.38,5.56,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;4.94,-8.18,;6.42,-8.61,;7.53,-7.55,;8.72,-7.9,;7.17,-6.05,;5.69,-5.62,;1.42,-3.96,)| Show InChI InChI=1S/C28H34FN5O3S/c29-24-2-1-21(36-16-28-5-3-20(37-28)4-6-28)13-22(24)23-15-34(27-25(23)26(30)31-17-32-27)19-11-18(12-19)14-33-7-9-38(35)10-8-33/h1-2,13,15,17-20H,3-12,14,16H2,(H2,30,31,32)/t18-,19+,20?,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158508
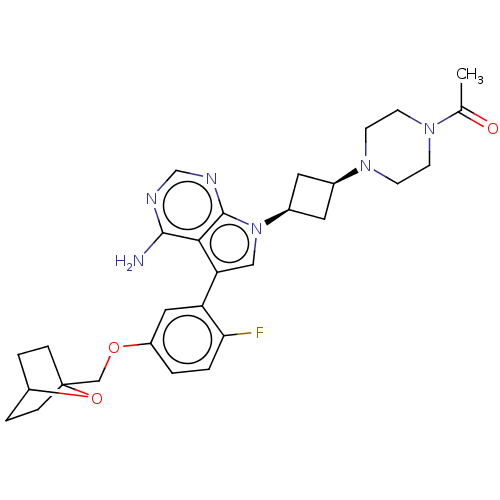
(CHEMBL3787271)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cc(OCC34CCC(CC3)O4)ccc2F)c2c(N)ncnc12 |r,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;8.5,4.87,;10.02,4.88,;10.78,6.2,;9.99,7.52,;8.47,7.51,;9.19,6.61,;3.38,5.56,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C29H35FN6O3/c1-18(37)34-8-10-35(11-9-34)19-12-20(13-19)36-15-24(26-27(31)32-17-33-28(26)36)23-14-22(2-3-25(23)30)38-16-29-6-4-21(39-29)5-7-29/h2-3,14-15,17,19-21H,4-13,16H2,1H3,(H2,31,32,33)/t19-,20+,21?,29? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of RET (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158505

(CHEMBL3786100)Show SMILES Nc1ncnc2n(cc(-c3cc(OCC45CCC(CC4)O5)ccc3F)c12)[C@@H]1C[C@H](CN2CC[S+]([O-])CC2)C1 |r,wD:26.30,28.33,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;8.5,4.87,;10.02,4.88,;10.78,6.2,;9.99,7.52,;8.47,7.51,;9.19,6.61,;3.38,5.56,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;4.94,-8.18,;6.42,-8.61,;7.53,-7.55,;8.72,-7.9,;7.17,-6.05,;5.69,-5.62,;1.42,-3.96,)| Show InChI InChI=1S/C28H34FN5O3S/c29-24-2-1-21(36-16-28-5-3-20(37-28)4-6-28)13-22(24)23-15-34(27-25(23)26(30)31-17-32-27)19-11-18(12-19)14-33-7-9-38(35)10-8-33/h1-2,13,15,17-20H,3-12,14,16H2,(H2,30,31,32)/t18-,19+,20?,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158505

