

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444496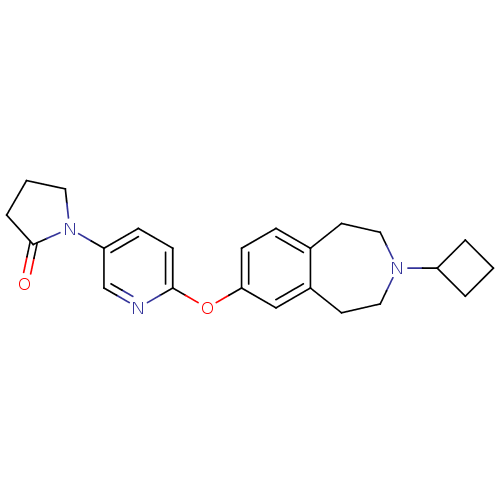 (CHEMBL3092650) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444491 (CHEMBL3092823) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403827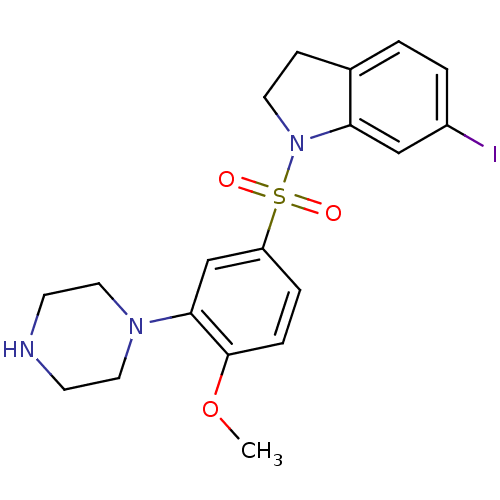 (CHEMBL414628) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403846 (CHEMBL356021) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50403827 (CHEMBL414628) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against human cloned 5-hydroxytryptamine 7 receptor in HEK 293 cells using [3H]5-CT as the radioligand (n... | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444496 (CHEMBL3092650) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444500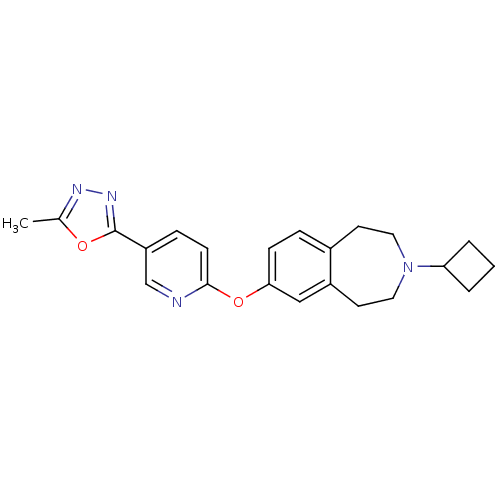 (CHEMBL3092834) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403851 (CHEMBL346338) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217828 (CHEMBL413707) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403850 (CHEMBL157454) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403839 (CHEMBL155717) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403840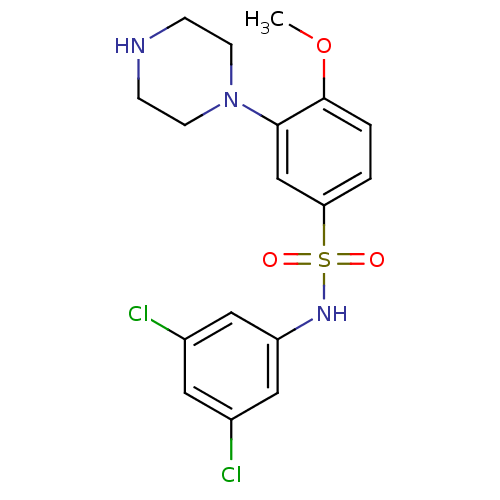 (CHEMBL345110) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444507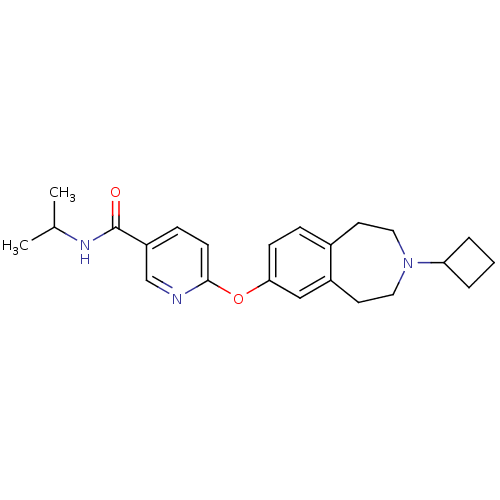 (CHEMBL3092826) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444505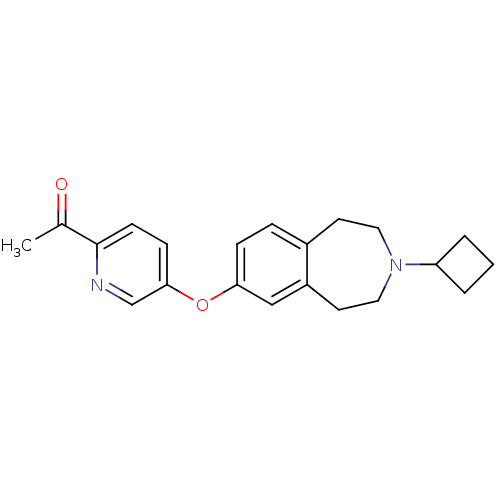 (CHEMBL3092828) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444509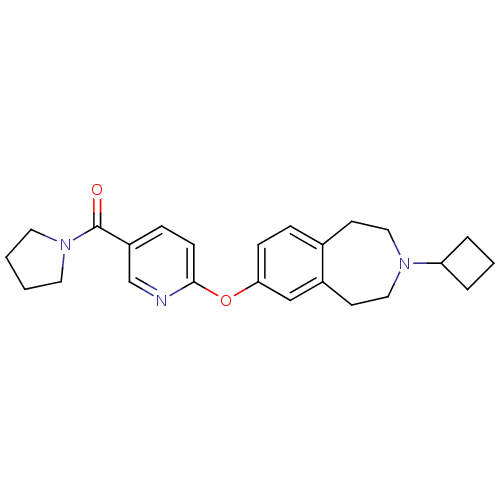 (CHEMBL3092840) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50247054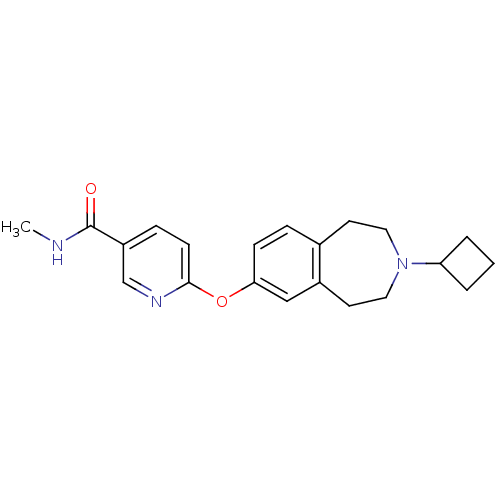 (6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217831 (CHEMBL430706) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217829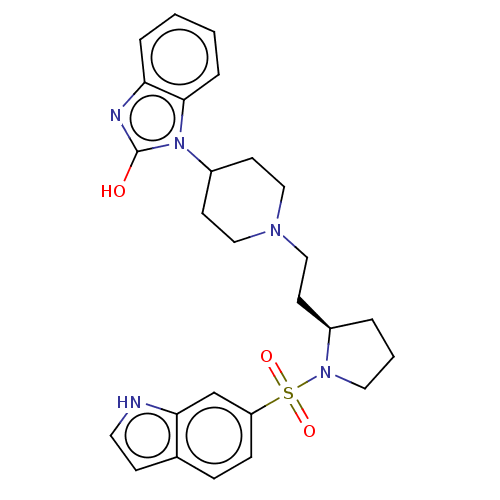 (CHEMBL115262) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444489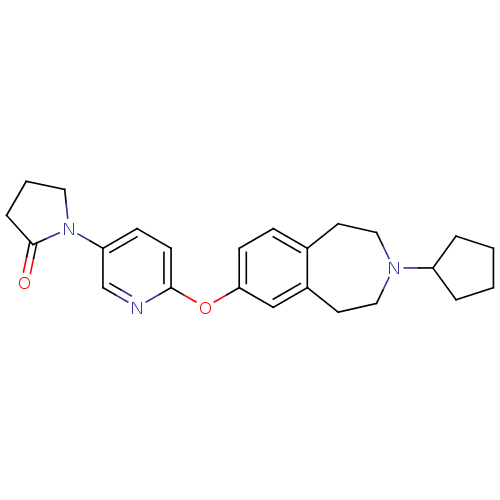 (CHEMBL3092825) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444495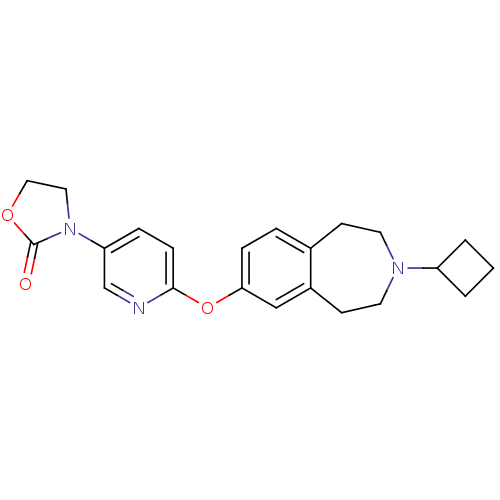 (CHEMBL3092651) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474384 (CHEMBL2113364) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403845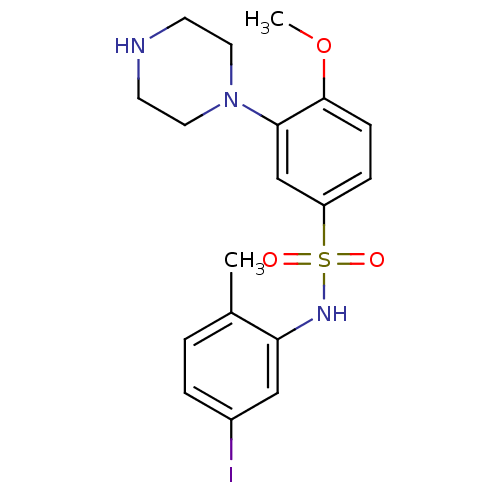 (CHEMBL154089) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403843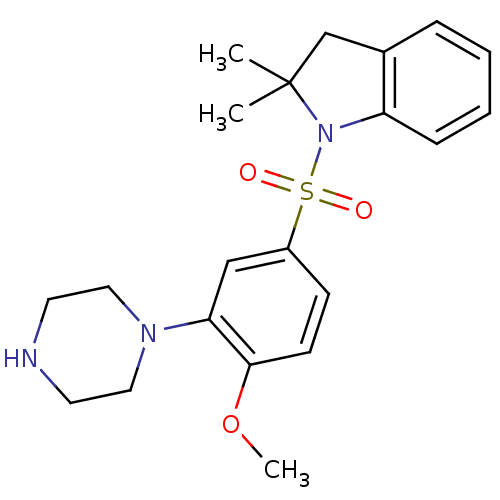 (CHEMBL151295) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217832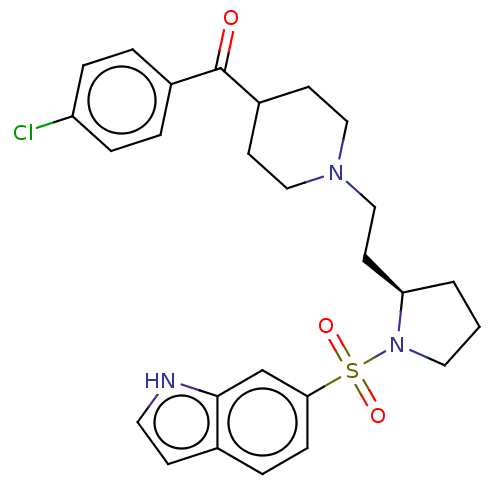 (CHEMBL116292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403861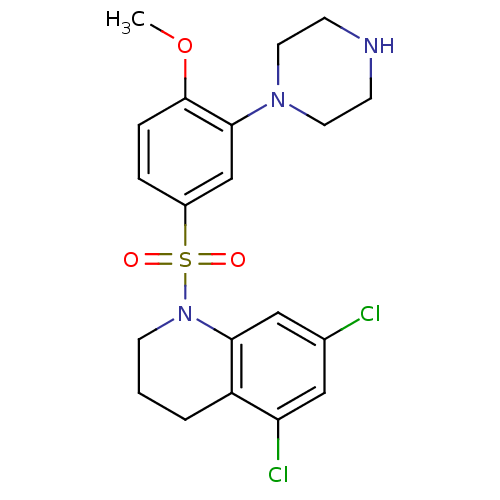 (CHEMBL151998) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50217835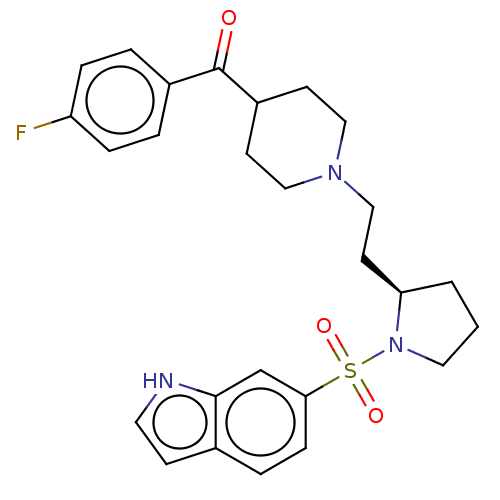 (CHEMBL114345) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346209 (5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474396 (CHEMBL2113356) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444506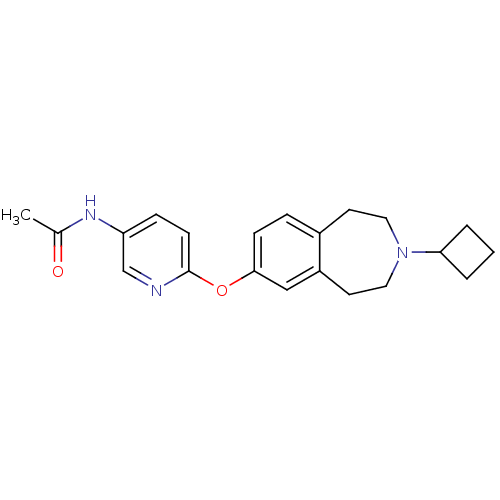 (CHEMBL3092827) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444494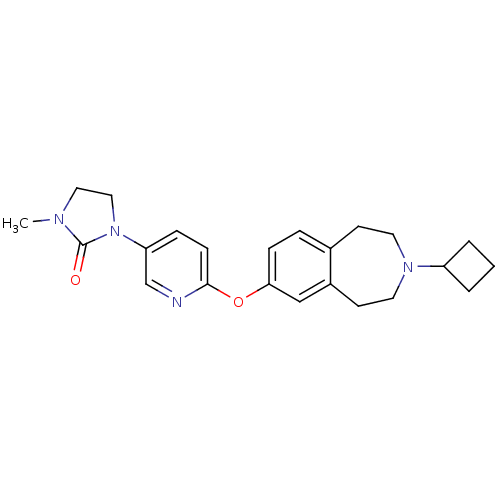 (CHEMBL3092820) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132022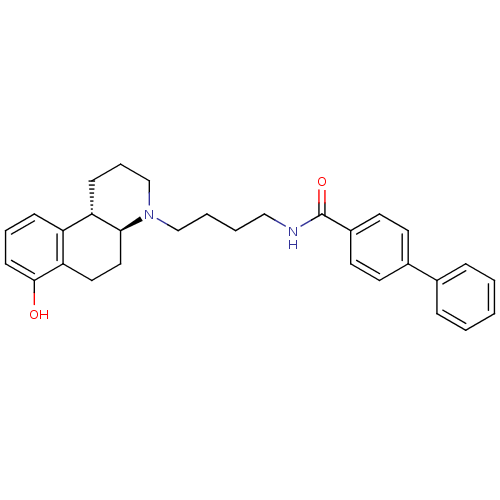 (Biphenyl-4-carboxylic acid [4-((4aS,10bS)-7-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50403826 (CHEMBL154274) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against human cloned 5-hydroxytryptamine 7 receptor in HEK 293 cells using [3H]5-CT as the radioligand(n=... | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403826 (CHEMBL154274) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403842 (CHEMBL154675) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474398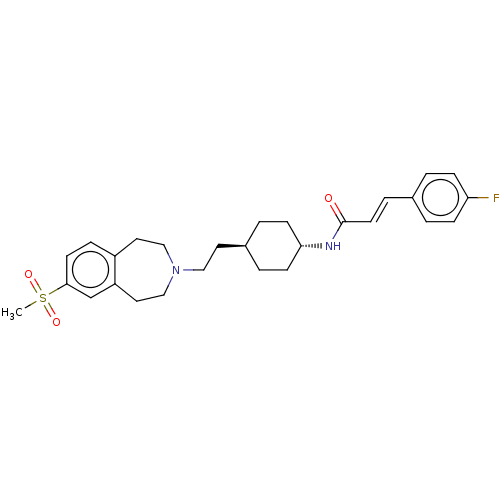 (CHEMBL2368629) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346209 (5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403837 (CHEMBL154367) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403838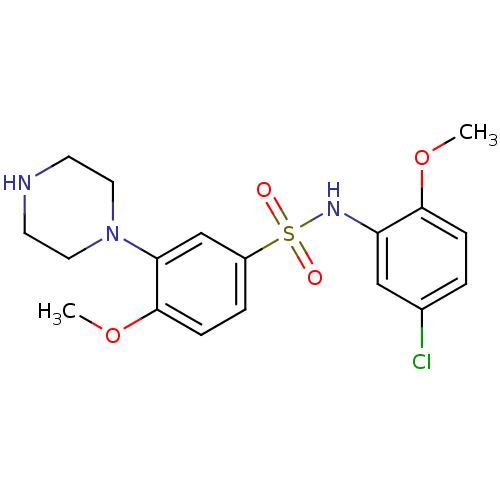 (CHEMBL154273) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474412 (CHEMBL2368622) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474389 (CHEMBL3084597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474399 (CHEMBL2368625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50217831 (CHEMBL430706) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1B adrenergic receptor | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474406 (CHEMBL3084598) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474403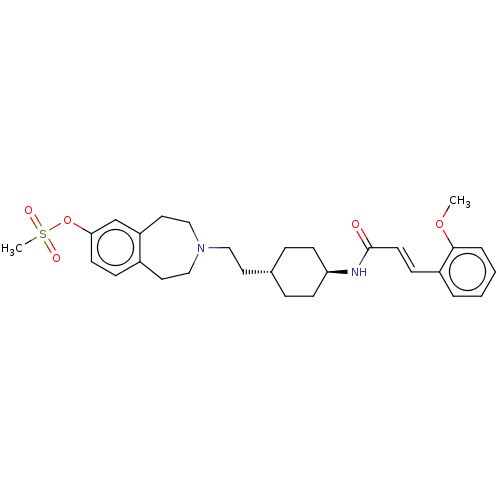 (CHEMBL2368633) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474395 (CHEMBL2368626) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50098551 ((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand | Bioorg Med Chem Lett 12: 3341-4 (2002) BindingDB Entry DOI: 10.7270/Q2FT8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403863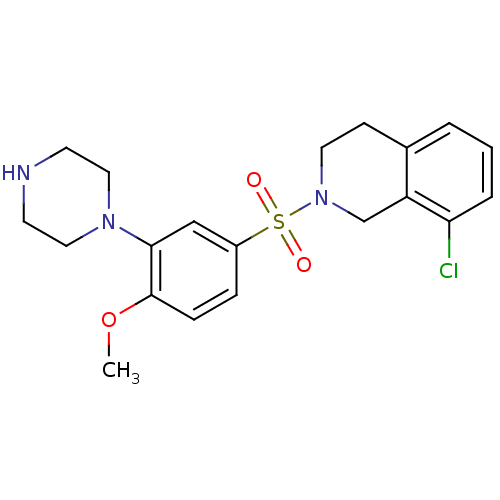 (CHEMBL157173) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50403859 (CHEMBL348870) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 6 receptor | Bioorg Med Chem Lett 11: 55-8 (2001) BindingDB Entry DOI: 10.7270/Q21R6RPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50444493 (CHEMBL3092821) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... | Bioorg Med Chem Lett 23: 6890-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.090 BindingDB Entry DOI: 10.7270/Q2PZ5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50000027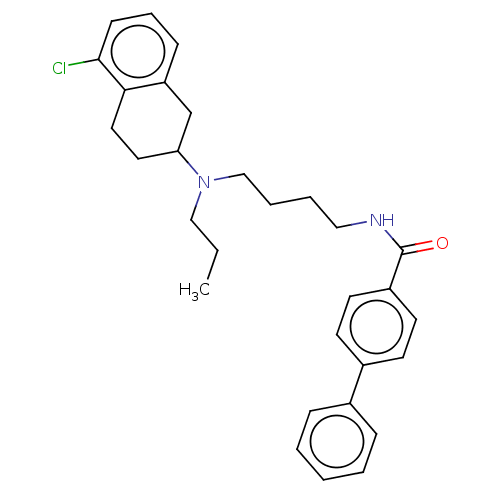 (CHEMBL51977) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | Bioorg Med Chem Lett 9: 179-84 (1999) BindingDB Entry DOI: 10.7270/Q2057J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 893 total ) | Next | Last >> |