(CHEMBL3786100)Show SMILES Nc1ncnc2n(cc(-c3cc(OCC45CCC(CC4)O5)ccc3F)c12)[C@@H]1C[C@H](CN2CC[S+]([O-])CC2)C1 |r,wD:26.30,28.33,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;8.5,4.87,;10.02,4.88,;10.78,6.2,;9.99,7.52,;8.47,7.51,;9.19,6.61,;3.38,5.56,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;4.94,-8.18,;6.42,-8.61,;7.53,-7.55,;8.72,-7.9,;7.17,-6.05,;5.69,-5.62,;1.42,-3.96,)| Show InChI InChI=1S/C28H34FN5O3S/c29-24-2-1-21(36-16-28-5-3-20(37-28)4-6-28)13-22(24)23-15-34(27-25(23)26(30)31-17-32-27)19-11-18(12-19)14-33-7-9-38(35)10-8-33/h1-2,13,15,17-20H,3-12,14,16H2,(H2,30,31,32)/t18-,19+,20?,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 st... |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158491
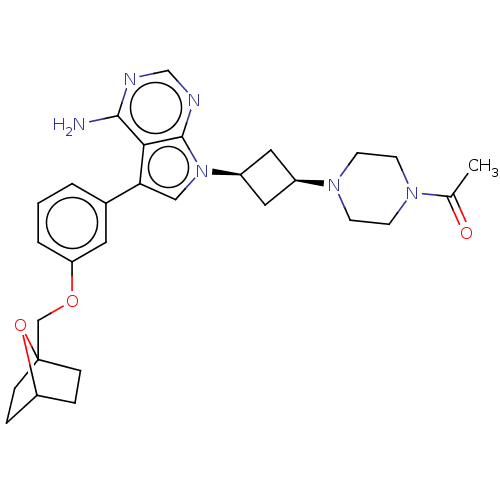
(CHEMBL3787636)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cccc(OCC34CCC(CC3)O4)c2)c2c(N)ncnc12 |r,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.78,8.6,;-1.87,9.66,;-1.5,11.15,;-.02,11.55,;1.07,10.49,;-.08,10.33,;1.29,3.91,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C29H36N6O3/c1-19(36)33-9-11-34(12-10-33)21-14-22(15-21)35-16-25(26-27(30)31-18-32-28(26)35)20-3-2-4-24(13-20)37-17-29-7-5-23(38-29)6-8-29/h2-4,13,16,18,21-23H,5-12,14-15,17H2,1H3,(H2,30,31,32)/t21-,22+,23?,29? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158492

(CHEMBL3785376)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cccc(OCC34CCC(CC3)O4)c2F)c2c(N)ncnc12 |r,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.78,8.6,;-1.87,9.66,;-1.5,11.15,;-.02,11.55,;1.07,10.49,;-.08,10.33,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C29H35FN6O3/c1-18(37)34-9-11-35(12-10-34)19-13-20(14-19)36-15-23(25-27(31)32-17-33-28(25)36)22-3-2-4-24(26(22)30)38-16-29-7-5-21(39-29)6-8-29/h2-4,15,17,19-21H,5-14,16H2,1H3,(H2,31,32,33)/t19-,20+,21?,29? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay |
Bioorg Med Chem Lett 28: 3404-3408 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.027
BindingDB Entry DOI: 10.7270/Q2C82D0H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158491

(CHEMBL3787636)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cccc(OCC34CCC(CC3)O4)c2)c2c(N)ncnc12 |r,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.78,8.6,;-1.87,9.66,;-1.5,11.15,;-.02,11.55,;1.07,10.49,;-.08,10.33,;1.29,3.91,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C29H36N6O3/c1-19(36)33-9-11-34(12-10-33)21-14-22(15-21)35-16-25(26-27(30)31-18-32-28(26)35)20-3-2-4-24(13-20)37-17-29-7-5-23(38-29)6-8-29/h2-4,13,16,18,21-23H,5-12,14-15,17H2,1H3,(H2,30,31,32)/t21-,22+,23?,29? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 st... |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158428
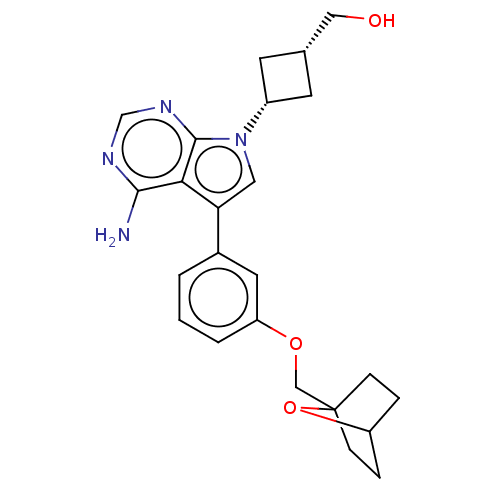
(CHEMBL3787567)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCC45CCC(CC4)O5)c3)c12)[C@@H]1C[C@H](CO)C1 |r,wD:25.29,27.32,(-1.03,2.79,;-1.03,1.56,;-2.38,.77,;-2.38,-.77,;-1.03,-1.56,;.3,-.77,;1.77,-1.24,;2.67,.02,;1.77,1.24,;2.24,2.71,;3.77,2.92,;4.35,4.36,;3.4,5.57,;1.87,5.35,;.91,6.57,;1.49,8.01,;.54,9.22,;-.78,8.63,;-1.88,9.69,;-1.51,11.18,;-.02,11.58,;1.07,10.52,;-.08,10.37,;1.29,3.92,;.3,.77,;2.25,-2.71,;3.55,-3.46,;2.75,-4.78,;3.11,-6.27,;4.29,-6.62,;1.43,-3.97,)| Show InChI InChI=1S/C24H28N4O3/c25-22-21-20(11-28(23(21)27-14-26-22)17-8-15(9-17)12-29)16-2-1-3-19(10-16)30-13-24-6-4-18(31-24)5-7-24/h1-3,10-11,14-15,17-18,29H,4-9,12-13H2,(H2,25,26,27)/t15-,17+,18?,24? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50156383

(CHEMBL3785951)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cc(OC[C@@H]3CCCO3)ccc2F)c2c(N)ncnc12 |r,wU:21.22,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;9.01,5.1,;10.39,5.78,;10.17,7.31,;8.66,7.57,;3.38,5.55,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33FN6O3/c1-17(35)32-6-8-33(9-7-32)18-11-19(12-18)34-14-23(25-26(29)30-16-31-27(25)34)22-13-20(4-5-24(22)28)37-15-21-3-2-10-36-21/h4-5,13-14,16,18-19,21H,2-3,6-12,15H2,1H3,(H2,29,30,31)/t18-,19+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50156375
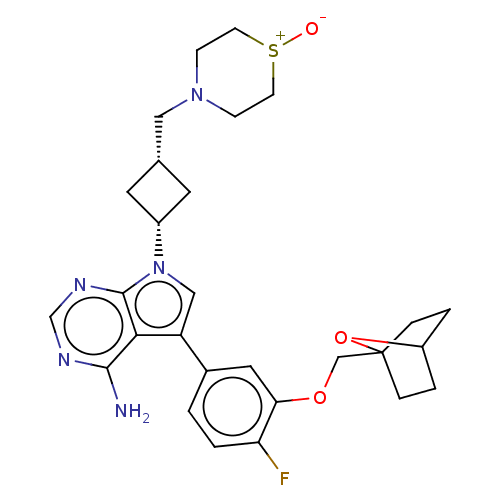
(CHEMBL3785995)Show SMILES Nc1ncnc2n(cc(-c3ccc(F)c(OCC45CCC(CC4)O5)c3)c12)[C@@H]1C[C@H](CN2CC[S+]([O-])CC2)C1 |r,wD:26.30,28.33,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;3.84,6.7,;1.86,5.34,;.91,6.55,;-.62,6.33,;-1.57,7.54,;-2.89,6.94,;-3.98,7.99,;-3.62,9.48,;-2.14,9.89,;-1.05,8.84,;-2.2,8.68,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;4.94,-8.18,;6.42,-8.61,;7.53,-7.55,;8.72,-7.9,;7.17,-6.05,;5.69,-5.62,;1.42,-3.96,)| Show InChI InChI=1S/C28H34FN5O3S/c29-23-2-1-19(13-24(23)36-16-28-5-3-21(37-28)4-6-28)22-15-34(27-25(22)26(30)31-17-32-27)20-11-18(12-20)14-33-7-9-38(35)10-8-33/h1-2,13,15,17-18,20-21H,3-12,14,16H2,(H2,30,31,32)/t18-,20+,21?,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157881
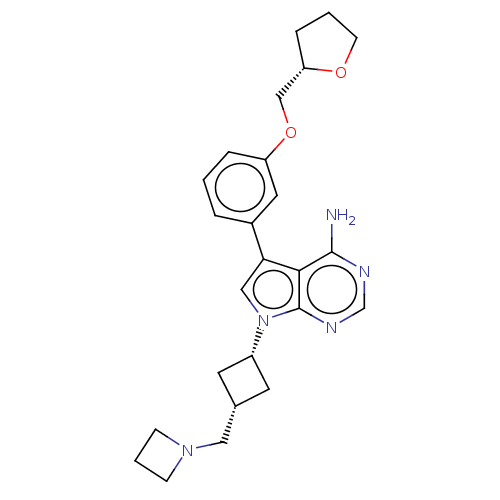
(CHEMBL3787614)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,16.15,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158508

(CHEMBL3787271)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cc(OCC34CCC(CC3)O4)ccc2F)c2c(N)ncnc12 |r,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;8.5,4.87,;10.02,4.88,;10.78,6.2,;9.99,7.52,;8.47,7.51,;9.19,6.61,;3.38,5.56,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C29H35FN6O3/c1-18(37)34-8-10-35(11-9-34)19-12-20(13-19)36-15-24(26-27(31)32-17-33-28(26)36)23-14-22(2-3-25(23)30)38-16-29-6-4-21(39-29)5-7-29/h2-3,14-15,17,19-21H,4-13,16H2,1H3,(H2,31,32,33)/t19-,20+,21?,29? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 st... |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50156383

(CHEMBL3785951)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cc(OC[C@@H]3CCCO3)ccc2F)c2c(N)ncnc12 |r,wU:21.22,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;9.01,5.1,;10.39,5.78,;10.17,7.31,;8.66,7.57,;3.38,5.55,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33FN6O3/c1-17(35)32-6-8-33(9-7-32)18-11-19(12-18)34-14-23(25-26(29)30-16-31-27(25)34)22-13-20(4-5-24(22)28)37-15-21-3-2-10-36-21/h4-5,13-14,16,18-19,21H,2-3,6-12,15H2,1H3,(H2,29,30,31)/t18-,19+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 st... |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158508

(CHEMBL3787271)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cc(OCC34CCC(CC3)O4)ccc2F)c2c(N)ncnc12 |r,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;8.5,4.87,;10.02,4.88,;10.78,6.2,;9.99,7.52,;8.47,7.51,;9.19,6.61,;3.38,5.56,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C29H35FN6O3/c1-18(37)34-8-10-35(11-9-34)19-12-20(13-19)36-15-24(26-27(31)32-17-33-28(26)36)23-14-22(2-3-25(23)30)38-16-29-6-4-21(39-29)5-7-29/h2-3,14-15,17,19-21H,4-13,16H2,1H3,(H2,31,32,33)/t19-,20+,21?,29? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM129797
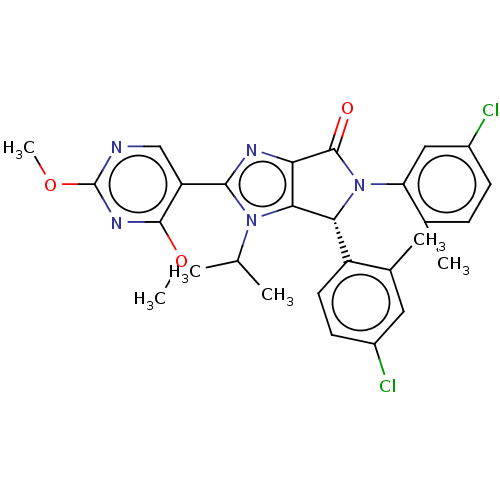
(US8815926, 76)Show SMILES COc1ncc(-c2nc3C(=O)N([C@@H](c3n2C(C)C)c2ccc(Cl)cc2C)c2cc(Cl)ccc2C)c(OC)n1 |r| Show InChI InChI=1S/C28H27Cl2N5O3/c1-14(2)34-24-22(32-25(34)20-13-31-28(38-6)33-26(20)37-5)27(36)35(21-12-18(30)8-7-15(21)3)23(24)19-10-9-17(29)11-16(19)4/h7-14,23H,1-6H3/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay |
Bioorg Med Chem Lett 28: 3404-3408 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.027
BindingDB Entry DOI: 10.7270/Q2C82D0H |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158429

(CHEMBL3785500)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCC45CCC(CC4)O5)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:25.29,27.32,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.92,;4.34,4.35,;3.39,5.56,;1.86,5.34,;.91,6.56,;1.49,7.99,;.54,9.2,;-.78,8.6,;-1.87,9.66,;-1.5,11.15,;-.02,11.55,;1.07,10.5,;-.08,10.34,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.26,;4.58,-6.69,;5.27,-8.03,;6.62,-7.29,;5.88,-5.94,;1.42,-3.96,)| Show InChI InChI=1S/C27H33N5O2/c28-25-24-23(19-3-1-4-22(13-19)33-16-27-7-5-21(34-27)6-8-27)15-32(26(24)30-17-29-25)20-11-18(12-20)14-31-9-2-10-31/h1,3-4,13,15,17-18,20-21H,2,5-12,14,16H2,(H2,28,29,30)/t18-,20+,21?,27? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158507
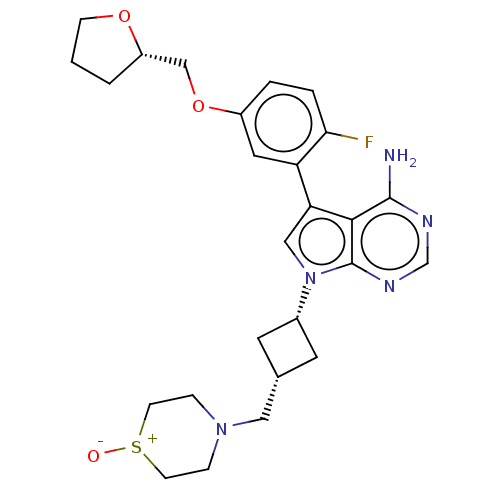
(CHEMBL3787436)Show SMILES Nc1ncnc2n(cc(-c3cc(OC[C@@H]4CCCO4)ccc3F)c12)[C@@H]1C[C@H](CN2CC[S+]([O-])CC2)C1 |r,wU:14.13,wD:24.27,26.30,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;9.01,5.1,;10.39,5.78,;10.17,7.31,;8.66,7.57,;3.38,5.55,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;4.94,-8.18,;6.42,-8.61,;7.53,-7.55,;8.72,-7.89,;7.17,-6.05,;5.69,-5.62,;1.42,-3.96,)| Show InChI InChI=1S/C26H32FN5O3S/c27-23-4-3-19(35-15-20-2-1-7-34-20)12-21(23)22-14-32(26-24(22)25(28)29-16-30-26)18-10-17(11-18)13-31-5-8-36(33)9-6-31/h3-4,12,14,16-18,20H,1-2,5-11,13,15H2,(H2,28,29,30)/t17-,18+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 st... |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157628
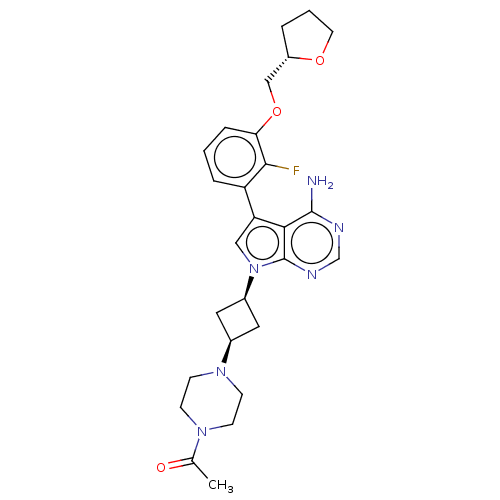
(CHEMBL3786167)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cccc(OC[C@@H]3CCCO3)c2F)c2c(N)ncnc12 |r,wD:11.14,23.24,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33FN6O3/c1-17(35)32-7-9-33(10-8-32)18-12-19(13-18)34-14-22(24-26(29)30-16-31-27(24)34)21-5-2-6-23(25(21)28)37-15-20-4-3-11-36-20/h2,5-6,14,16,18-20H,3-4,7-13,15H2,1H3,(H2,29,30,31)/t18-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157628

(CHEMBL3786167)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cccc(OC[C@@H]3CCCO3)c2F)c2c(N)ncnc12 |r,wD:11.14,23.24,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33FN6O3/c1-17(35)32-7-9-33(10-8-32)18-12-19(13-18)34-14-22(24-26(29)30-16-31-27(24)34)21-5-2-6-23(25(21)28)37-15-20-4-3-11-36-20/h2,5-6,14,16,18-20H,3-4,7-13,15H2,1H3,(H2,29,30,31)/t18-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 st... |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157628

(CHEMBL3786167)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cccc(OC[C@@H]3CCCO3)c2F)c2c(N)ncnc12 |r,wD:11.14,23.24,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33FN6O3/c1-17(35)32-7-9-33(10-8-32)18-12-19(13-18)34-14-22(24-26(29)30-16-31-27(24)34)21-5-2-6-23(25(21)28)37-15-20-4-3-11-36-20/h2,5-6,14,16,18-20H,3-4,7-13,15H2,1H3,(H2,29,30,31)/t18-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length insulin receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 ... |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50156383

(CHEMBL3785951)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cc(OC[C@@H]3CCCO3)ccc2F)c2c(N)ncnc12 |r,wU:21.22,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;9.01,5.1,;10.39,5.78,;10.17,7.31,;8.66,7.57,;3.38,5.55,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33FN6O3/c1-17(35)32-6-8-33(9-7-32)18-11-19(12-18)34-14-23(25-26(29)30-16-31-27(25)34)22-13-20(4-5-24(22)28)37-15-21-3-2-10-36-21/h4-5,13-14,16,18-19,21H,2-3,6-12,15H2,1H3,(H2,29,30,31)/t18-,19+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158507

(CHEMBL3787436)Show SMILES Nc1ncnc2n(cc(-c3cc(OC[C@@H]4CCCO4)ccc3F)c12)[C@@H]1C[C@H](CN2CC[S+]([O-])CC2)C1 |r,wU:14.13,wD:24.27,26.30,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;9.01,5.1,;10.39,5.78,;10.17,7.31,;8.66,7.57,;3.38,5.55,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;4.94,-8.18,;6.42,-8.61,;7.53,-7.55,;8.72,-7.89,;7.17,-6.05,;5.69,-5.62,;1.42,-3.96,)| Show InChI InChI=1S/C26H32FN5O3S/c27-23-4-3-19(35-15-20-2-1-7-34-20)12-21(23)22-14-32(26-24(22)25(28)29-16-30-26)18-10-17(11-18)13-31-5-8-36(33)9-6-31/h3-4,12,14,16-18,20H,1-2,5-11,13,15H2,(H2,28,29,30)/t17-,18+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50158491

(CHEMBL3787636)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cccc(OCC34CCC(CC3)O4)c2)c2c(N)ncnc12 |r,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.78,8.6,;-1.87,9.66,;-1.5,11.15,;-.02,11.55,;1.07,10.49,;-.08,10.33,;1.29,3.91,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C29H36N6O3/c1-19(36)33-9-11-34(12-10-33)21-14-22(15-21)35-16-25(26-27(30)31-18-32-28(26)35)20-3-2-4-24(13-20)37-17-29-7-5-23(38-29)6-8-29/h2-4,13,16,18,21-23H,5-12,14-15,17H2,1H3,(H2,30,31,32)/t21-,22+,23?,29? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data